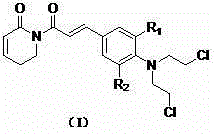
Application of nitrogen mustard based piperlongumine compound in medicine
A technology of piperonamides and compounds is applied to the application field of nitrogen mustardyl piperonamide compounds in medicine, and can solve problems such as the application of nitrogen mustardyl piperonamide compounds which have not been seen before.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
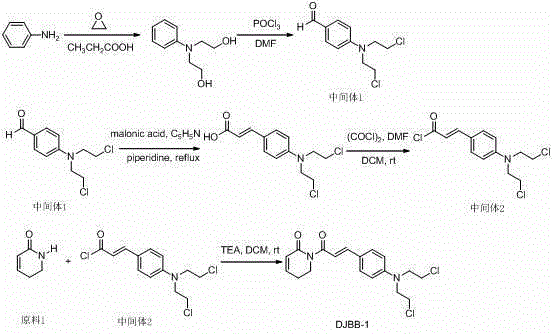
[0017] Example 1: Compound DJBB-1 preparation of
[0018] The synthetic route is as follows:
[0019]
[0020] For the synthetic route of intermediate 1, please refer to the reference (Liu Wenhu et al., Acta Pharmaceutica Sinica, 2014, 49(2):217-224).
[0021] For the synthetic route of intermediate 2, please refer to the reference (Shoujiao Pengetal, J. Med. Chem. 2015, 58, 5242? 5255).
[0022] The synthetic route of compound DJBB-1 can be found in the reference (ShoujiaoPengetal, J.Med.Chem.2015, 58, 5242? 5255):
[0023] Add raw material 110mmol, intermediate 210mmol, CH 2 Cl 2 10ml, 10ml of triethylamine, stirred at room temperature for 10h. Then add saturated NH 4 Washed with Cl solution, CH 2 Cl 2 Extraction, washing with saturated brine, MgSO 4 Dried and concentrated. The crude product was separated by column chromatography with a yield of 65%.
[0024] 1 HNMR (400MHz, CDCl 3 ): δ7.89(d,J=15.6Hz,1H),7.60-7.54(m,2H),7.34(d,J=15.6Hz,1H),6.94-6.90(m,3H),6...
Embodiment 2
[0026] Embodiment 2: compound DJBB-2 preparation of
[0027] The synthetic route is the same as compound DJBB-1.
[0028] The yield of compound DJBB-2 was 60%.
[0029] 1 HNMR (400MHz, CDCl 3 ): δ7.90(d,J=15.6Hz,1H),7.32(d,J=15.6Hz,1H),6.96-6.92(m,3H),6.02(t,J=9.6Hz,1H),4.06 (t, J=6.4Hz, 2H), 3.89(s, 6H), 3.86(t, J=7.6Hz, 4H), 3.74(t, J=7.6Hz, 4H), 2.42(m, 2H).
[0030] MS-ESI (m / z): 449.10 (M+Na 十 ).
Embodiment 3
[0031] Embodiment 3: compound DJBB-3 preparation of
[0032] The synthetic route is the same as compound DJBB-1.
[0033] The yield of compound DJBB-3 was 56%.
[0034] 1 HNMR (400MHz, CDCl 3 ): δ7.92(d,J=15.6Hz,1H),7.36(d,J=15.6Hz,1H),6.96-6.92(m,3H),6.03(t,J=9.6Hz,1H),4.03 (t, J = 6.4Hz, 2H), 3.82 (t, J = 7.6Hz, 4H), 3.76 (t, J = 7.6Hz, 4H), 2.46 (m, 2H).
[0035] MS-ESI (m / z): 425.06 (M+Na 十 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com