Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Nitrogen mustard derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
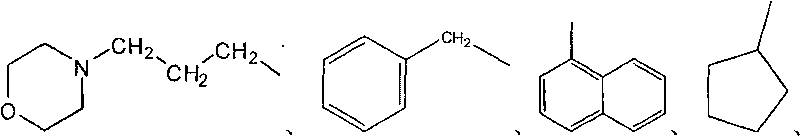
Nitrogen Mustard Derivatives. The most representative and better studied substances of this group are the nitrogen mustard derivatives, characterized by the 2-haloethyl amine group (Fig. 107) attached to a radical which can be aliphatic or aromatic.
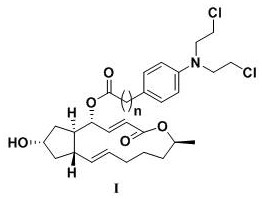
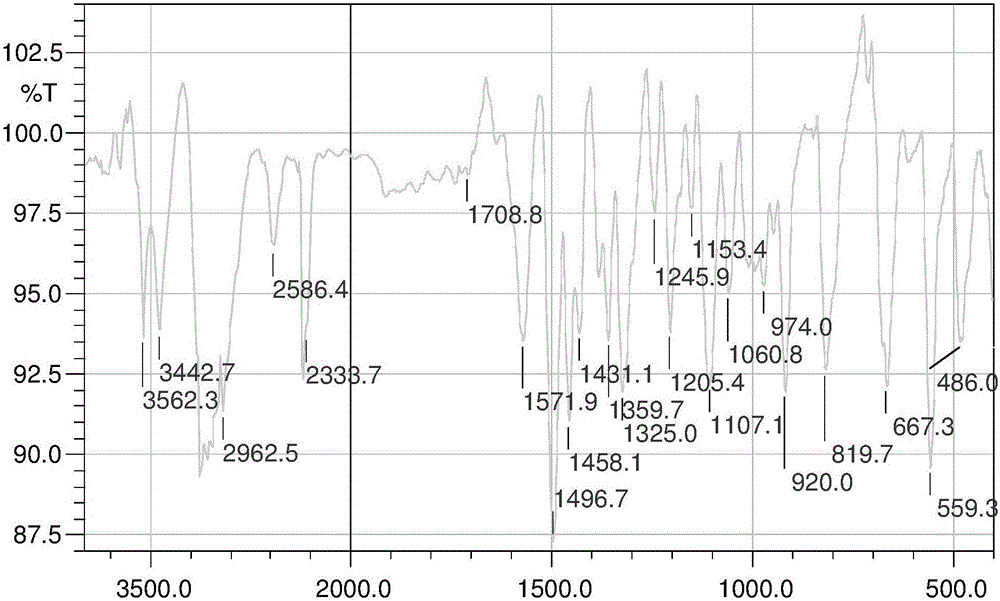
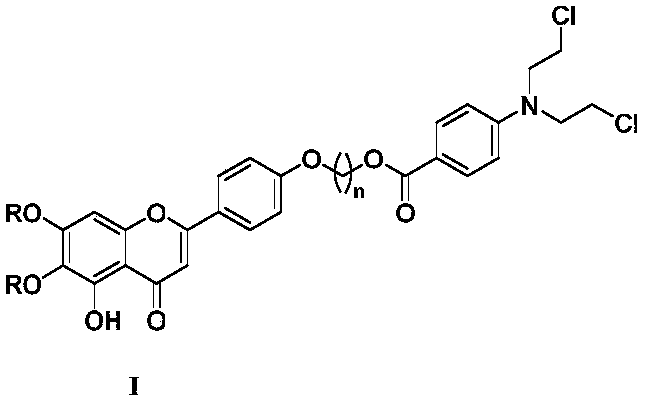
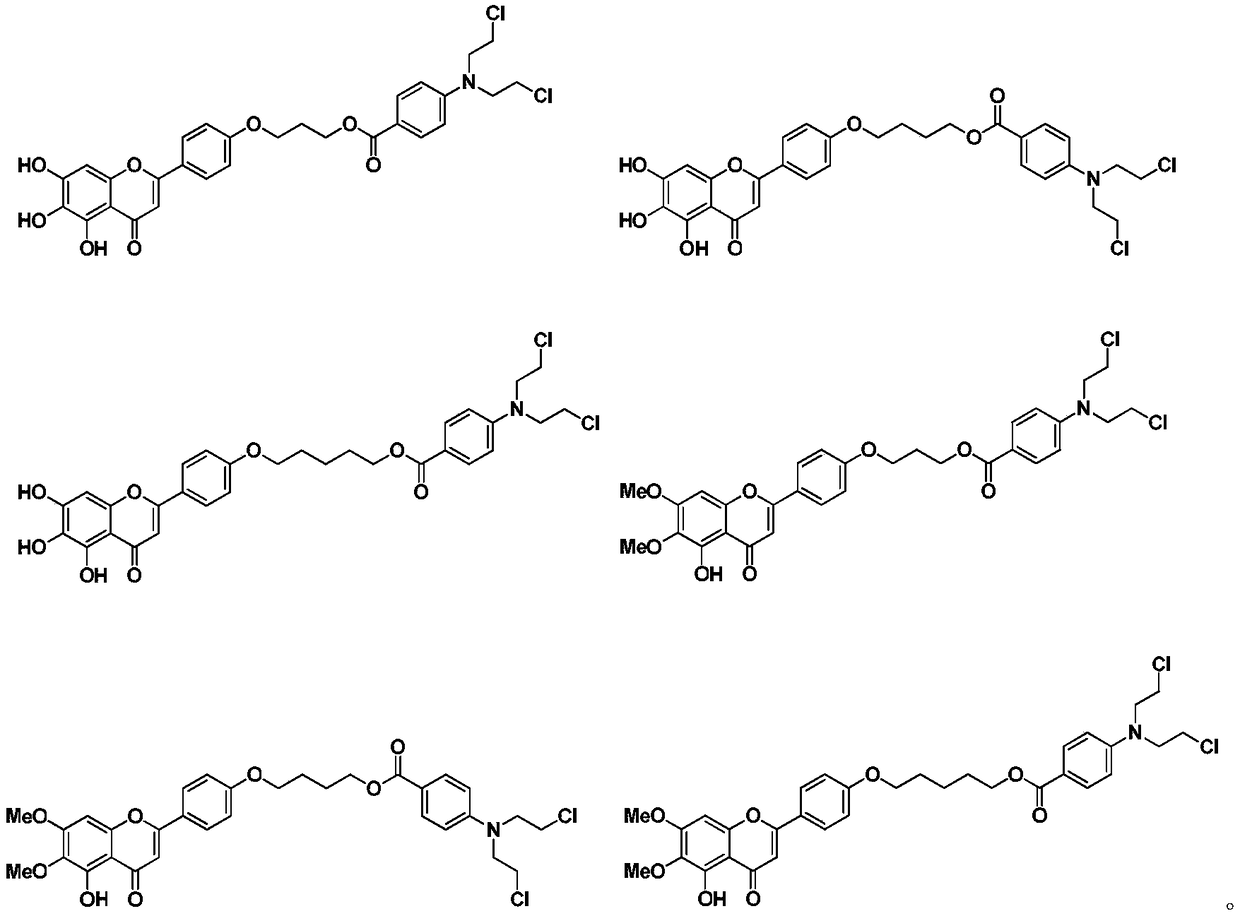
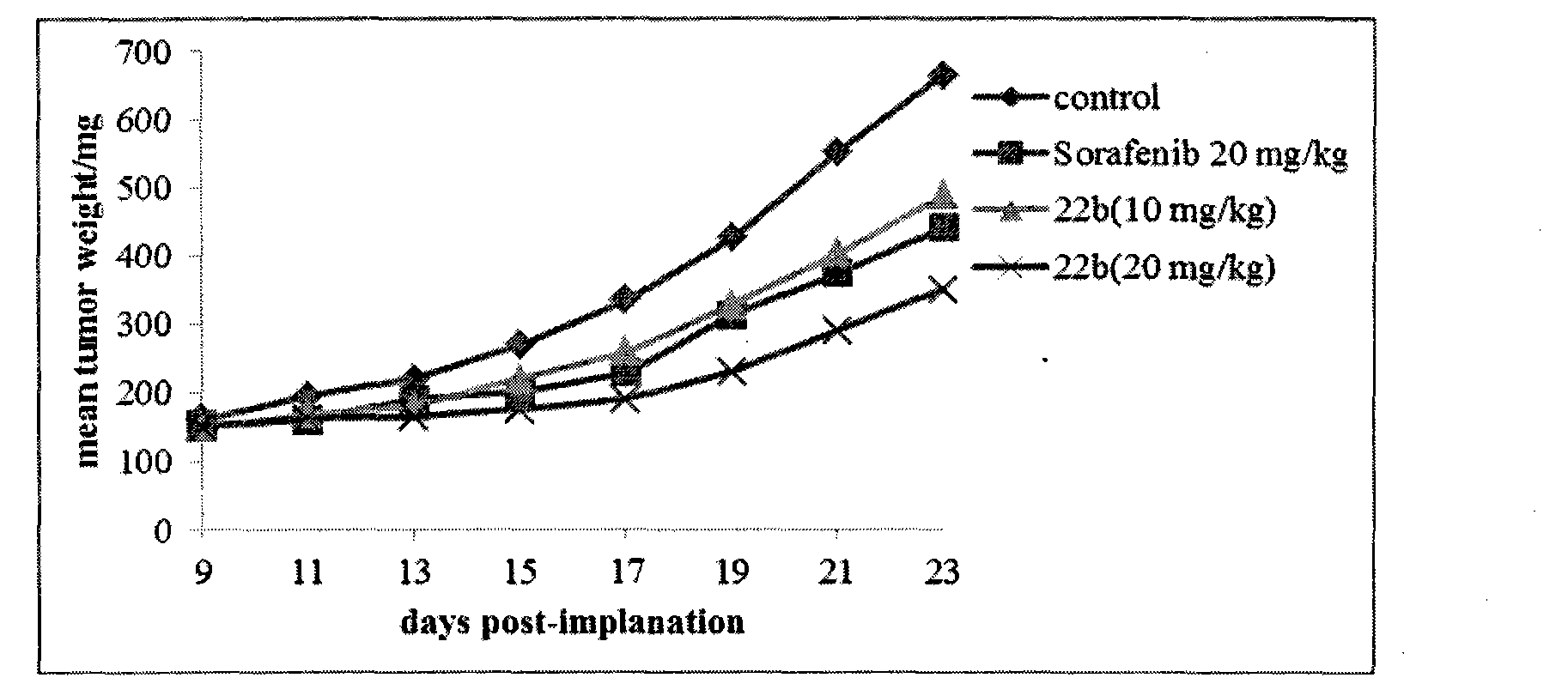
Scutellarin aglycone nitrogen mustard derivative and preparation method and application thereof
ActiveCN108864024AGood antitumor activityGood choiceOrganic active ingredientsOrganic chemistryBenzoic acidAglycone
The invention belongs to the field of natural medicine and medicinal chemistry and relates to a scutellarin aglycone nitrogen mustard derivative and a preparation method and application thereof, in particular to a scutellarin aglycone nitrogen mustard derivative of which 4'-OH is combined with benzoic acid nitrogen mustard and a preparation method and antitumor activity thereof. The structure of the scutellarin aglycone nitrogen mustard derivative and pharmaceutically acceptable salt thereof is as shown in the general formula I, wherein R and n are as described in claims and the specification.The formula I is shown in the description.
Owner:SHENYANG PHARMA UNIVERSITY
Two nitrogen mustard derivatives, as well as preparation method and application therefore in tumor treatment
InactiveCN103450096AImprove anti-tumor effectSynthetic steps with high yieldsOrganic active ingredientsOrganic chemistryAlkyl transferIn vivo
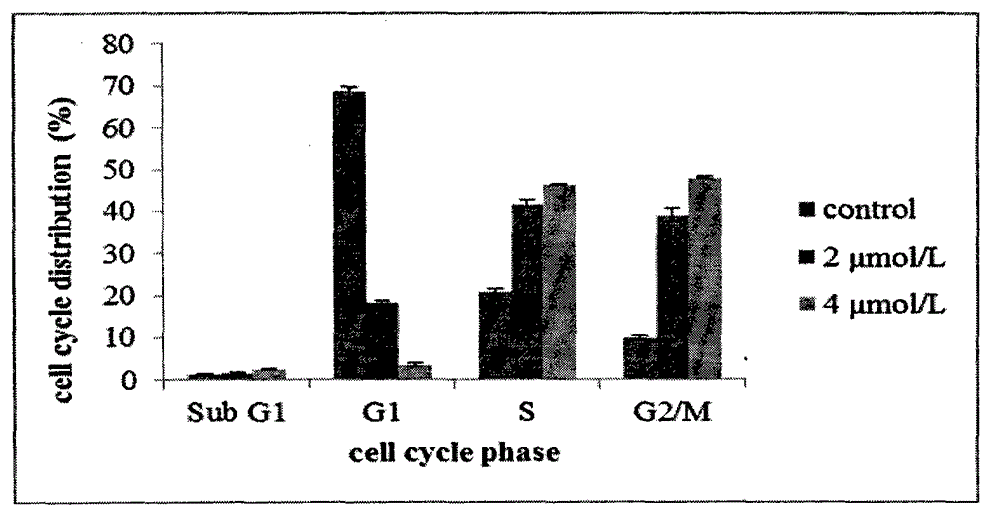
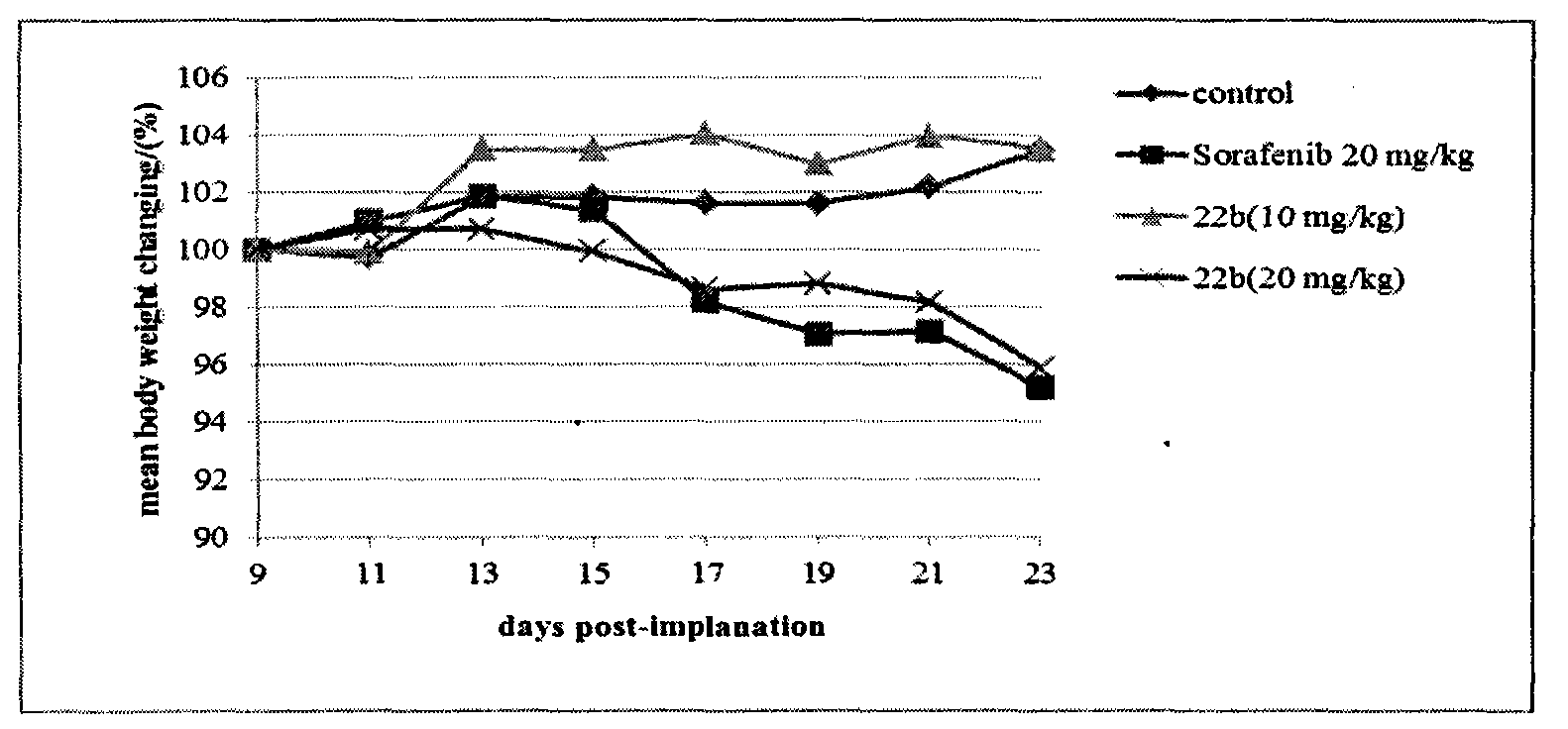
The invention discloses novel nitrogen mustard derivatives. The nitrogen mustard derivatives are characterized in that a nitrogen mustard alkylation group is at one end, a 6,7-substituted quinazoline structure is at the other end, the substituent R1 is located at the 4th position of the quinazoline main body and is 2-nitrogen mustard group or 3-nitrogen mustard group, morpholinepropoxy and methoxy are located at the 6th position and the 7th position of the quinazoline main body, and the structural formula is shown in formula A. Experiments prove that the compounds can inhibit the cell cycle at the G2 / M period and are di-functional alkylation agents. The in-vivo anti-tumor activity experiments prove that the compounds are quite good in activity. In addition, the compounds have the superiority to other nitrogen mustard medicines, namely, the compounds are quite low in toxicity. Simultaneously, the compounds are easy to synthesize, and quite high in total yield. All the above advantages prove that the compounds have great potential to function as medicines for treating tumors.
