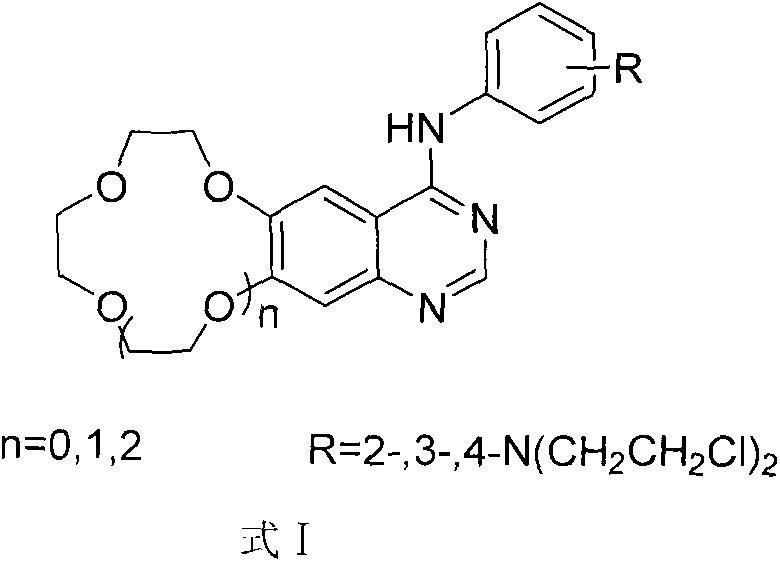
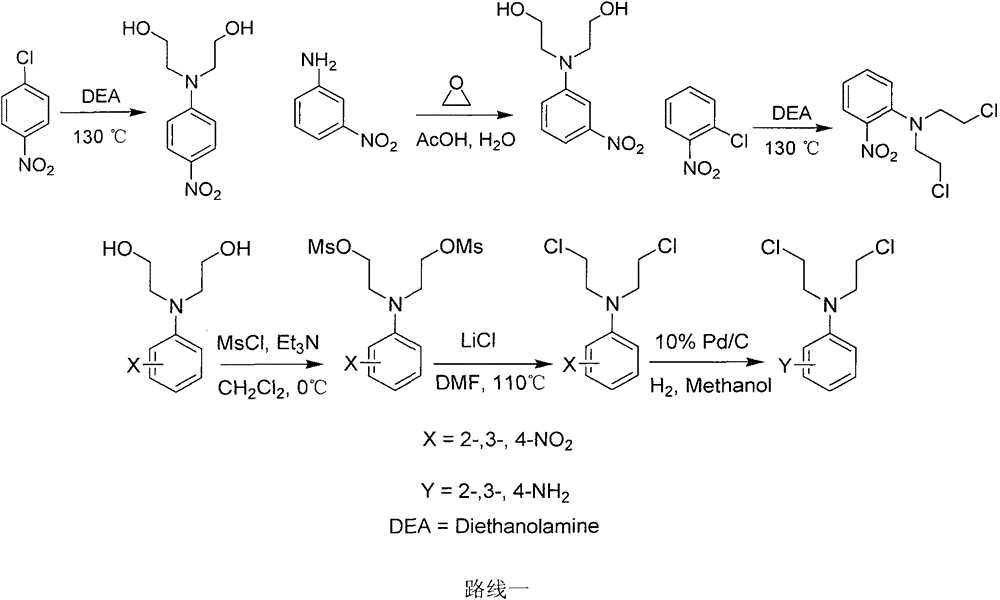
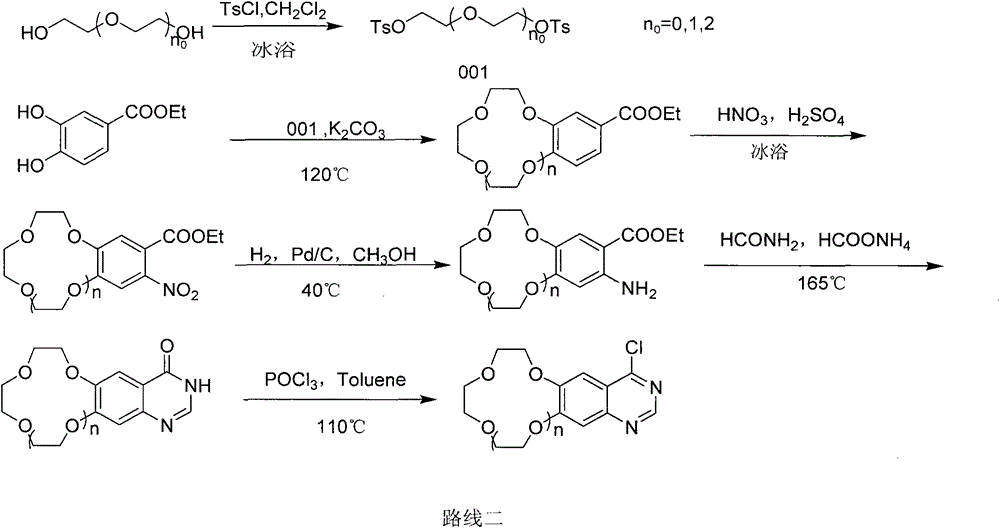
Crown ether ring-shaped quinazoline nitrogen mustard compound, and preparation method and application thereof in tumor treatment
A technology of quinazoline mustards and compounds, which is applied in the field of crown ether cyclic quinazoline mustards and their preparation and tumor treatment applications, can solve the problems of not fully tapping the potential and achieve good anti-tumor Activity and selectivity, fewer synthetic steps, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] N 1 , N 1 -Bis(2-chloroethyl)-N 2 -(6,7-Benzo-9-crown-3-quinazolin-4-yl) o-phenylenediamine (A 1 )
[0026]
[0027] 1.1 Synthesis of N,N-bis(2-hydroxyethyl)-2-nitroaniline
[0028] Add 1.58g (10mmol) of o-nitrochlorobenzene and 2.12g (20mmol) of diethanolamine into a 25mL round-bottomed flask, and then heat it to 115-120°C to react. TCL detects the reaction process. After 7 hours, the reaction is basically complete, and the reaction is stopped. The entire reaction system was poured into 90°C hot water while it was hot and stirred vigorously. The product was precipitated in the form of oil, and then the oil was extracted with ethyl acetate, washed with water, dried, and purified by column chromatography (petroleum ether: ethyl acetate). Ester = 1:1) separation and purification to obtain a purple oily product with a yield of 39%. The obtained spectrum is consistent with that reported in the literature.
[0029]
[0030] 1.2 Synthesis of N,N-bis(2-methylsulfon...
Embodiment 2
[0061] N 1 , N 1 -Bis(2-chloroethyl)-N 3 -(6,7-benzo-12-crown-4-quinazolin-4-yl) m-phenylenediamine (B 2 )
[0062]
[0063] 2.1 Synthesis of N,N-bis(2-hydroxyethyl)-3-nitroaniline
