Novel quinazoline nitrogen mustard compound, and preparation method and application thereof to treatment of cancer
A technology for quinazoline mustard and tumor treatment, which is applied in the fields of antitumor drugs, organic chemistry, and drug combinations, and can solve the problems of not fully tapping the potential of anticancer drugs, and achieve good anticancer activity and high yield , low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
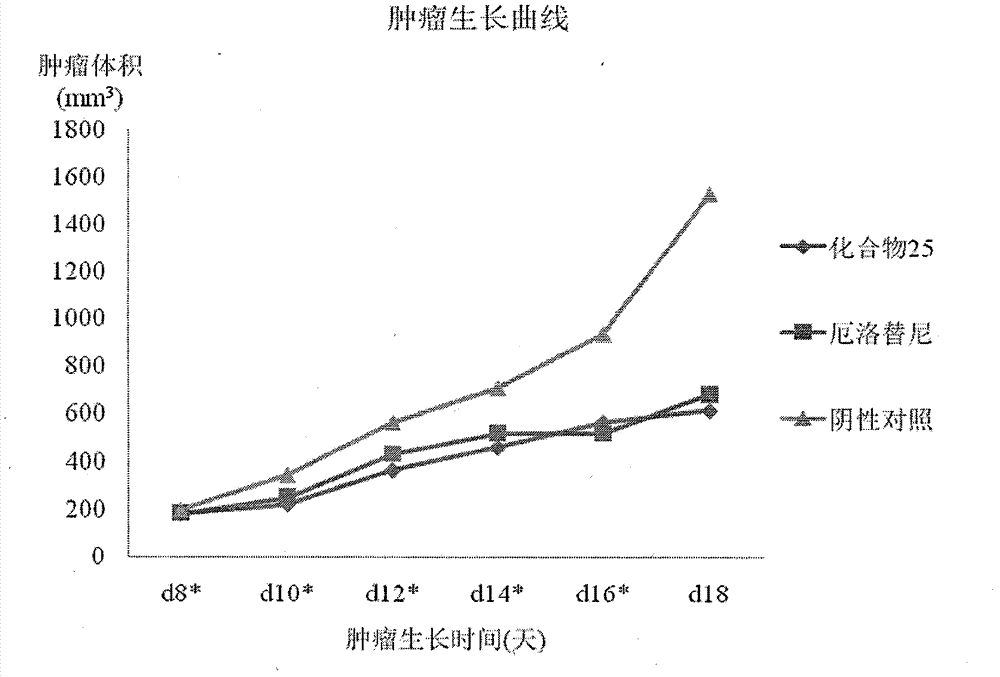
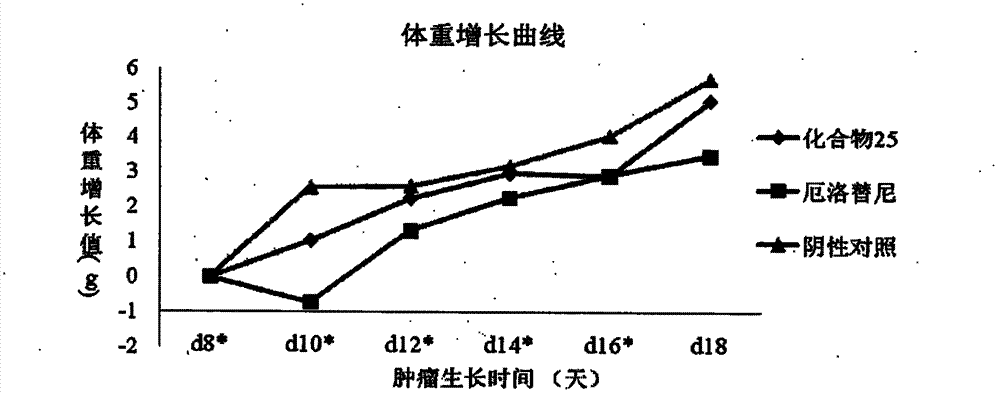
[0092] According to the following steps, the substituents R1=meta nitrogen mustard, R2=methoxyethoxy, R2=methoxyethoxy compounds in formula A are prepared.
[0093] 1.1N, the synthesis of N-bis(2-hydroxyethyl)-3-nitroaniline
[0094] Take 5.52 g (0.04 mol) of 3-nitroaniline, add it to 80 mL of 25% acetic acid aqueous solution, and add 10 mL of ethylene oxide dropwise under ice cooling. Warm up to room temperature, stir for 72 hours, filter with suction, wash the filter cake with water, and dry to obtain 8.02 g of a yellow-orange solid, with a yield of 88%. mp 94-96°C, 1 H NMR (400MHz, CDCl 3 )δ7.53 (d, J=8.0Hz, 1H, ArH), 7.48 (s, 1H, ArH), 7.33 (t, J=8.2Hz, 1H, ArH), 6.97 (dd, J=8.4, 2.2Hz , 1H, ArH), 3.89 (t, J=4.8Hz, 4H, -NCH 2 CH2-), 3.65(t, J=4.8Hz, 4H, -CH 2 CH 2 OH). 13 C NMR (100MHz, CDCl 3 )δ149.43, 148.63, 129.84, 118.10, 111.34, 106.48, 60.35, 55.06.MS (ESI + ) m / z: 227.3 (M+H + ).IR(KBr pellet, cm -1 ): 3265, 3173, 2959, 2863, 1618, 1565, 1525, 1486, 1341...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com