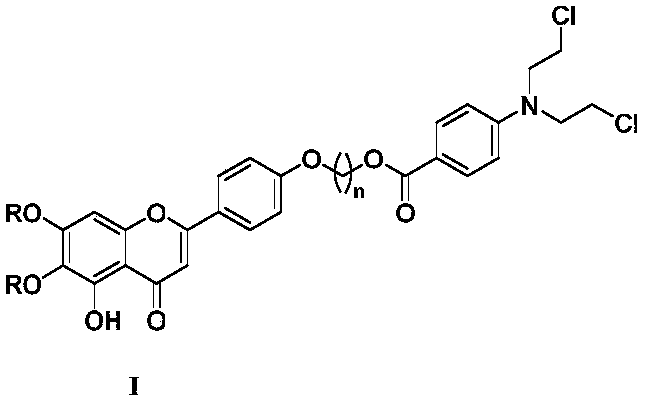
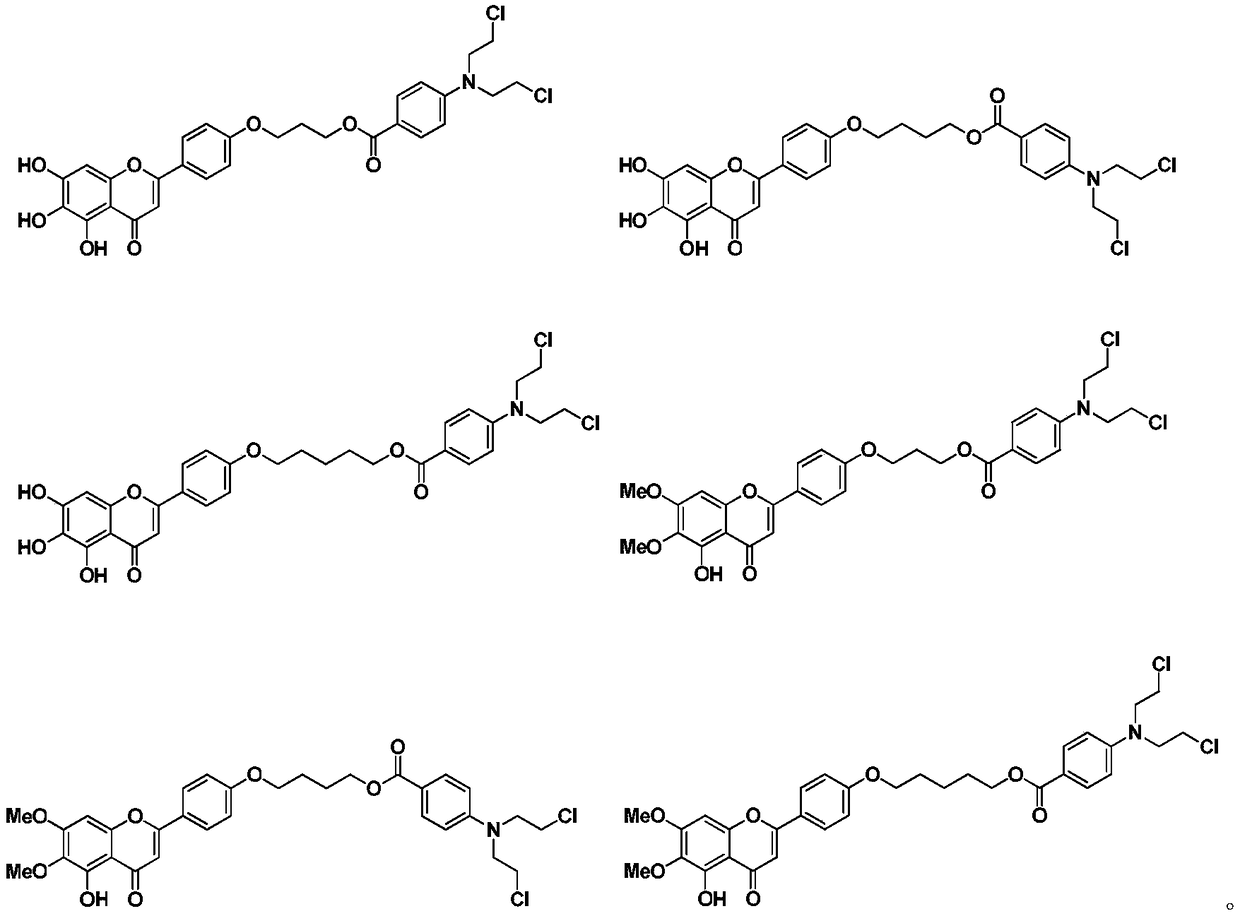
Scutellarin aglycone nitrogen mustard derivative and preparation method and application thereof
A technology of scutellarin aglycone nitrogen mustards and derivatives, which is applied in the field of medicinal chemistry, can solve the problems of high toxicity and side effects, and achieve obvious anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
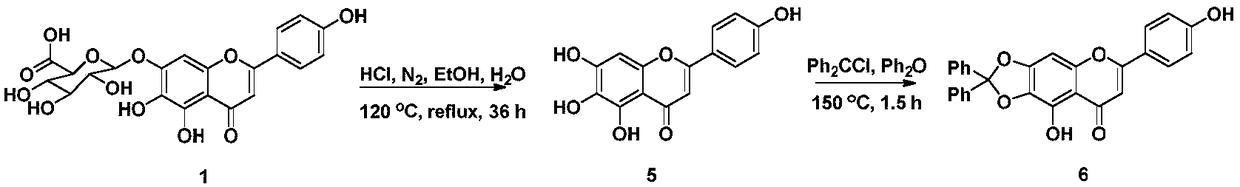
[0031] Add scutellarin 1 (10g, 21.6mmol) to 120mL absolute ethanol, 120mL concentrated hydrochloric acid and 10mL H 2 O mixture. in N 2 Under the condition of protection, reflux for 36h. After cooling at room temperature, the reaction solution was poured into an equal volume of water, suction filtered, washed with water until neutral, dried, and the crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate 1:1) to obtain scutellarin as a yellow solid Yuan 5 1.05g, yield 17%. 1 H NMR (DMSO-d 6 ,400MHz)δ(ppm):12.80(s,1H,5-OH),10.47(s,1H,7-OH),10.32(s,1H,4′-OH),8.75(s,1H,6- OH), 7.91(d, 2H, J=8.9Hz, H-2′, 6′), 6.92(d, 2H, J=8.9Hz, H-3′, 5′), 6.75(s, 1H, H -8), 6.57(s, 1H, H-3).
Embodiment 2
[0033]
[0034] Dissolve scutellarin aglycone 5 (1 g, 3.5 mmol) in 50 mL of diphenyl ether, and add dichlorodiphenylmethane (1009 μL, 5.25 mmol). in N 2 Protection, under the condition of 175°C, react for 1.5h. After cooling at room temperature, the reaction solution was poured into 500 mL of petroleum ether, suction filtered, and dried. The crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate 2:1) to obtain 937 mg of a yellow solid with a yield of 59%. 