Analytic application and method of thiol nucleophilic substitution derivatization reagent
A derivatization reagent and derivatization technology, applied in the field of analytical chemistry, can solve the problems of difficult real content, difficult and accurate determination of mustard gas prototypes, and unsuitable biological sample analysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0070] Preparation Example 1: Preparation and Characterization of S,S'-Diacetyltrithiodiglycol
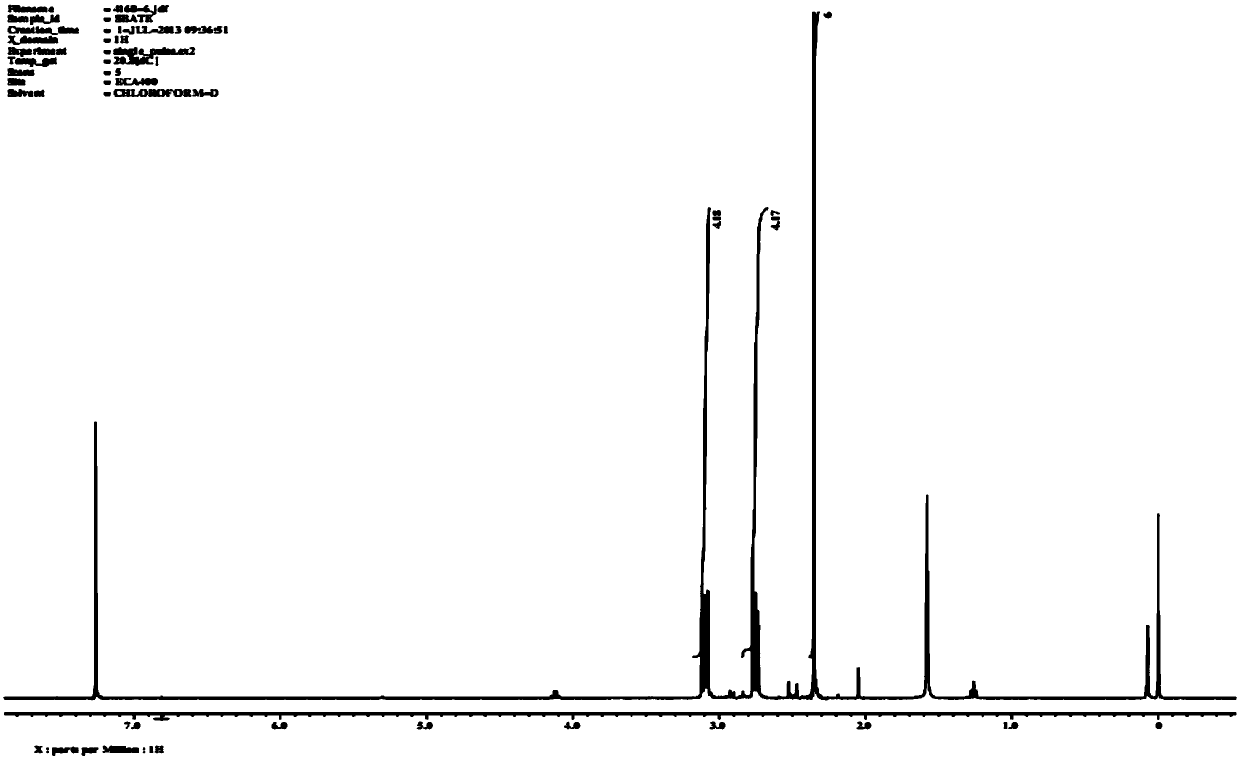
[0071] 5% mustard gas acetonitrile solution was added to 0.1g / mL PTA aqueous solution in portions or once, sealed with a polytetrafluoroethylene (PTFE) bottle cap, and reacted at 100°C for 1 hour; the reaction mixture was extracted with 2mL dichloromethane , the extract was subjected to silica gel column chromatography, the eluting solvent was n-hexane:dichloromethane=1:3, the collected fractions were subjected to vacuum distillation to obtain the derivative product S,S'-diacetyl trisulfide of mustard gas and PTA Diethylene glycol.