Owner:BEIJING NORMAL UNIVERSITY
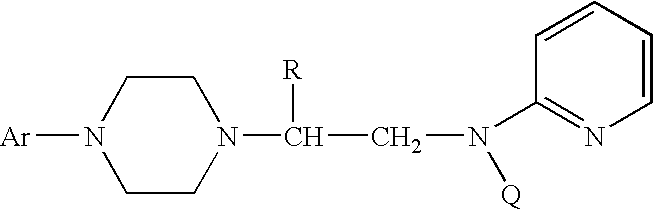
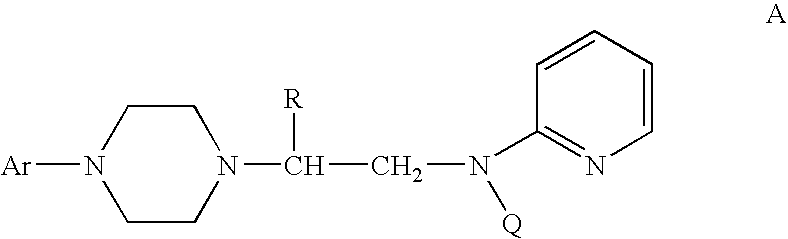
Process for making chiral 1,4-disubstituted piperazines
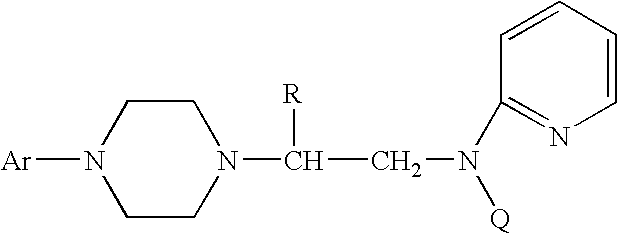
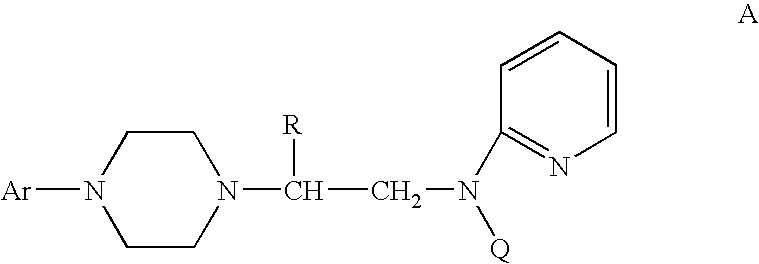
A process for a stereoselective preparation of novel chiral nitrogen mustard derivatives useful in synthesizing optically active 1,4-disubstituted piperazines of formula: wherein R, Ar, and Q are defined as set forth herein, and intermediate compounds therefor. The 1,4-disubstituted piperazines act as 5HT1A receptor binding agents useful in the treatment of Central Nervous System (CNS) disorders.
Owner:WYETH LLC
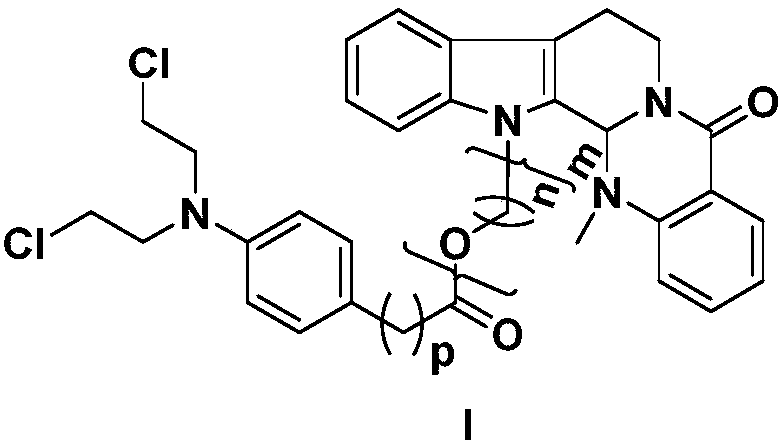
Preparation method and application of evodiamine combined nitrogen mustard derivatives with antineoplastic activities
ActiveCN107674076AImprove pharmacological activityOrganic chemistryAntineoplastic agentsEvodiamineMedicinal chemistry
The invention relates to the fields of natural drugs and medicinal chemistry, particularly a preparation method of evodiamine N-13 combined nitrogen mustard derivatives and application of the evodiamine N-13 combined nitrogen mustard derivatives in preparing antineoplastic drugs. The structure of the evodiamine combined nitrogen mustard derivatives and pharmaceutically acceptable salts thereof isdisclosed as a general formula I, wherein n, m and p are as described in the claims and specification. The evodiamine derivatives provided by the invention have better antineoplastic effects, and canbe used for further preparing antineoplastic drugs.
Owner:SHENYANG PHARMA UNIVERSITY
O-nitro aryl nitrogen mustard derivative and preparation methods and application thereof
InactiveCN102675140ANot prone to cytotoxicityHigh selectivityOrganic compound preparationCarboxylic acid amides preparationSilica gelAcetone
The invention discloses an o-nitro aryl nitrogen mustard derivative and a preparation method and application thereof. The o-nitro aryl nitrogen mustard derivative is figured in the following formula; and in the formula, R is o-nitro phenyl or o-nitro phenoxyl. The preparation method of the o-nitro aryl nitrogen mustard derivative is that sodium carbonate is adopted as acid-binding agent, acyl chloride is subjected to reaction, extraction and silica gel column chromatography in dry acetone to obtain dihydroxy compound, and then the dihydroxy compound and thionyl chloride reacts with each other in methylene chloride to obtain the target substance. The invention further provides another preparation method of the o-nitro aryl nitrogen mustard derivative.
Owner:LANZHOU UNIVERSITY
Benzoic acid nitrogen mustard derivative as well as preparation method and application thereof
InactiveCN101693670AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryBenzoic acidCancer cell
The invention relates to a benzoic acid nitrogen mustard derivative which has the following formula: R1 is 4-CH3-C6H5, 4-CH3O-C6H5, 4-CH(CH3)2-C6H5, 4-F-C6H5, 4-Cl-C6H5, 4-Br-C6H5, 4-NO2-C6H5, 2-F-C6H5, 2-Cl-C6H5, 2-Br-C6H5, n-butyl, tertiary butyl and n-dodecyl, and R2 is H; or R1 is CH3-, and R2 is C6H5-; or R1 and R2 are both n-propyl and n-butyl. The benzoic acid nitrogen mustard derivative has remarkable inhibiting effect for human oral cavity epicuticula cancer cell strain (KB) and human leukaemia cell strain (K562) so that the benzoic acid nitrogen mustard derivative can be applied in preparing antitumor medicaments. The invention also discloses a preparation method of the benzoic acid nitrogen mustard derivative.
Owner:南京大学中国医药城研发中心
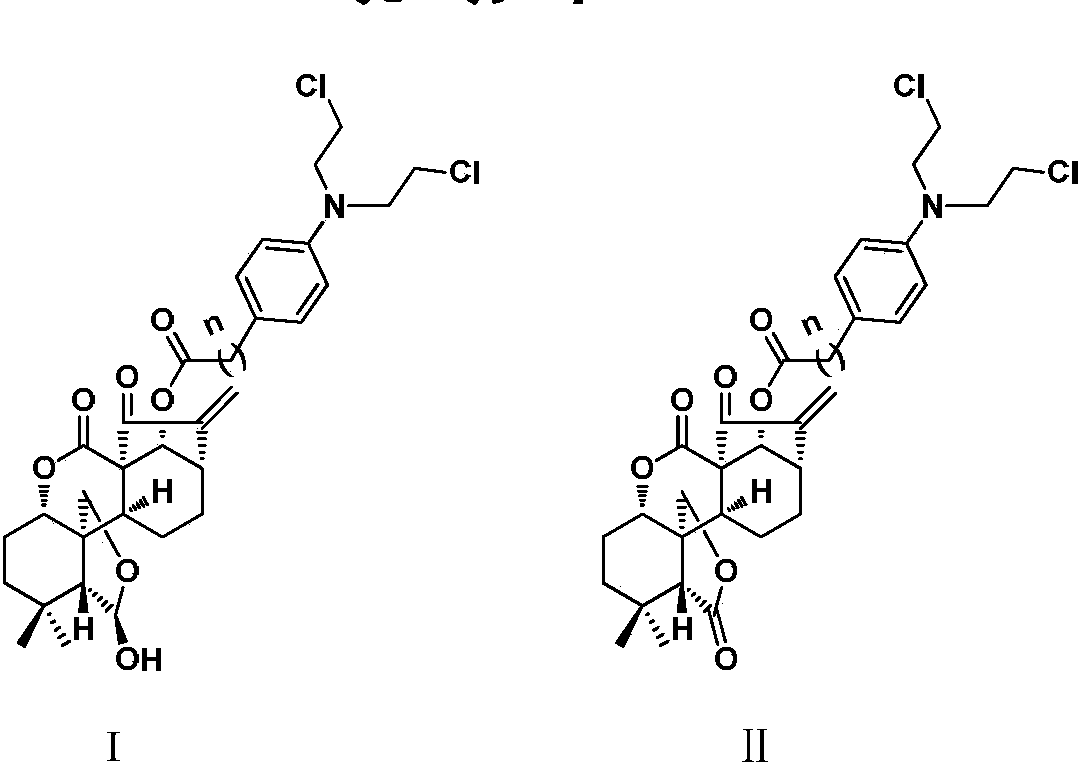
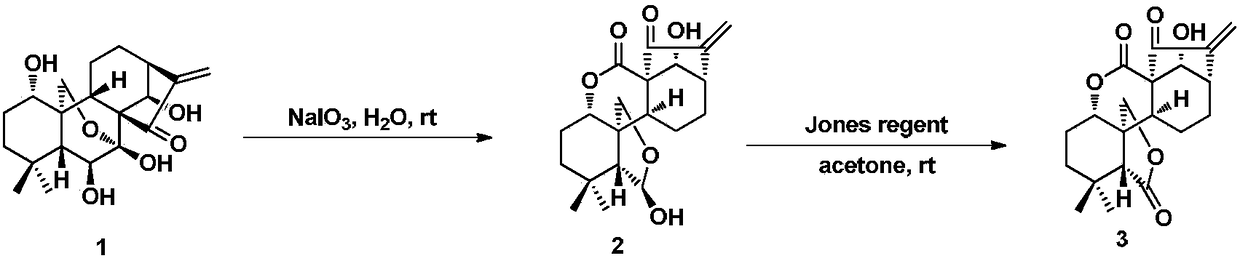
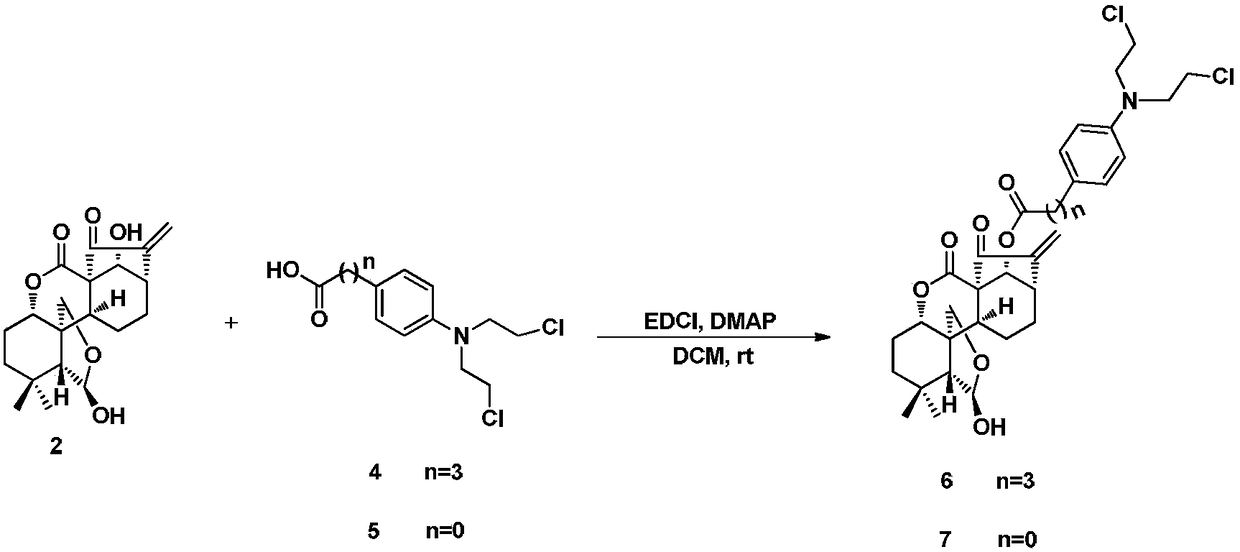
Enmein-type ent-kaurane diterpenoid spliced nitrogen mustard derivatives and preparation method and application thereof
ActiveCN108276424AHigh activityImprove pharmacological activityOrganic chemistryAntineoplastic agentsEnt kaurane diterpenoidMedicinal chemistry
The invention relates to the technical field of medicines, and relates to enmein-type ent-kaurane diterpenoid spliced nitrogen mustard derivatives and a preparation method and application thereof, inparticular to 14-OH spliced nitrogen mustard derivatives of enmein-type ent-kaurane diterpenoid, and a preparation method thereof and application thereof to preparation of anti-tumor medicines. The enmein-type ent-kaurane diterpenoid spliced nitrogen mustard derivatives and pharmaceutically acceptable salts thereof adopt a structural general formula I or II, wherein n is described in claims and adescription.