1 H NMR (DMSO-d 6 ,400MHz)δ(ppm):13.17(s,1H,5-OH),10.41(s,1H,4′-OH),7.93(d,2H,J=8.7Hz,H-2′,6′) ,7.57-7.46(m,10H,Ar-H),7.06(s,1H,H-8),6.93(d,2H,J=8.7Hz,H-3′,5′),6.87(s,1H , H-3).
Embodiment 3
[0036]
[0037] Intermediate 6 (450mg, 1mmol) was dissolved in 30mL of acetone, and K 2 CO 3 (417mg, 3mmol) and 1,3-dibromopropane (420μL, 3mmol) were refluxed for 8h. After cooling at room temperature, it was filtered with suction, and the filtrate was concentrated and separated through a silica gel column (petroleum ether: ethyl acetate 6:1) to obtain 473 mg of light yellow powder 7a with a yield of 83%. 7a (285mg, 0.5mmol), dissolved in 5mL of DMF, added K 2 CO 3 (139mg, 1mmol) and benzoen mustard 4 (152mg, 0.5mmol), react at room temperature for 24h. The reaction solution was poured into 30 mL of H 2 O, extracted with ethyl acetate (3×20mL), washed with saturated saline solution, dried over anhydrous sodium sulfate, filtered, concentrated the filtrate, and separated by silica gel column chromatography (petroleum ether: ethyl acetate 4:1) to obtain light yellow powder 8a 323 mg, 86% yield. 8a was added to 10 mL of aqueous acetic acid, at 170 ℃ After reflux reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com