[0072] figure 1 S,S'-Diacetyltrithiodiglycol 1 H NMR spectrum. The characterization results showed that the purity of the refined S,S'-diacetyltrithiodiglycol could meet the experimental requirements.
preparation example 2
[0073] Preparation Example 2: Preparation and Characterization of 3,5-Dimercaptomethyl-Phenoxyacetic Acid
[0074] The preparation method of 3,5-dimercaptomethyl-aromatic compound is as follows:
[0075]
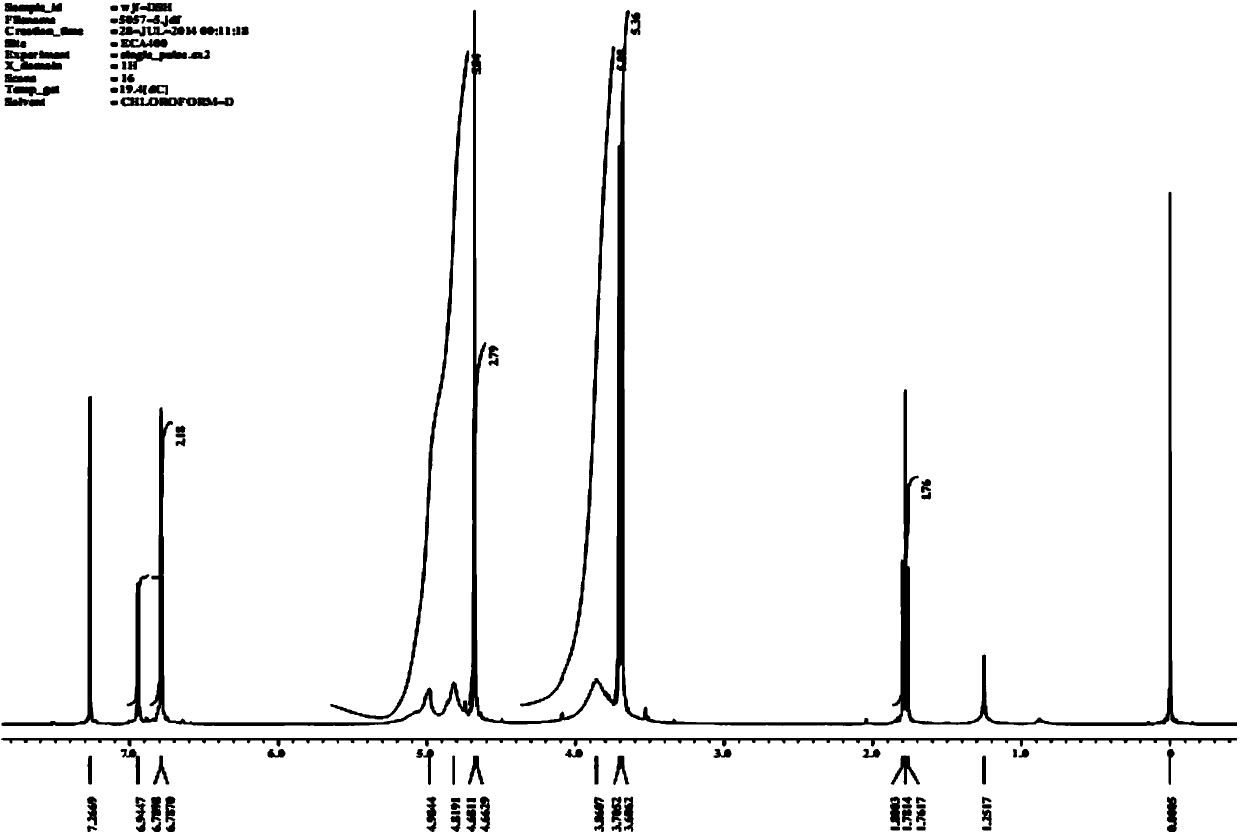
[0076] The dithiol derivatization reagent 3,5-dimercaptomethyl-phenoxyacetic acid (DSH) was prepared and purified according to the above synthetic route.
[0077] figure 2 for DSH 1 H NMR spectrum. The characterization results showed that the purity of the prepared DSH could meet the experimental requirements.
Embodiment 1
[0078] Embodiment 1: the instrument parameter optimization of PTA derivatization method
[0079] Prepare PTA aqueous solution and d4-mustard acetonitrile internal standard solution with a concentration of 0.1 mg / mL and 1 mg / mL respectively, mix the sample containing mustard gas with PTA solution and internal standard solution, vortex and mix, and place in a water bath at 50°C Reacted for 30min, the reaction solution was centrifuged at 14000rpm for 10 minutes, and 50 μL of the supernatant was put into the intubation tube of the sampling bottle, and the chromatograms of mustard gas and d4-mustard gas were measured with a triple quadrupole linear ion trap tandem mass spectrometer (LC / MS Hyphenated instrument 5500QTRAP, equipped with TurboV electrospray ionization source (ESI) and ACQUITY TM ultra-high performance liquid chromatography (UPLC).
[0080] The detection conditions of liquid chromatography-mass spectrometry are as follows:
[0081] Chromatographic conditions Chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com