Owner:SHENYANG PHARMA UNIVERSITY
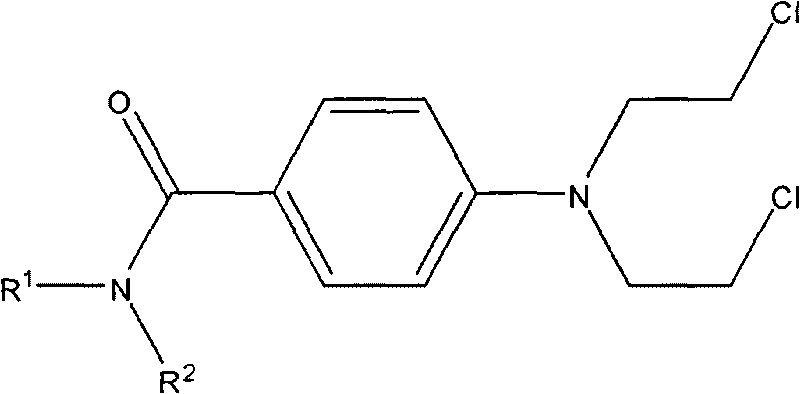
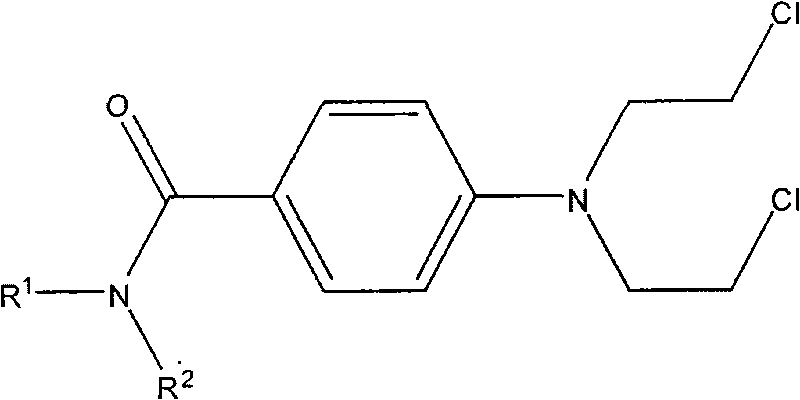
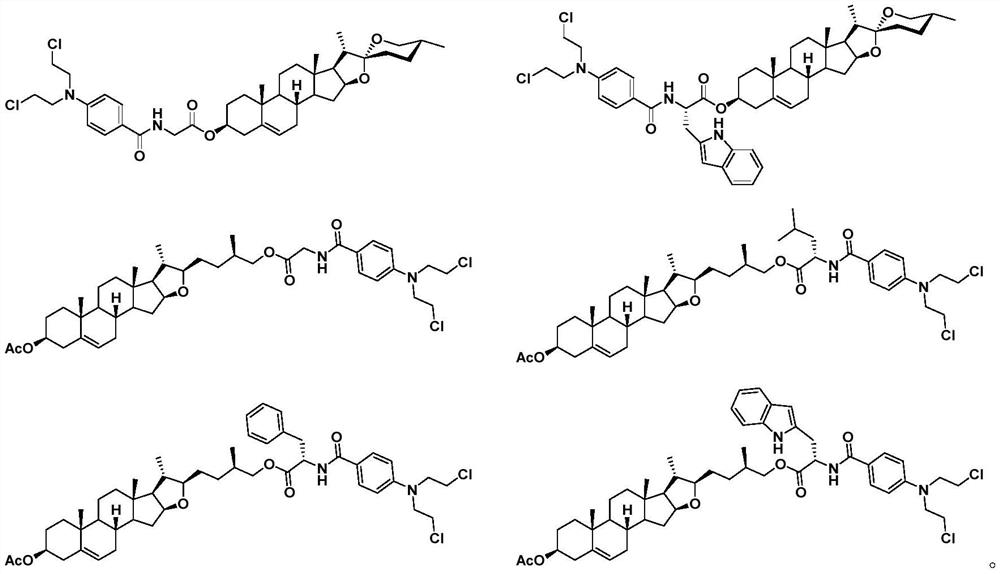
Aryl nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-propionyl-1,4-phenylenediamine and preparation method thereof
InactiveCN106631857AImprove therapeutic indexSmall toxicityAntipyreticOrganic compound preparationSide effectDistillation
The invention particularly discloses a structural formula and preparation method of an aryl nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-propionyl-1,4-phenylenediamine. The method comprises the following steps: preparing N,N-di(2-chloroethyl)-1,4-phenylenediamine; adding the N,N-di(2-chloroethyl)-1,4-phenylenediamine, dichloromethane and triethylamine into a reactor, cooling in an ice water bath, stirring, and dropwisely adding a propionyl chloride-dichloromethane mixed solution into the reactor; after finishing the dropwise addition, removing the ice water bath, and reacting at room temperature for 4-14 hours; and after the reaction finishes, washing the reaction solution, drying with anhydrous cupric sulfate, carrying out atmospheric distillation, and purifying the filter cake. The aryl nitrogen mustard derivative can effectively lower the toxic and side effects of nitrogen mustards on the premise of enhancing the therapeutic index of the nitrogen mustards, and has the curative effects of killing germs and diminishing inflammation, thereby lowering the risk of complications caused by decrease in immunity of the patient after chemotherapy.
Owner:CHANGAN UNIV
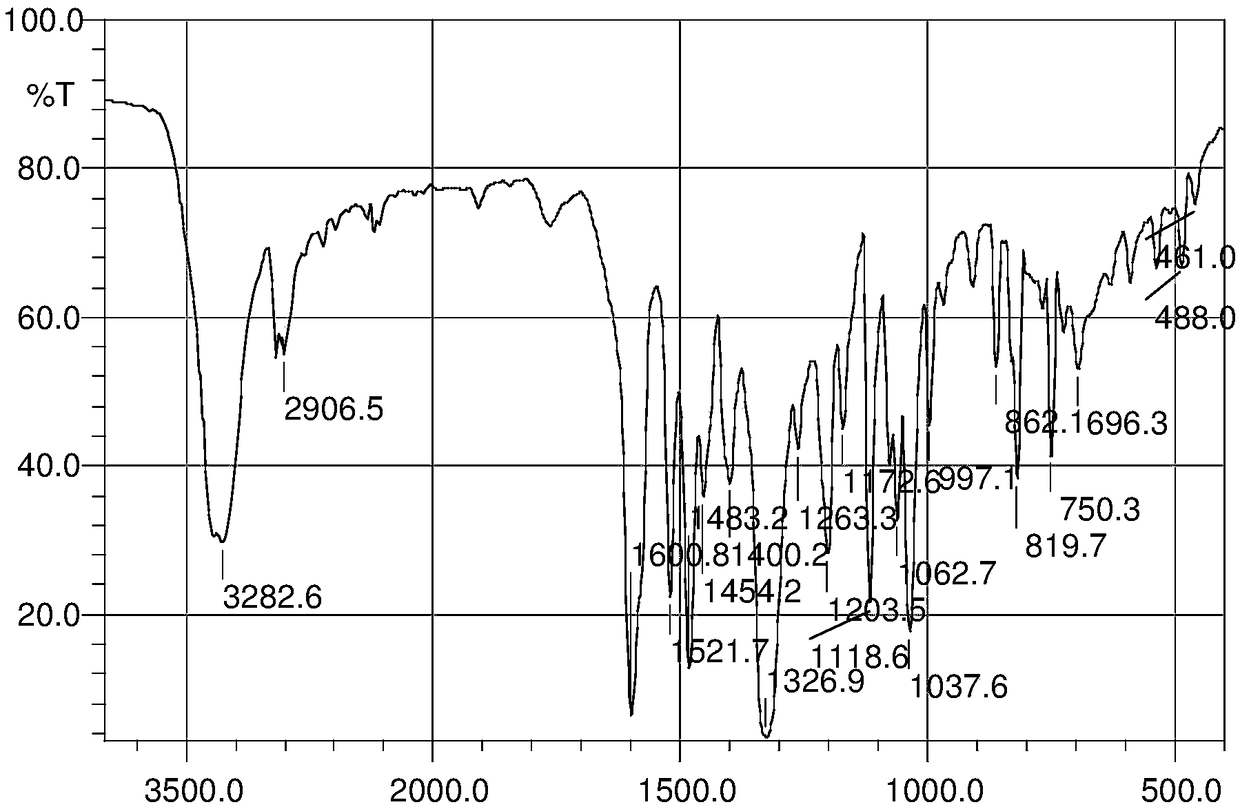
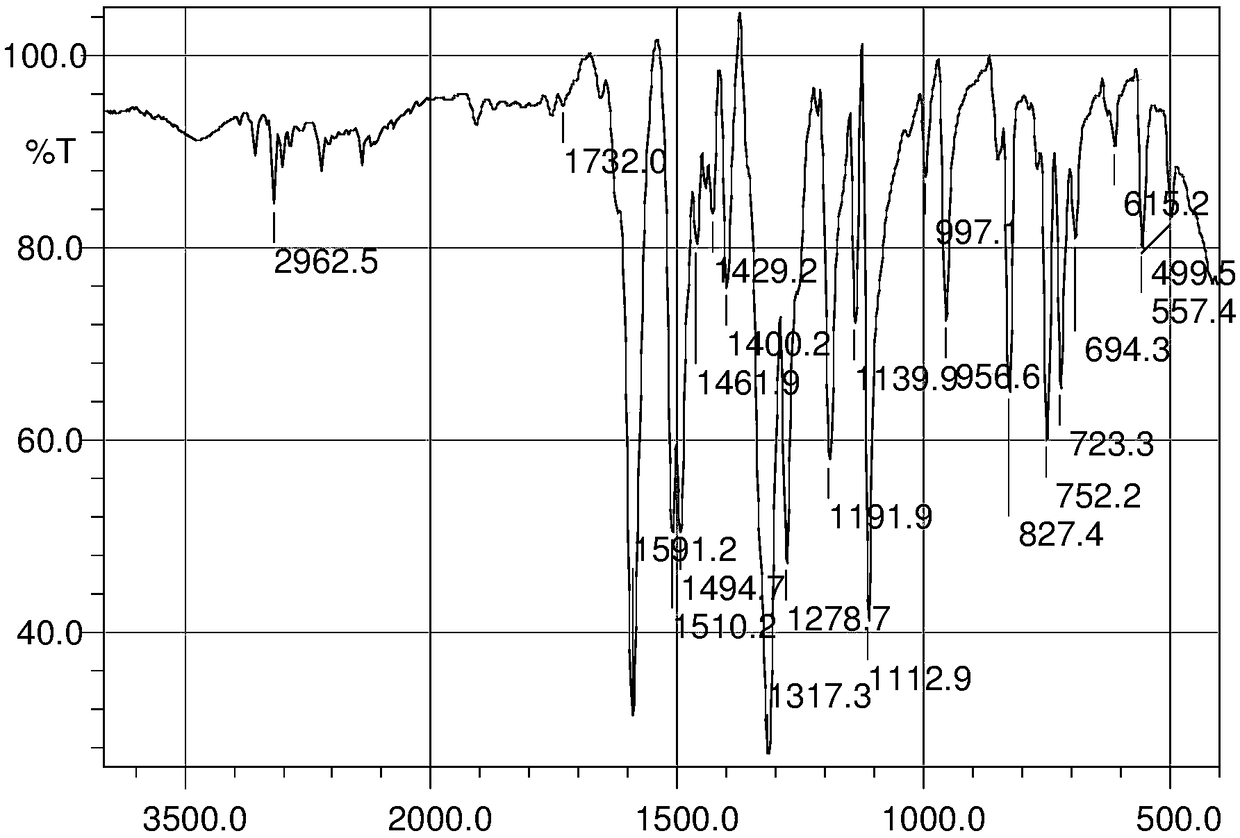
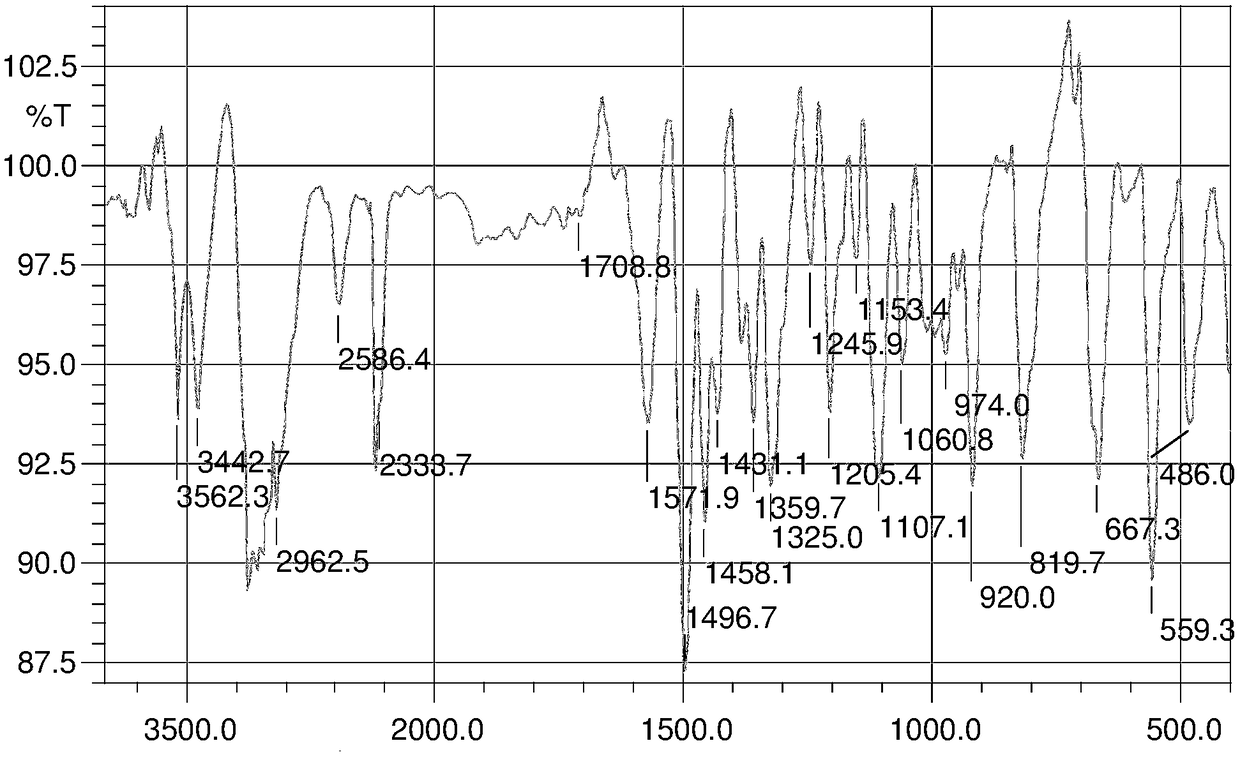
Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy
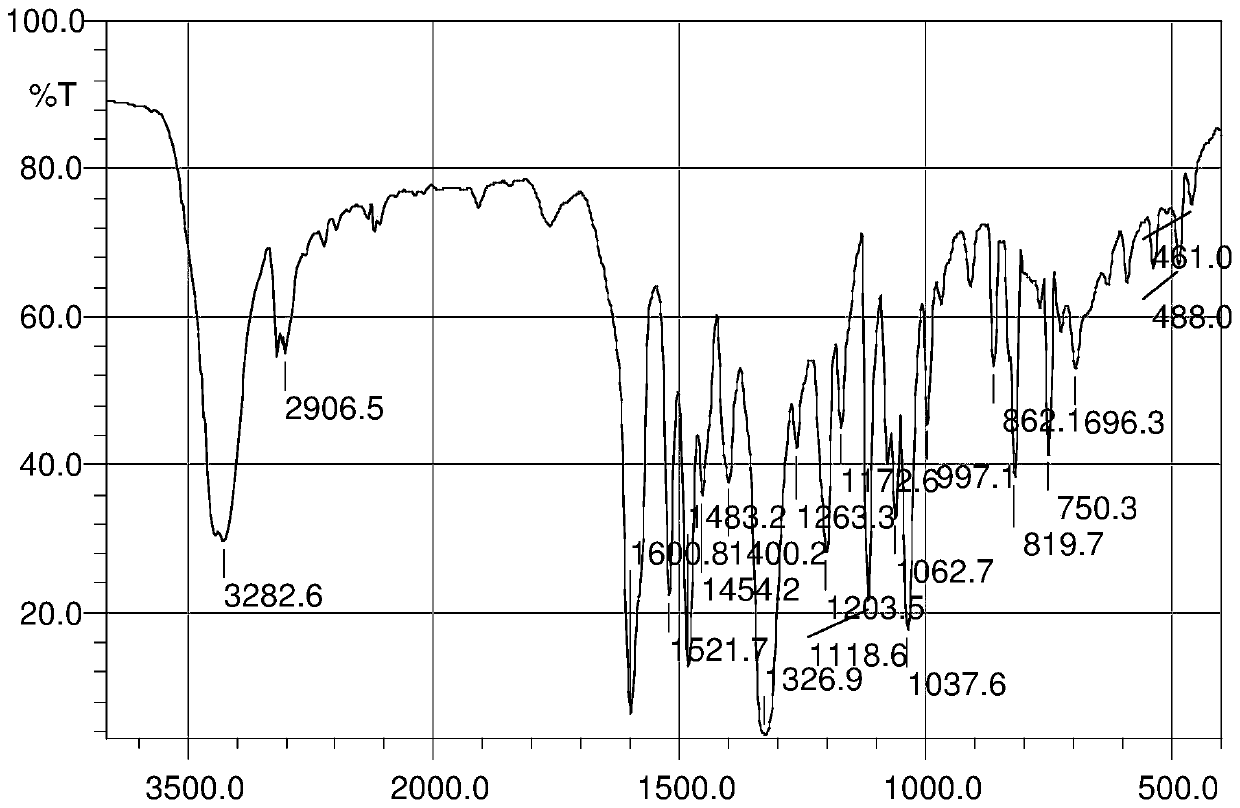
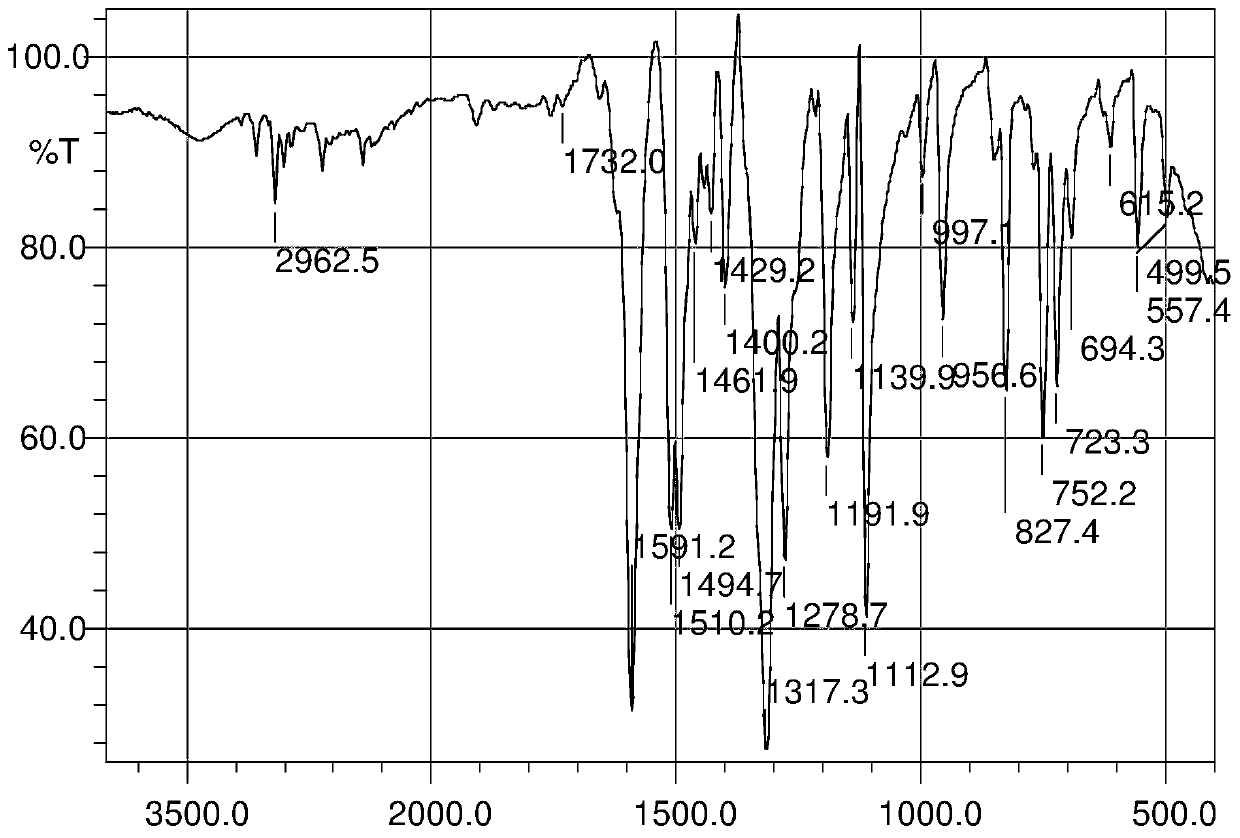
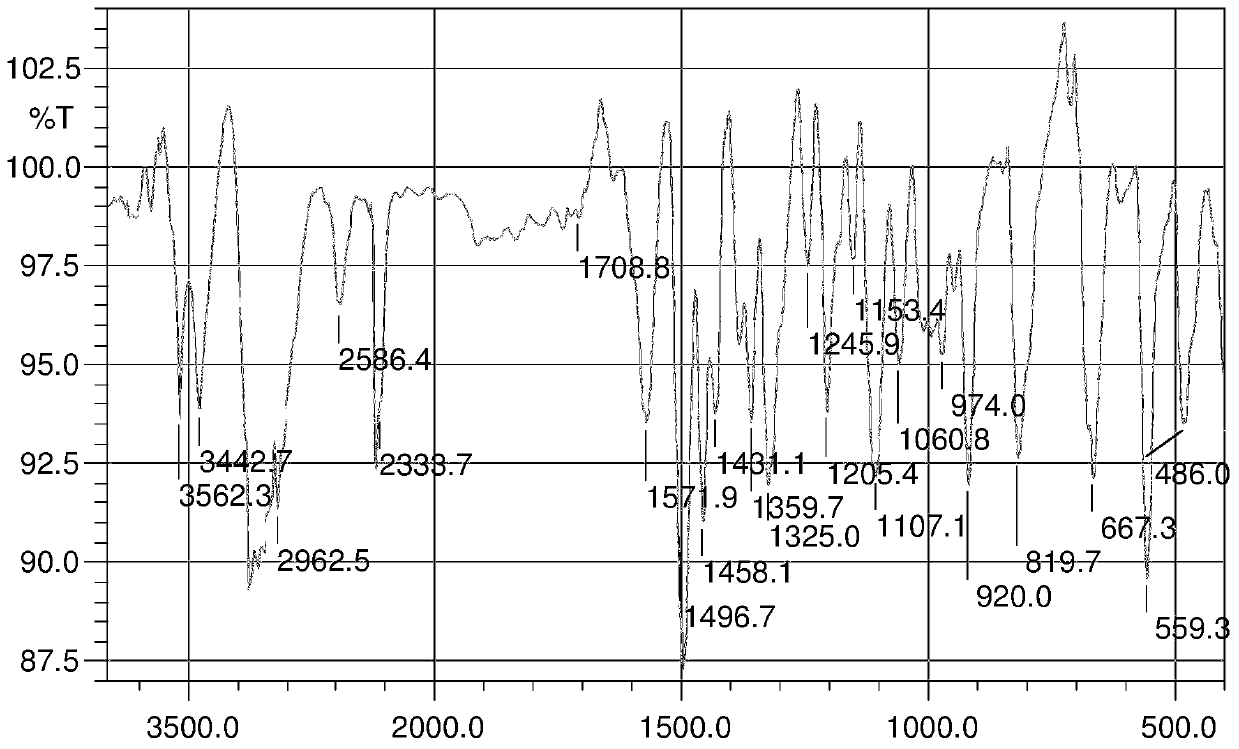