[0064] Take 5.52 g (0.04 mol) of 3-nitroaniline, add it to 80 mL of 25% acetic acid aqueous solution, and add 10 mL of ethylene oxide dropwise under ice cooling. Warm up to room temperature, stir for 72 hours, filter with suction, wash the filter cake with water, and dry to obtain 8.02 g of a yellow-orange solid with a yield of 88%. The obtained spectrum is consistent with that reported in the literature.
[0065]
[0066] 2.2 Synthesis of N,N-bis(2-methylsulfonate ethyl)-3-nitroaniline
[0067] Take 2.26g (0.01mol) of N,N-bis(2-hydroxyethyl)-3-nitroaniline, add it to 30mL redistilled dichloromethane, add 3.32mL (0.025mol) triethylamine under ice-cooling, Then 1.93 mL (0.025 mol) of methanesulfonyl chloride was added. After 10 minutes, TLC tracked until the reaction was complete. The...
Embodiment 3
[0097] N 1 , N 1 -Bis(2-chloroethyl)-N 4 -(6,7-benzo-15-crown-5-quinazolin-4-yl)-p-phenylenediamine (C 3 )
[0098]
[0099] synthetic route:
[0100] 3.1 Synthesis of N,N-bis(2-hydroxyethyl)-4-nitroaniline
[0101] Add 1.58g (10mmol) of p-nitrochlorobenzene and 2.12g (20mmol) of diethanolamine to a 25mL round-bottomed flask, and then heat it to 115-120°C to react. TCL detects the reaction process. After 10 hours, the reaction is basically complete, and the reaction is stopped. The entire reaction system was poured into hot water at 90°C while it was hot and stirred vigorously. The product was precipitated in the form of a solid, which was filtered to obtain a bright yellow solid with a yield of 39%. The obtained spectrum is consistent with that reported in the literature.
[0102]
[0103] 3.2 Synthesis of N,N-bis(2-methylsulfonate ethyl)-4-nitroaniline
[0104] Dissolve 400mg of N,N-bis(2-hydroxyethyl)-4-nitroaniline in 13.25mL THF / CH 2 Cl 2 = 1:3 mixed solven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com