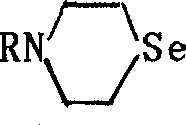
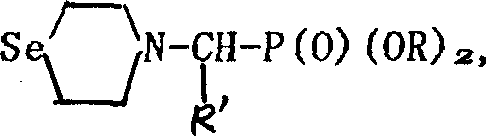Seleno morpholine, its derivative and their prepn. and use
A technology of selenomorpholine and its derivatives, applied in application, fertilizer mixture, organic chemistry, etc., can solve the problems of low selenium content in soil, selenium content in grain cannot meet nutritional needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: In a 500ml there-necked flask, add 7.9g (0.10mol) of selenium and 6.0g (0.11mol) of potassium borohydride, add 100ml of absolute ethanol and stir and heat until it is a colorless liquid, cool to room temperature, and add 4.4g (0.11mol) sodium hydroxide, then dropwise add 200ml absolute ethanol solution containing 17.8g (0.10mol) nitrogen mustard hydrochloride, and reflux for 4hr after the dropwise addition. The reaction mixture was cooled and filtered, the solvent was distilled off, the residue was distilled under reduced pressure, and the 61-63°C / 7mmHg fraction was collected to obtain 7.5g of colorless liquid selenomorpholine, with a yield of 50.0%.
Embodiment 2
[0015] Example 2: In a 100ml three-necked flask, add 0.9g (6mmol) of selenomorpholine, 1.6g (6mmol) of 1-bromoundecanoic acid, dissolve in 30ml of dichloromethane, and add 1.25ml in batches under ice-salt bath cooling. g (6.1 mmol) of dicyclohexylcarbodiimide was added in about 10 minutes, stirred in ice bath for 30 minutes, and then stirred at room temperature for 4 hours. After the reaction, filter, wash the solid with a solvent, wash with dilute hydrochloric acid and dilute sodium hydroxide solution until neutral, dry, evaporate the solvent, and recrystallize the crude product from absolute ethanol to obtain 2.08 g of white solid 4-(1- Bromoundecanoyl) selenomorpholine, yield 87.3%. m.p.70-72°C.
Embodiment 3
[0016] Example 3: In a 100ml there-necked flask, add 0.75ml (20mol) of formic acid, 3.0g (20mmol) of selenomorpholine, dissolve in 30ml of dichloromethane, and under ice-salt bath cooling, add 4.1g (20mmol) of dicyclohexyl Carbodiimide was added to the reaction liquid in batches, and the addition was completed in about 10 minutes. After stirring in an ice bath for 30 minutes, continue stirring at room temperature for 4 hours. The resulting white solid dicyclohexyl urea was removed by filtration, washed with a small amount of dichloromethane, and the filtrate was washed with dilute hydrochloric acid and dilute sodium hydroxide solution until neutral, dried, and evaporated to remove the solvent. 3.24 g of colorless liquid 4-formylselenomorpholine was obtained with a yield of 91.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com