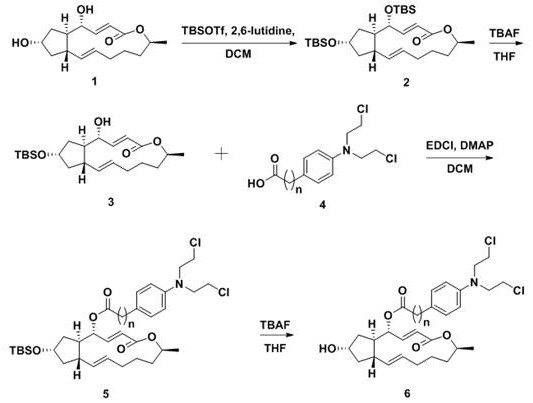
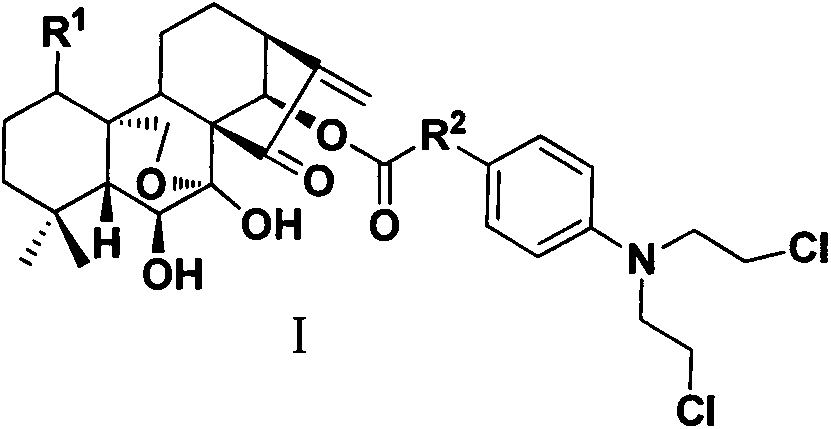
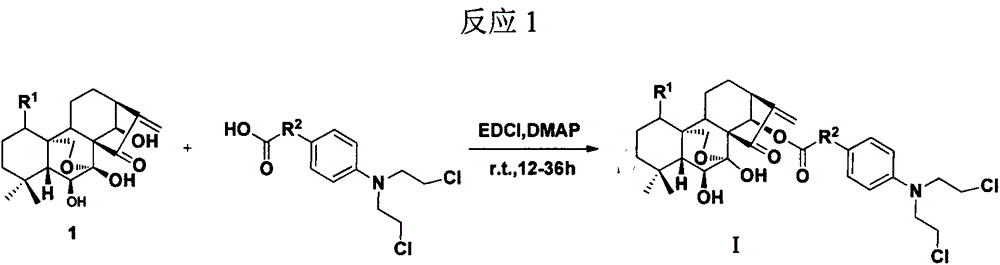
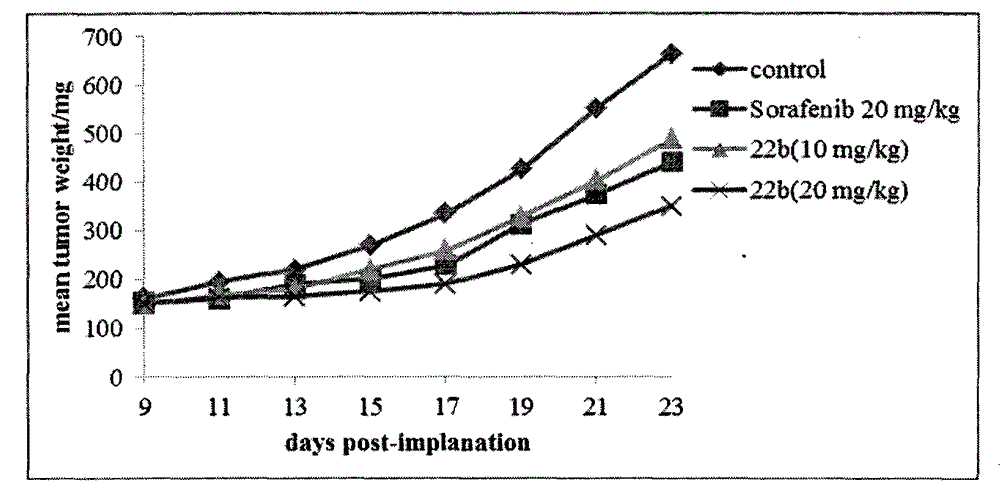
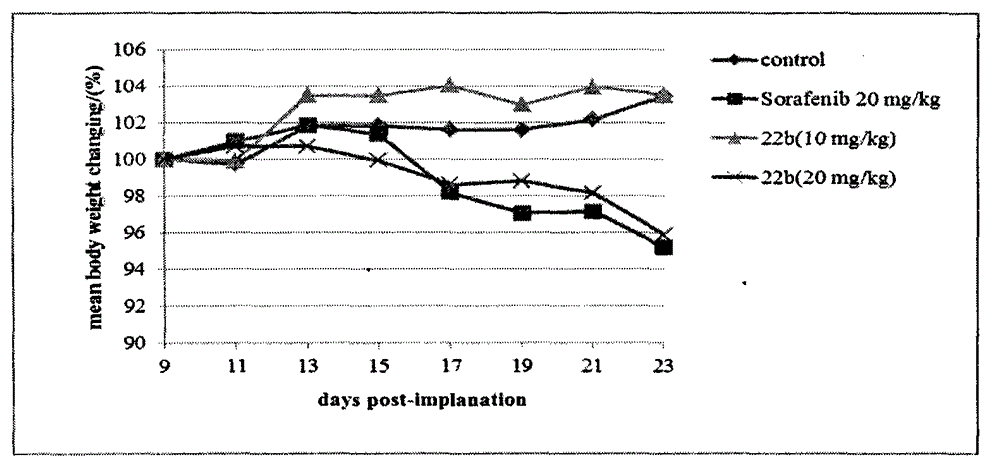
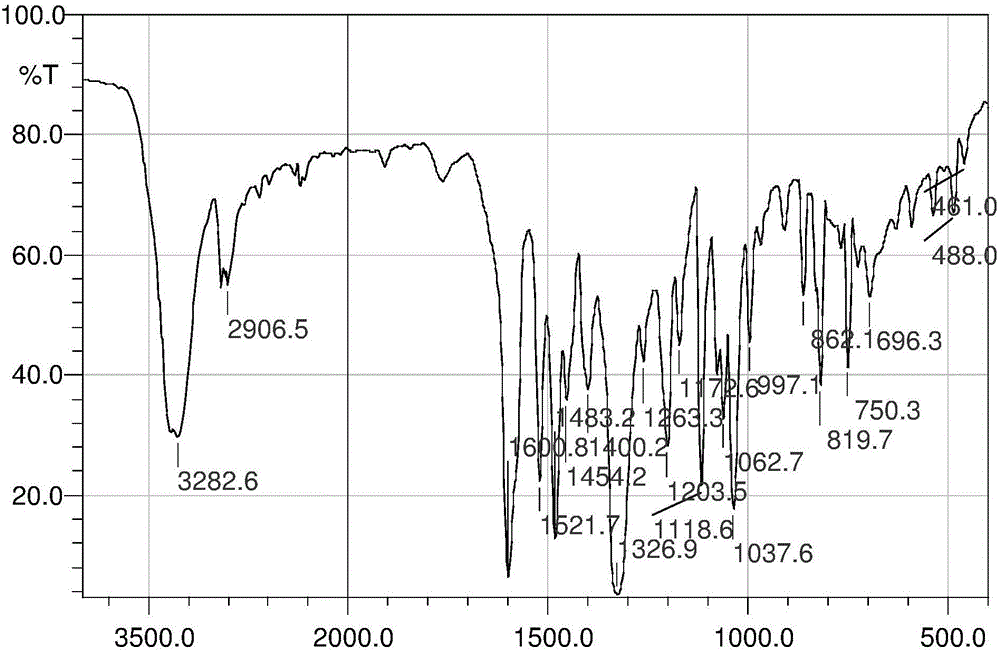
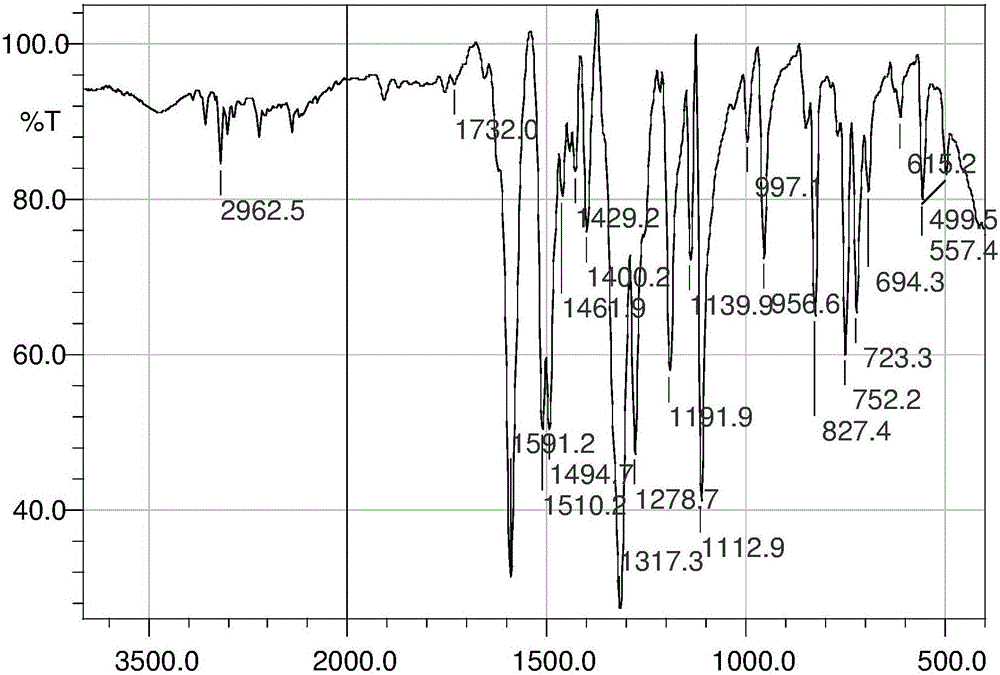
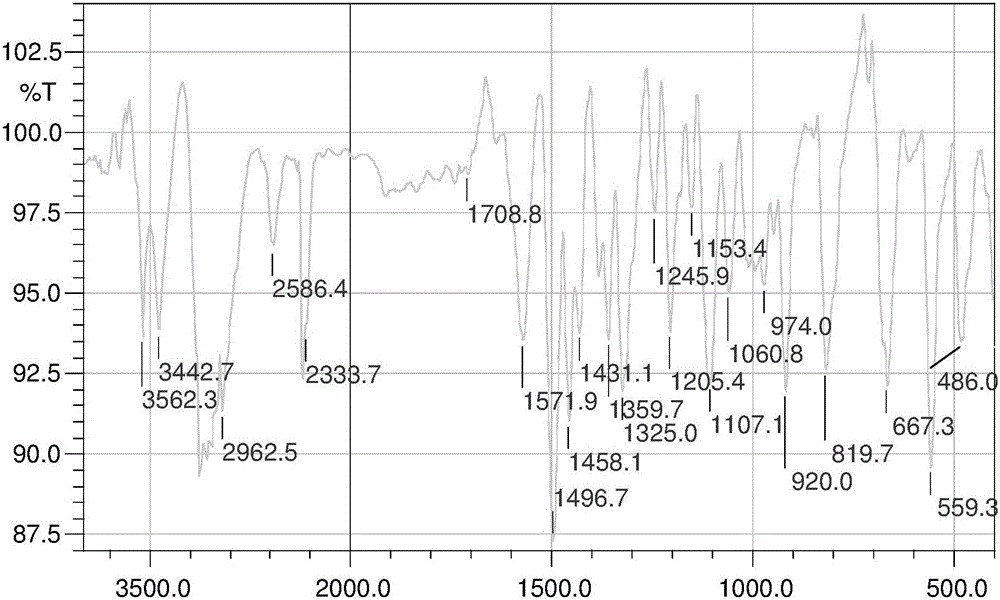
InactiveCN103626776AGood antitumor activityGood choiceOrganic active ingredientsOrganic chemistryInhibitory effectNitrogen mustard derivative
The invention relates to pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives or medical salts thereof, as well as application of the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives or the medical salts thereof. The pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives and the medical salts thereof have the structure shown as the formula I. Pharmacological experiments show that the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives and the medical salts thereof have inhibiting effects on the proliferation of various tumor cells. Moreover, the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives are small in toxicity, have the advantages of selectivity on tumor cells, and are dual-functional anti-tumor drugs. Meanwhile, the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives are easy to synthesize, and the overall yields are high. All the advantages show that the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives have great potential of being anti-tumor drugs.
Owner:BEIJING NORMAL UNIVERSITY
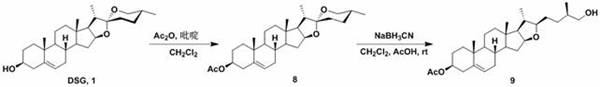
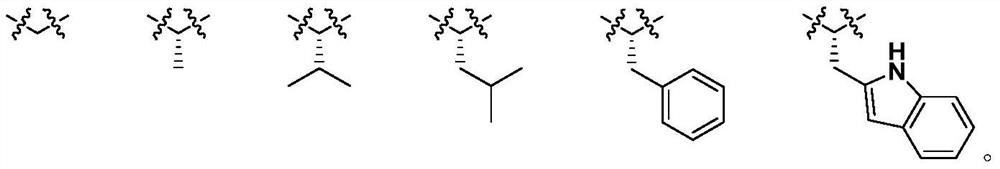
Diosgenin combined nitrogen mustard derivative with anti-tumor activity as well as preparation method and application thereof diosgenin combined nitrogen mustard derivative
ActiveCN112979744AHigh activityStrong cytotoxicityOrganic active ingredientsSteroidsBenzoic acidPharmaceutical drug
