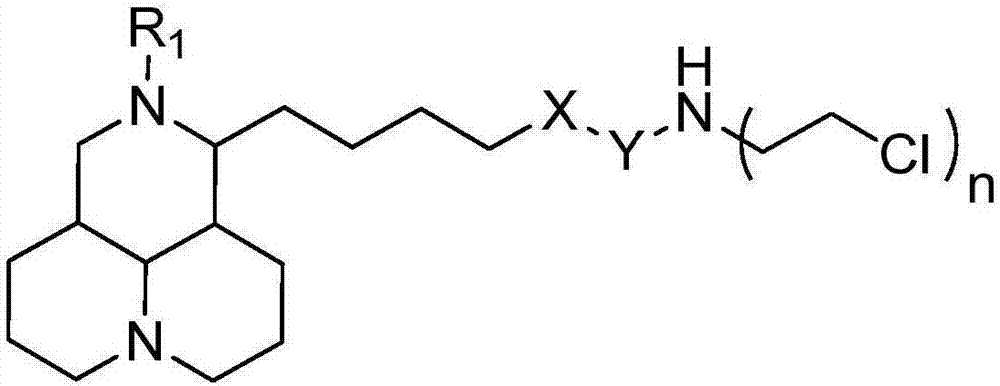
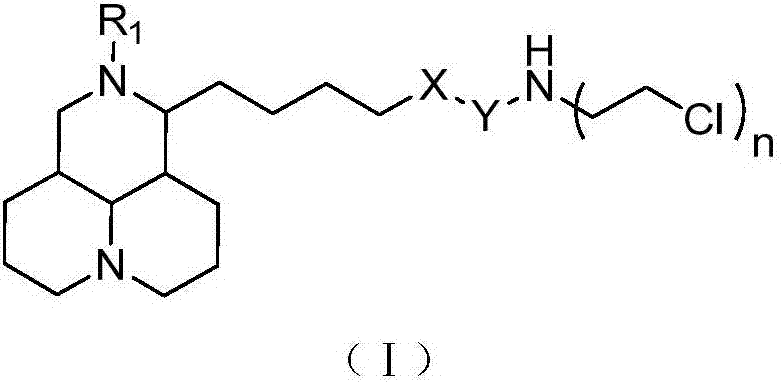
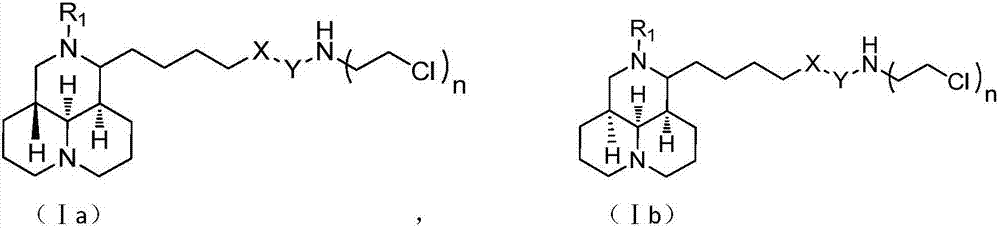
Sophoridine derivative with nitrogen mustard as well as preparation method and application of sophoridine derivative
A definition and compound technology, applied in the field of medicinal chemistry, can solve the problems of limited anti-tumor activity of sophoridine, inability to be widely used, and strong water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: 4-((3aR,3a1S,10aS)-2-toluenesulfonyl dodecahydropyridin[3,2,1-ij][1,6]naphthyridin-1-yl)butylbis(2 Preparation of -chloroethyl)butyl carbamate
[0038]
[0039] Step 1: Preparation of Sophoridine
[0040]
[0041] Weigh 1.0g of sophoridine, place it in 20mL of 5N potassium hydroxide aqueous solution, heat and reflux for 12h, after the reaction is completed, cool to room temperature, add 5N hydrochloric acid solution, control the pH to be less than 6, and precipitate a large amount of white solid, filter, and after the filtrate is concentrated, residual The substance was fully dissolved with methanol, and the inorganic salt was removed by filtration to obtain a methanol solution of sophoridine. After being spin-dried, the crude product of sophoridine was obtained, and then recrystallized with methanol to separate out the pure product of sophoridine. 13 C NMR (101MHz, CDCl 3 )δ 177.13, 67.62, 58.99, 53.08, 48.78, 41.72, 35.94, 34.64, 33.47, 28.38, 26.46...
Embodiment 2
[0055] Example 2: 4-((3aR,3a1S,10aS)-2-((3,4-dimethylphenyl)sulfonyl)dodecahydropyridine[3,2,1-ij][1,6] Preparation of naphthyridin-1-yl)butylbis(2-chloroethyl)butylcarbamate
[0056]
[0057] 13 C NMR (101MHz, CDCl 3 )δ159.28,139.48,136.26,134.69,131.22,131.12,125.91,66.82,66.08,60.86,53.08,48.91,47.01,41.63,40.87,36.03,31.39,29.69,28.30,26.29,24.09,23.33,23.13,20.55,19.62 .HRMS: calcd for C 28 h 43 Cl 2 N 3 O 4 S:587.2153,found:587.2153.
Embodiment 3
[0058] Example 3: 4-((3aR,3a1S,10aS)-2-((4-chlorophenyl)sulfonyl)dodecahydropyridin[3,2,1-ij][1,6]naphthyridine-1 Preparation of -yl)butyl bis(2-chloroethyl)butyl carbamate
[0059]
[0060] 13 C NMR (101MHz, CDCl 3 )δ159.28, 140.36, 139.69, 130.44, 129.52, 66.82, 66.08, 60.86, 53.08, 48.91, 47.01, 41.63, 40.87, 36.03, 31.39, 29.69, 28.30, 26.29, 24.09 for CMS. HR, 23.3 26 h 38 Cl 3 N 3 O 4 S:593.3212,found:593.3212.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com