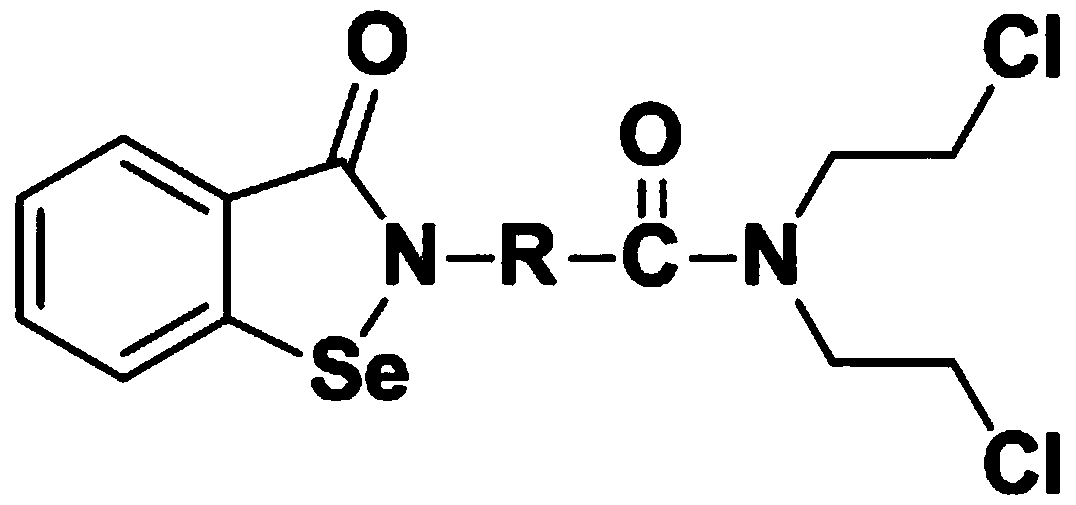
N,N-bis(2-chloroethyl)-2-(benzoisoselenazole-3-one)-amide compound with anti-tumor activity
An amide compound, benzisoselenazole technology, applied in antitumor drugs, organic chemistry, drug combination and other directions, can solve the problems affecting clinical use, poor selectivity of antitumor drugs, etc., to reduce drug resistance, improve Sensitivity, the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
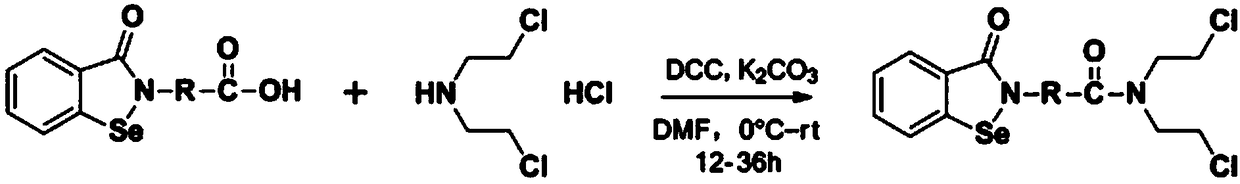
[0038] A method for preparing N,N-bis(2-chloroethyl)-2-(benzisoselazol-3-one)-amide compounds with antitumor activity, the preparation method specifically includes the following steps:
[0039] Step 1. Weigh 1.0 molar equivalent of benzisoselazol-3-one-2-aminocarboxylic acid, dissolve it in 200-1000 mL of organic solvent, and add 1.0~ 1.2 molar equivalent of condensation reagent, after stirring for 5-20min, add 1.0~3.0 molar equivalent of catalyst or / and 0.2~1.2 molar equivalent of additive, then add 0.8~5.0 molar equivalent of di(2-chloroethyl)amine Hydrochloride, stirred at room temperature for 12-36h to obtain the stirred product A;
[0040] Step 2, filter the product A stirred in step 1, then wash the filter cake with an organic solvent for 2-5 times, combine the organic phase after washing, remove the solvent under reduced pressure, use petroleum ether and ethyl acetate as the eluent, and use Separation on a silica gel column and evaporation of the solvent under reduced ...
Embodiment 1
[0048] Example 1: Preparation of N,N-bis(2-chloroethyl)-2-(benzisoselazol-3-one)-formamide
[0049]
[0050] Weigh 1.0 molar equivalent of benzisoselazol-3-one-2-carboxylic acid, dissolve in 200mL dimethylformamide (DMF) solution, add 1.0 molar equivalent of dicyclohexylcarbodiimide ( After DCC) was added, stir for 5 min, then respectively add 1.0 molar equivalent of potassium carbonate and 0.8 molar equivalent of di(2-chloroethyl)amine hydrochloride and stir at room temperature for 12 h, filter, and wash the filter cake with DMF (20 mL washing 3 times) , combined the organic phases, removed the solvent under reduced pressure, petroleum ether:ethyl acetate (V:V=3:1~8:1) was used as the eluent, separated on a silica gel column, and evaporated the solvent under reduced pressure to obtain the target compound. Yield 60%. HR-MS: 366.9514 (C12H12Cl2N2O2Se, [M+H]+).
Embodiment 2
[0051] Example 2: Preparation of N,N-bis(2-chloroethyl)-2-(benzisoselazol-3-one)-acetamide
[0052]
[0053] Weigh 1.0 molar equivalent of benzisoselazol-3-one-2-acetic acid, dissolve it in 500 mL of dimethylformamide (DMF) solution, add 1.1 molar equivalent of N,N'-diisopropyl After adding carbodiimide (DIC), stir for 20 min, then add 2.0 molar equivalents of potassium carbonate, 0.4 molar equivalents of 1-hydroxybenzotriazole (HOBt) and 1.0 molar equivalents of bis(2-chloroethyl ) amine hydrochloride, stirred at room temperature for 24h, filtered, washed the filter cake with DMF (20mL washed 3 times), combined the organic phases, removed the solvent under reduced pressure, petroleum ether:ethyl acetate (V:V=3:1~8:1) As the eluent, it was separated on a silica gel column, and the solvent was evaporated under reduced pressure to obtain the target compound. Yield 85%. HR-MS: 380.9670 (C13H14Cl2N2O2Se, [M+H]+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com