The invention belongs to the field of natural medicines and medicinal chemistry, and relates to a diosgenin combined nitrogen mustard derivative as well as a preparation method and application thereof. The invention particularly relates to a preparation method for introducing a benzoic acid nitrogen mustard derivative to a 3-OH or 26-OH site of a diosgenin mother nucleus structure and application of the derivative in preparation of anti-tumor drugs. The diosgenin combined nitrogen mustard derivative disclosed by the invention has a good anti-tumor effect and can be used for further preparing anti-tumor drugs.
Owner:QIQIHAR MEDICAL UNIVERSITY
Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application
InactiveCN103626776BSynthetic steps with high yieldsEasy to makeOrganic active ingredientsOrganic chemistryChemical compoundTumor therapy
The invention relates to pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives or medical salts thereof, as well as application of the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives or the medical salts thereof. The pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives and the medical salts thereof have the structure shown as the formula I. Pharmacological experiments show that the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives and the medical salts thereof have inhibiting effects on the proliferation of various tumor cells. Moreover, the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives are small in toxicity, have the advantages of selectivity on tumor cells, and are dual-functional anti-tumor drugs. Meanwhile, the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives are easy to synthesize, and the overall yields are high. All the advantages show that the pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives have great potential of being anti-tumor drugs.
Owner:BEIJING NORMAL UNIVERSITY
Arylamine nitrogen mustard derivative n,n-bis(2-chloroethyl)-n'-acetyl-1,4-phenylenediamine and its preparation method
InactiveCN106631874BImprove therapeutic indexSmall toxicityAntipyreticOrganic compound preparationSide effectIce water
Owner:CHANGAN UNIV
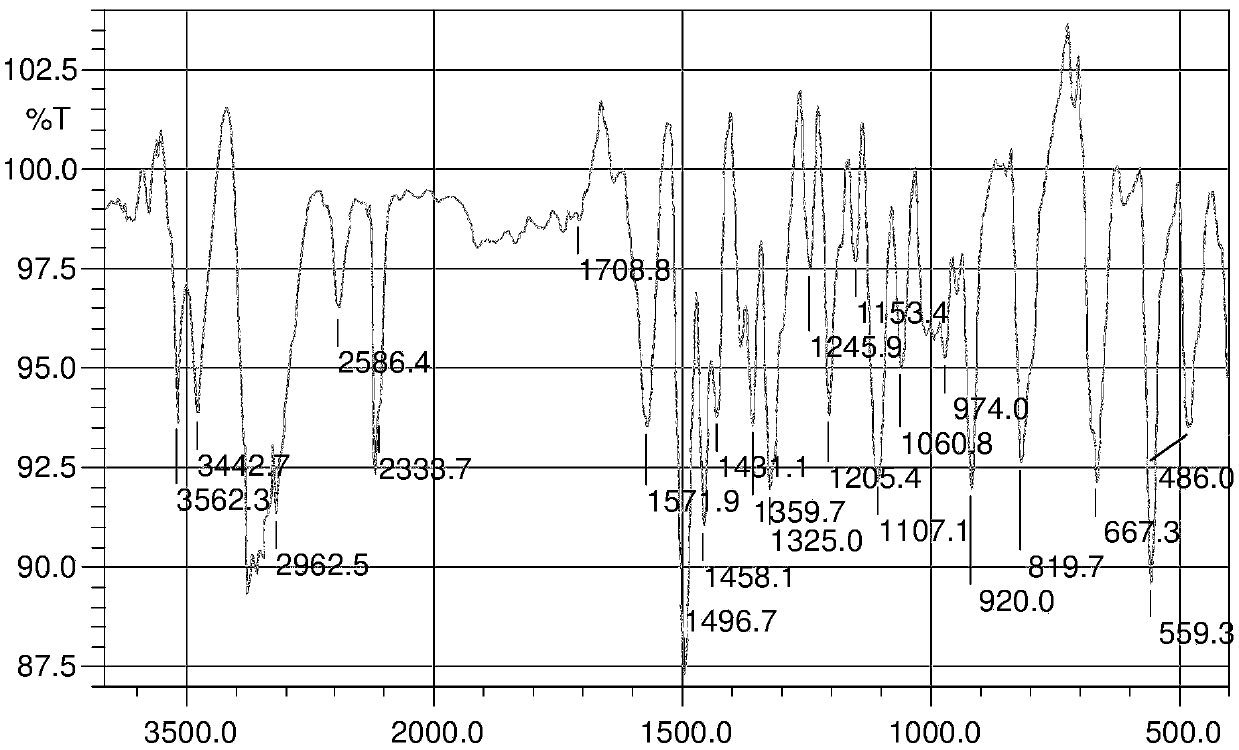
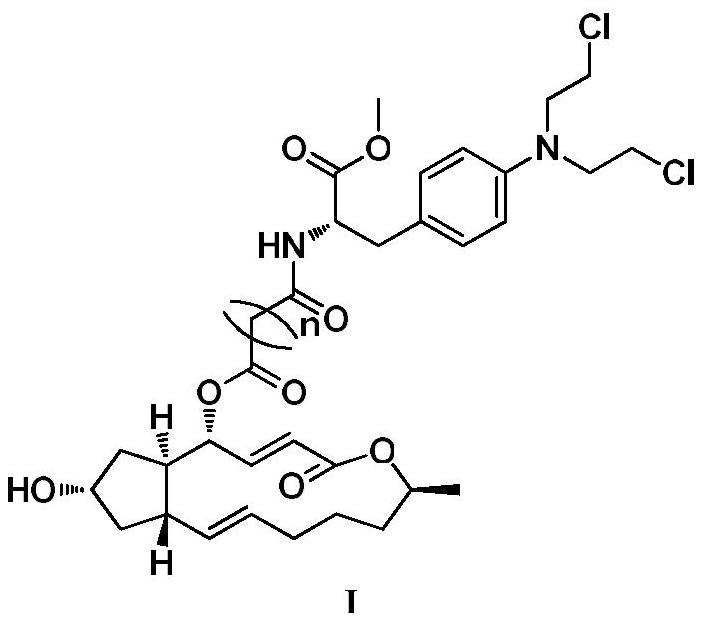
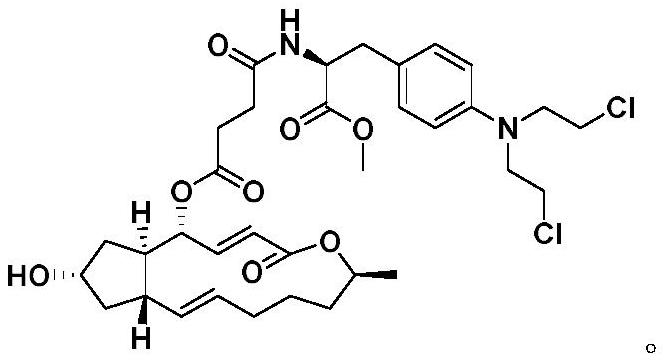
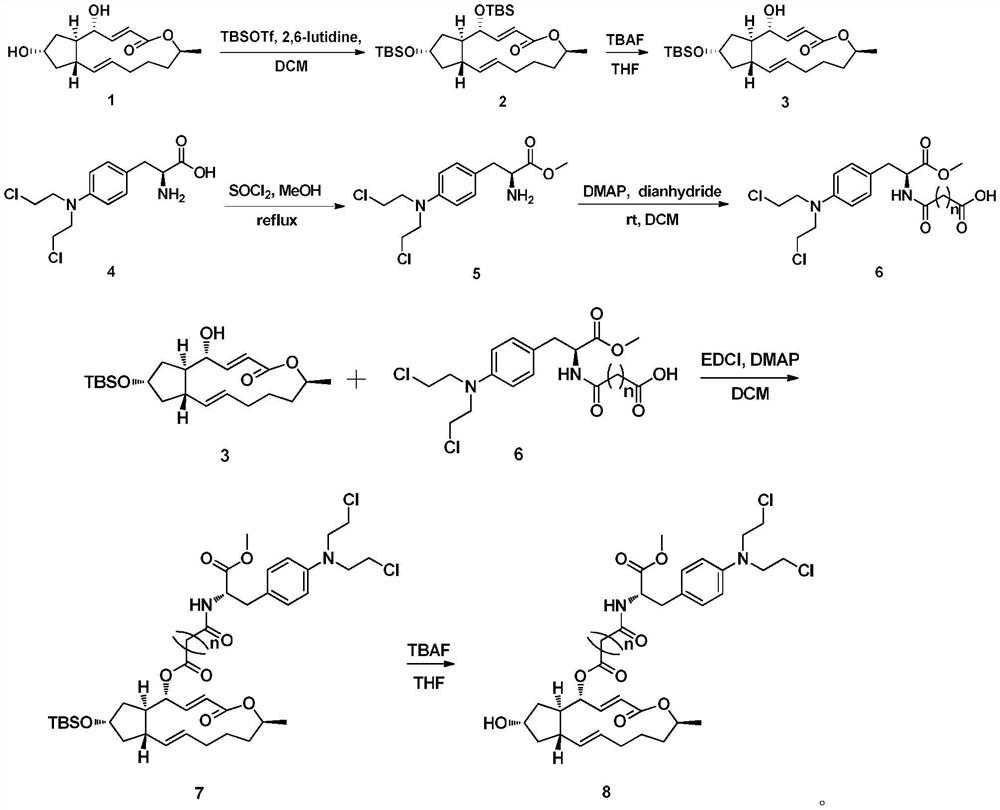
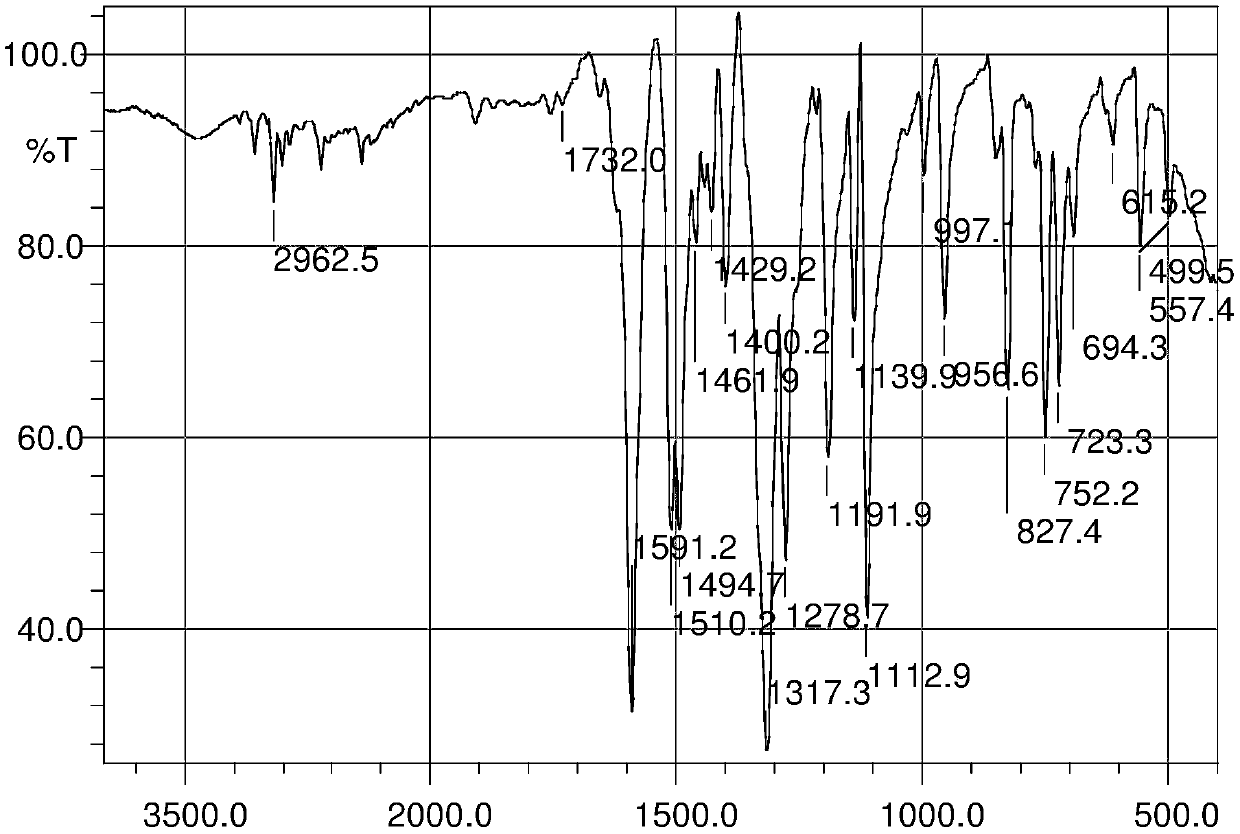
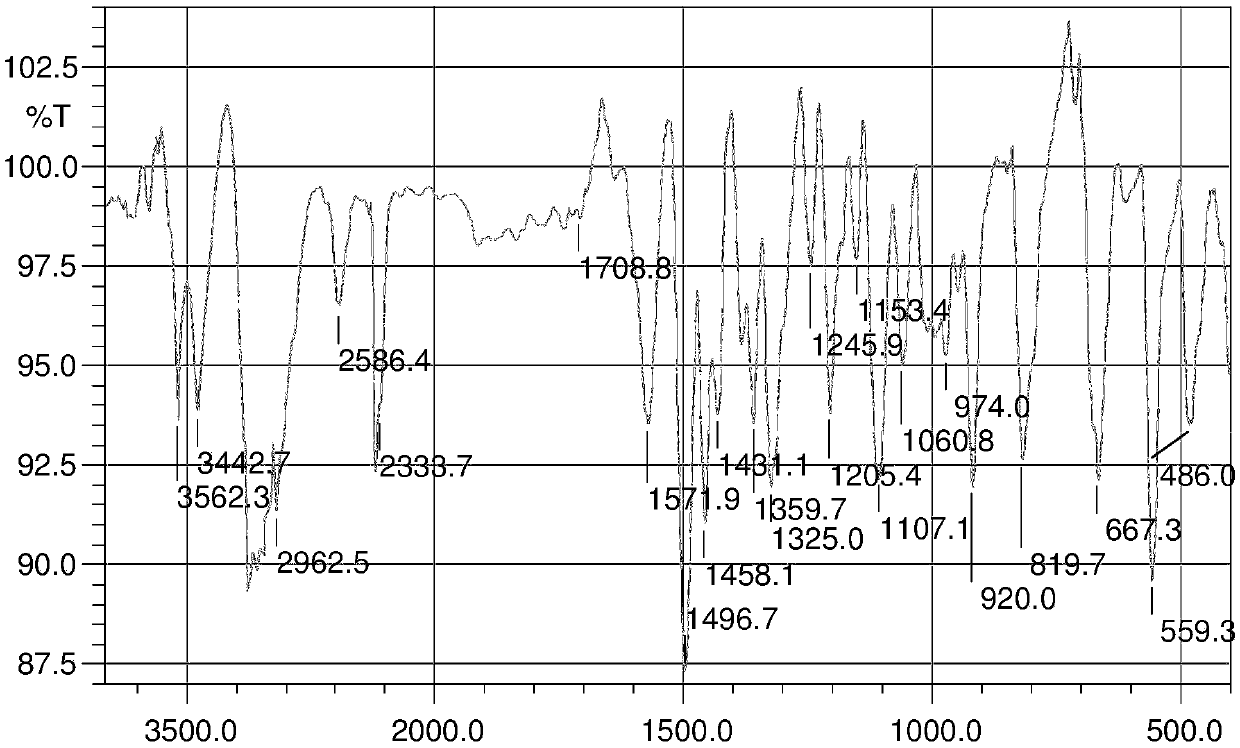
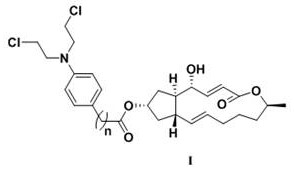
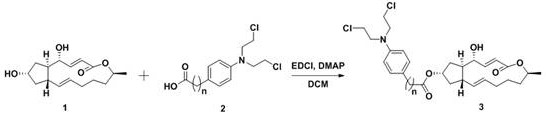
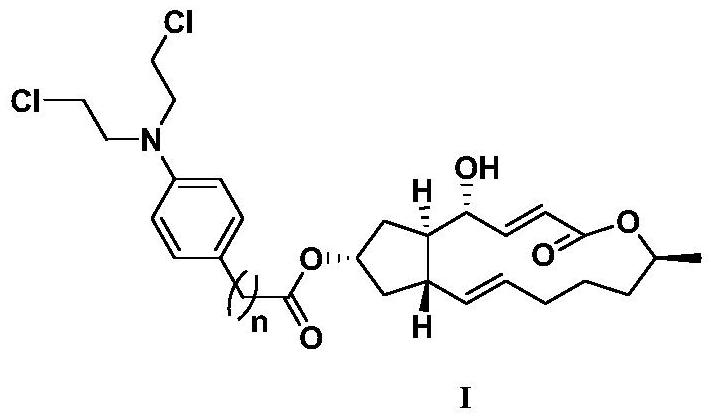
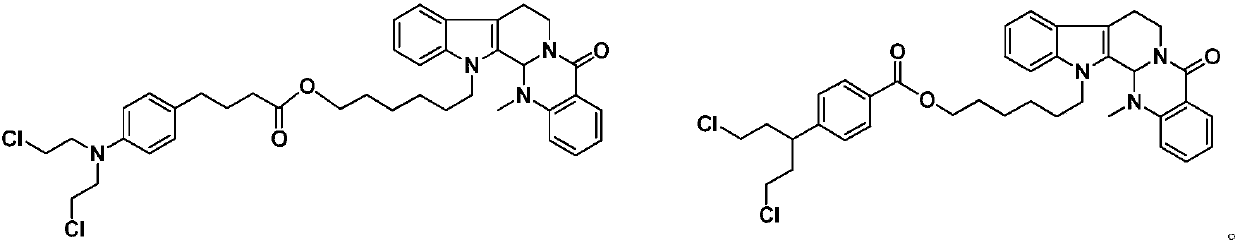
A kind of preparation method and application of 4-position splicing nitrogen mustard derivatives of brefeldin a
ActiveCN110028477BImprove pharmacological activityOrganic active ingredientsOrganic chemistryPharmaceutical drugPharmaceutical medicine
The invention belongs to the field of natural medicine and medicinal chemistry, and relates to a preparation method and uses of a class of brefeldin A derivative containing chlormethine linked at site4 and having antitumor activity, wherein the brefeldin A derivative containing chlormethine linked at site 4, and the pharmaceutically acceptable salt have a structure represented by the following general formula I, and n is defined in the claim and specification.
Owner:SHENYANG PHARMA UNIVERSITY
Arylamine nitrogen mustard derivative N,N-bis(2-chloroethyl)-N'-acetyl-1,4-phenylenediamine and preparation method thereof
InactiveCN106631874AImprove therapeutic indexSmall toxicityAntipyreticOrganic compound preparationIce waterStructural formula
The invention specifically discloses a structural formula of an arylamine nitrogen mustard derivative N,N-bis(2-chloroethyl)-N'-acetyl-1,4-phenylenediamine and a preparation method of the derivative. The method comprises the following steps: preparing N,N-bis(2-chloroethyl)-1,4-phenylenediamine; filling a reactor with N,N-bis(2-chloroethyl)-1,4-phenylenediamine, dichloromethane and triethylamine, cooling in an ice-water bath, stirring, dropwise adding a mixed solution of acetyl chloride and dichloromethane into the reactor, removing the ice-water bath after dropwise adding, reacting at the room temperature for 2-14 hours, washing, drying and distilling after the reaction is completed, purifying the distilled filter cake, thereby obtaining the product. The arylamine nitrogen mustard derivative disclosed by the invention can effectively reduce the toxic or side effects of nitrogen mustard on the premise of enhancing the therapeutic index of the nitrogen mustard and has the curative effects of sterilizing and diminishing inflammation so as to reduce the risk of causing complications due to decrease in immunity after chemotherapy of a patient.
Owner:CHANGAN UNIV
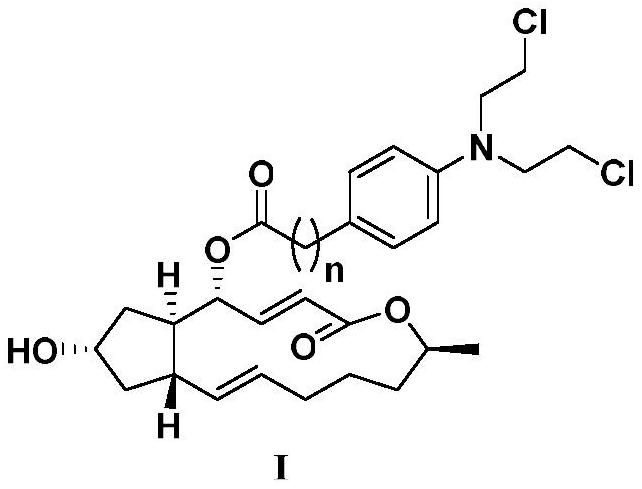
A kind of scutellarin aglycone nitrogen mustard derivatives and its preparation method and application
ActiveCN108864024BGood antitumor activityGood choiceOrganic active ingredientsOrganic chemistryBenzoic acidAglycone
The invention belongs to the field of natural medicine and medicinal chemistry and relates to a scutellarin aglycone nitrogen mustard derivative and a preparation method and application thereof, in particular to a scutellarin aglycone nitrogen mustard derivative of which 4'-OH is combined with benzoic acid nitrogen mustard and a preparation method and antitumor activity thereof. The structure of the scutellarin aglycone nitrogen mustard derivative and pharmaceutically acceptable salt thereof is as shown in the general formula I, wherein R and n are as described in claims and the specification.The formula I is shown in the description.
Owner:SHENYANG PHARMA UNIVERSITY
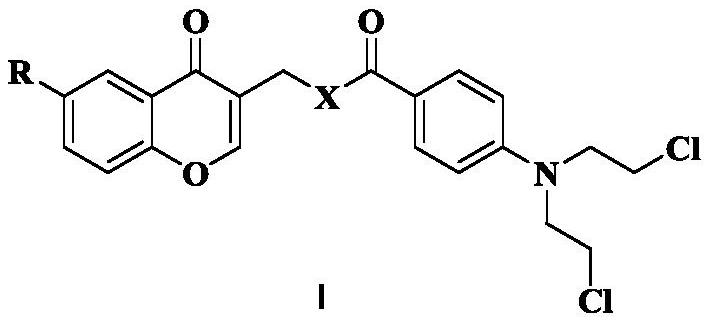
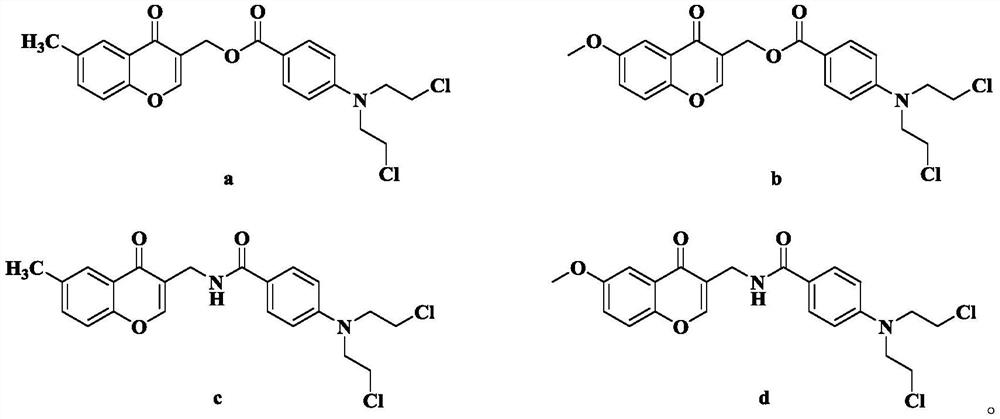
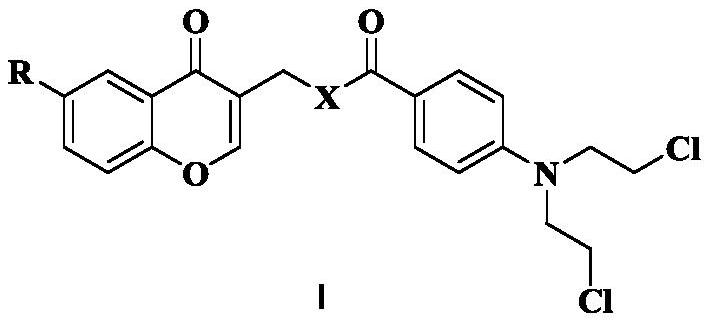
Chromone nitrogen mustard derivative and anti-tumor application
ActiveCN113788810AGood anti-tumor cell proliferation effectTest biological activityOrganic active ingredientsOrganic chemistryPharmaceutical drugPharmaceutical medicine
The invention discloses a chromone nitrogen mustard derivative and anti-tumor application, and belongs to the field of natural medicines and medicinal chemistry. The invention particularly relates to a preparation method of a series of chromone nitrogen mustard derivatives with anti-tumor activity and novel application of the chromone nitrogen mustard derivatives in anti-tumor drugs. The chromone nitrogen mustard derivative and the pharmaceutically acceptable salt thereof disclosed by the invention are shown in a general formula I in the specification. R and X are described in the claims and the specification.
Owner:SHENYANG PHARMA UNIVERSITY
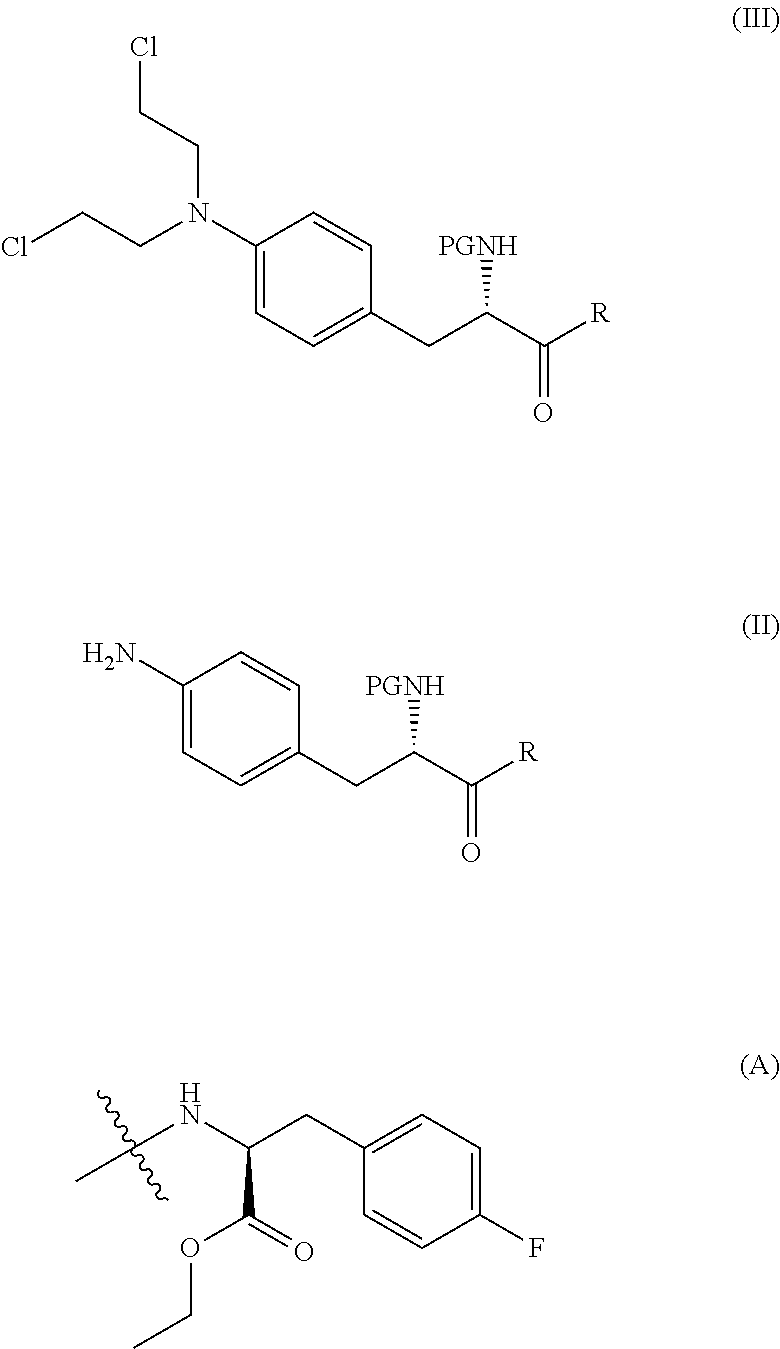
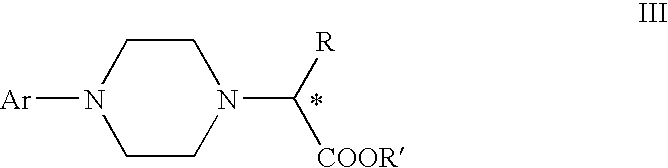
Process for preparation of nitrogen mustard derivatives
ActiveUS10287316B2High yieldHigh purityCarbamic acid derivatives preparationPeptide/protein ingredientsBiochemical engineeringChemical compound
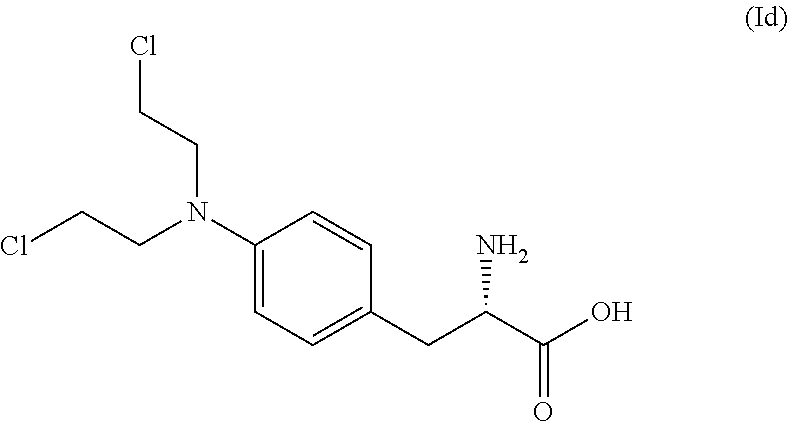
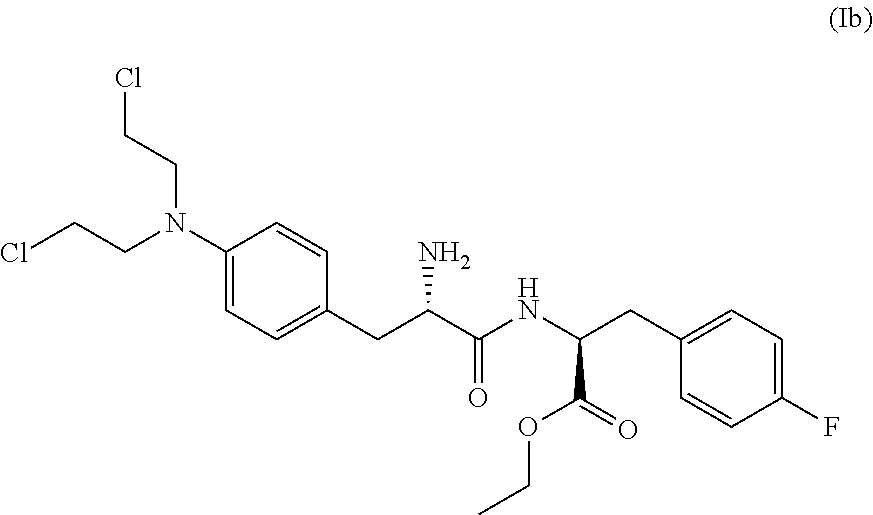
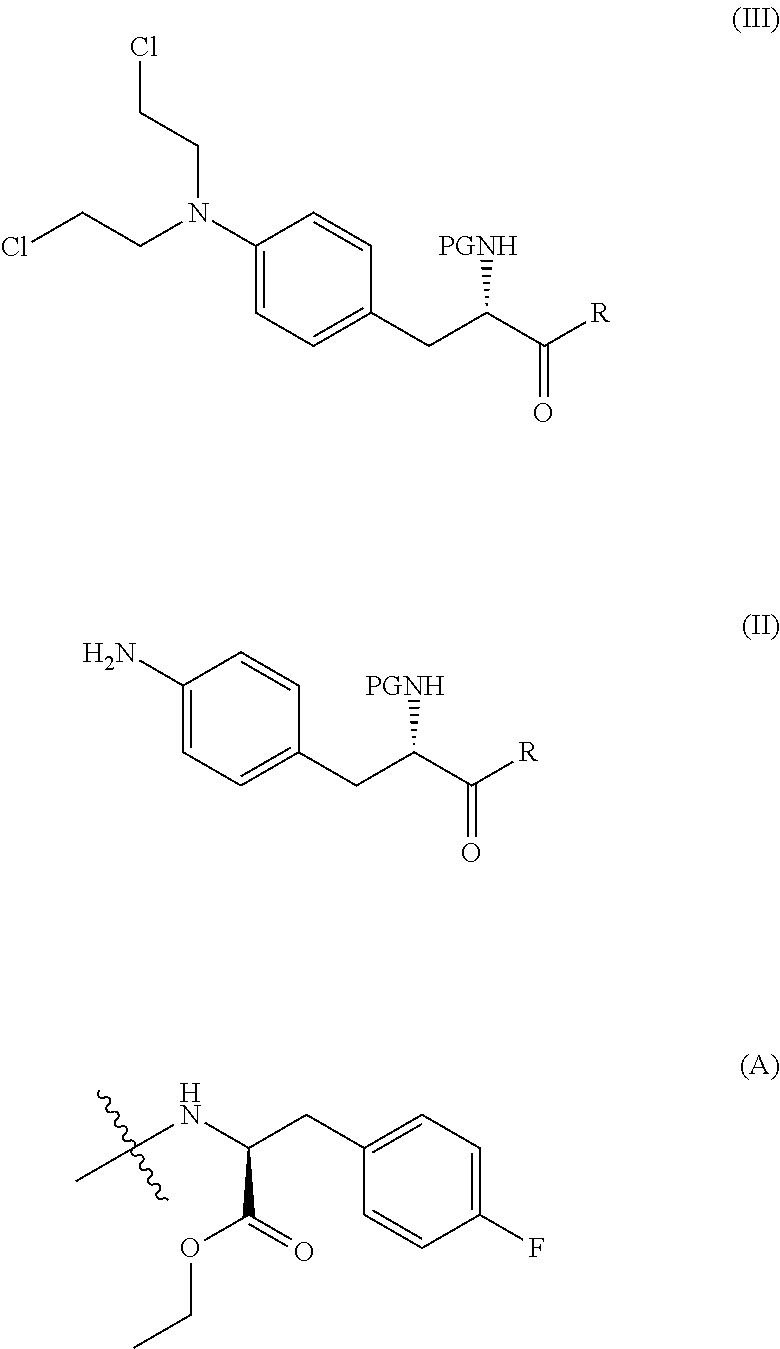
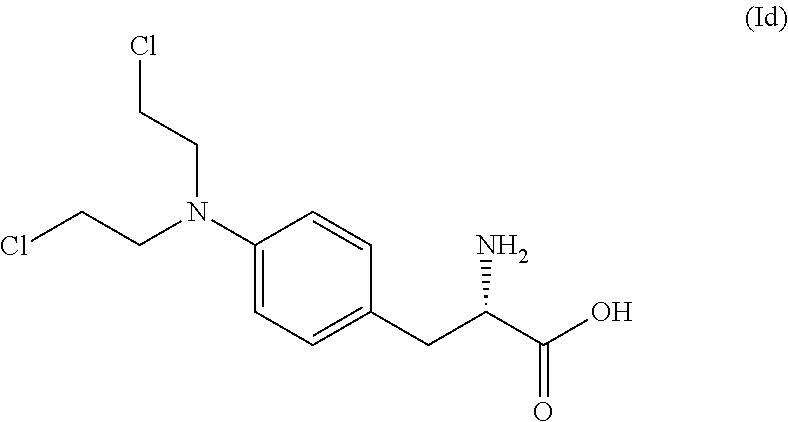
The present invention provides a process for the production of compound (III) or a deprotected product thereof: comprising reacting compound (II) with chloroacetic acid, in the presence of a reducing agent; wherein PG is a protecting group and R is OH in a suitably protected form or (A). The invention further provides intermediate compounds formed in the process of the invention, and processes for the production of intermediate compounds.
Owner:ONCOPEPTIDES
Process for preparation of nitrogen mustard derivatives
ActiveUS20180148473A1Good yieldHigh purityCarbamic acid derivatives preparationPeptide/protein ingredientsAcetic acidNitrogen
The present invention provides a process for the production of compound (III) or a deprotected product thereof: comprising reacting compound (II) with chloroacetic acid, in the presence of a reducing agent; wherein PG is a protecting group and R is OH in a suitably protected form or (A). The invention further provides intermediate compounds formed in the process of the invention, and processes for the production of intermediate compounds.
Owner:ONCOPEPTIDES
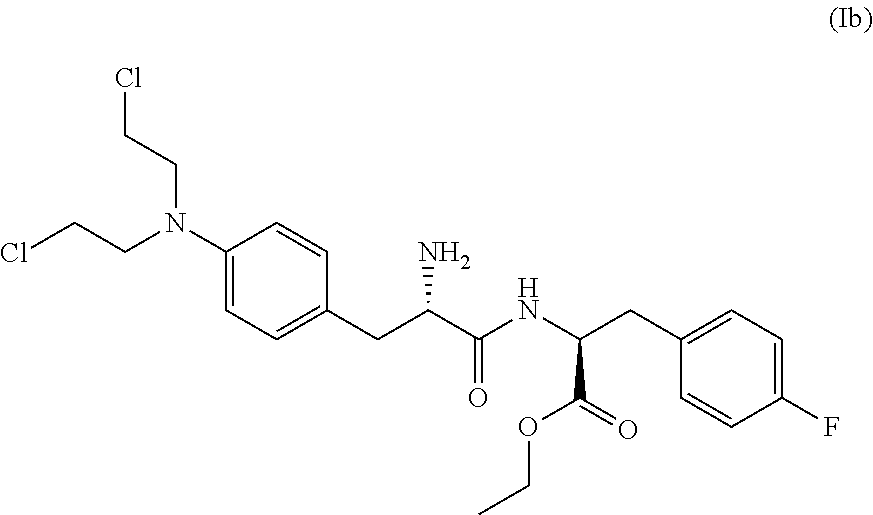
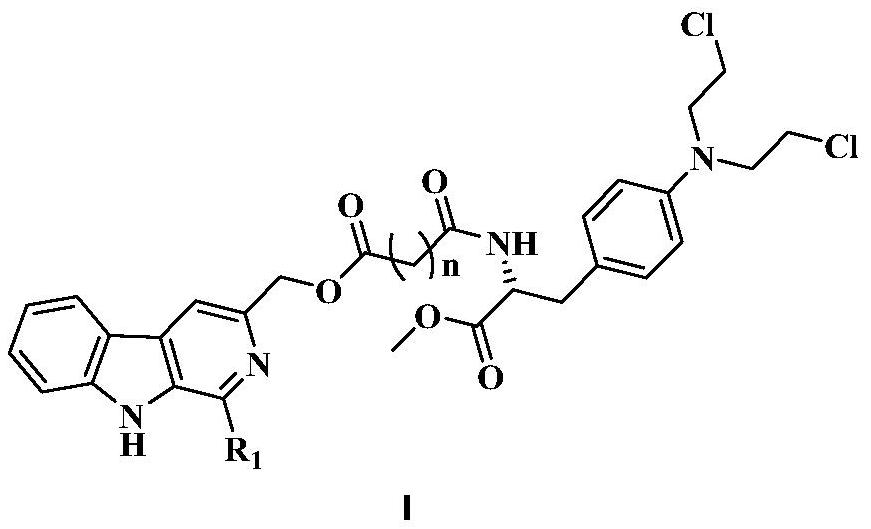
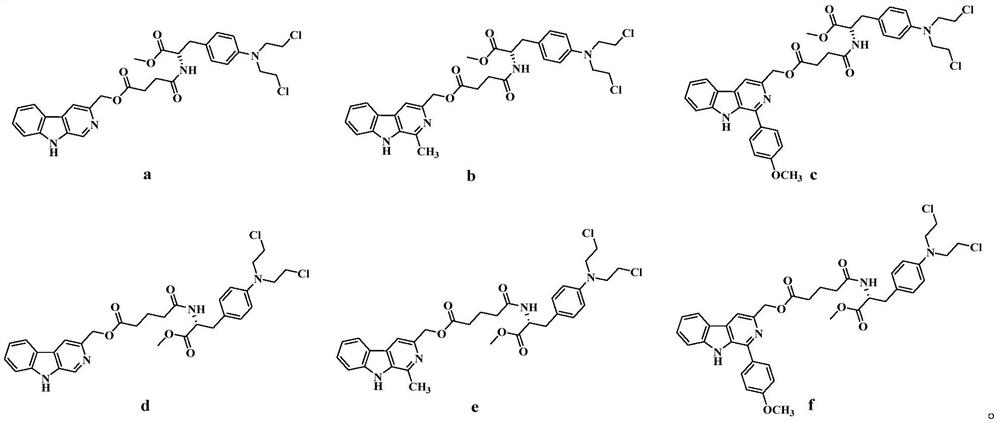
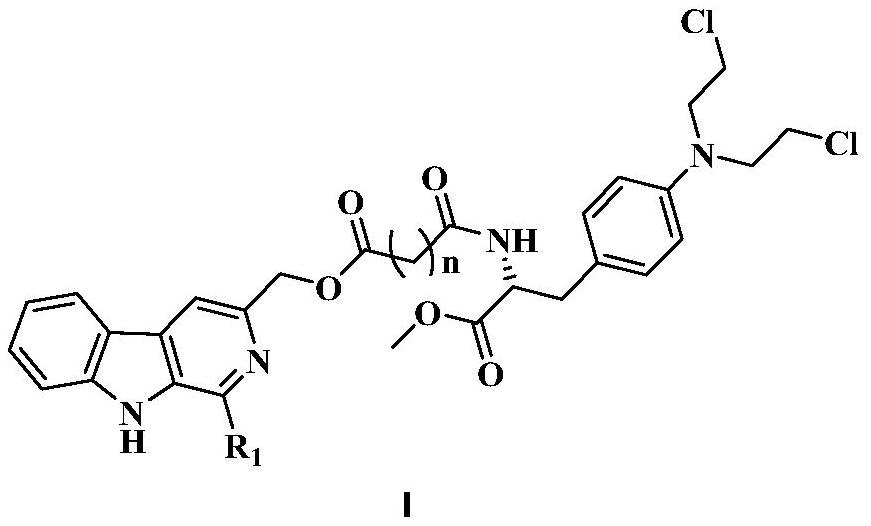
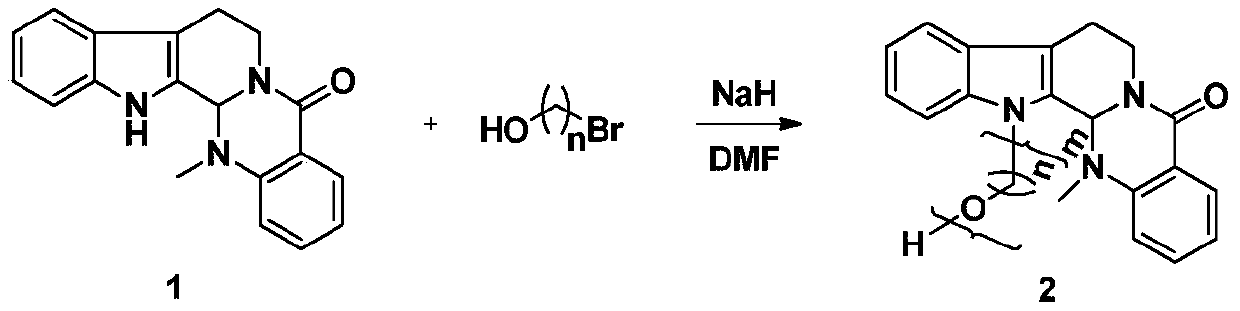
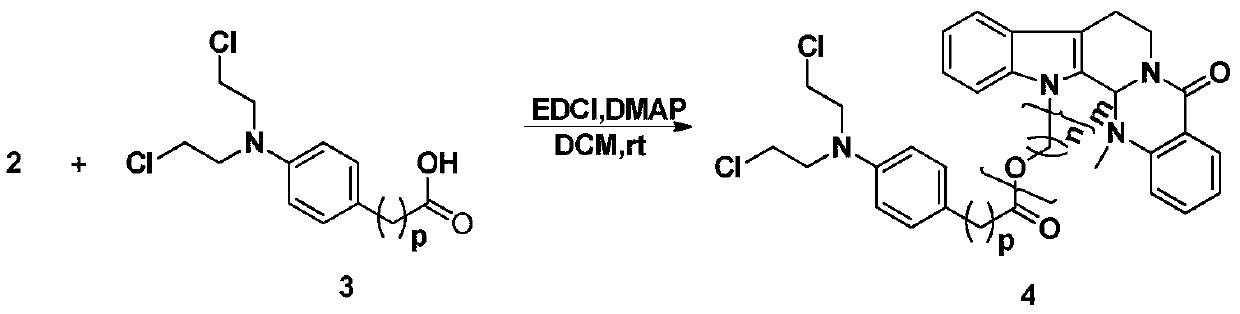
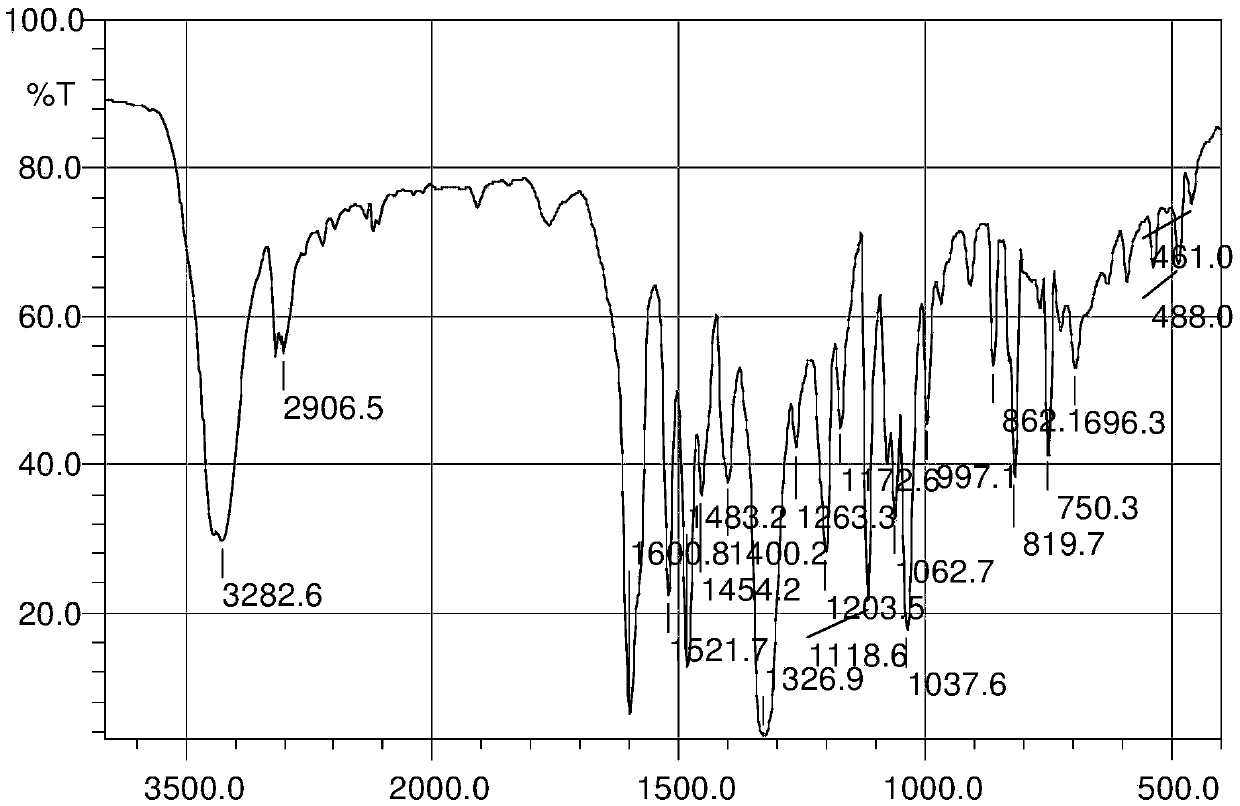
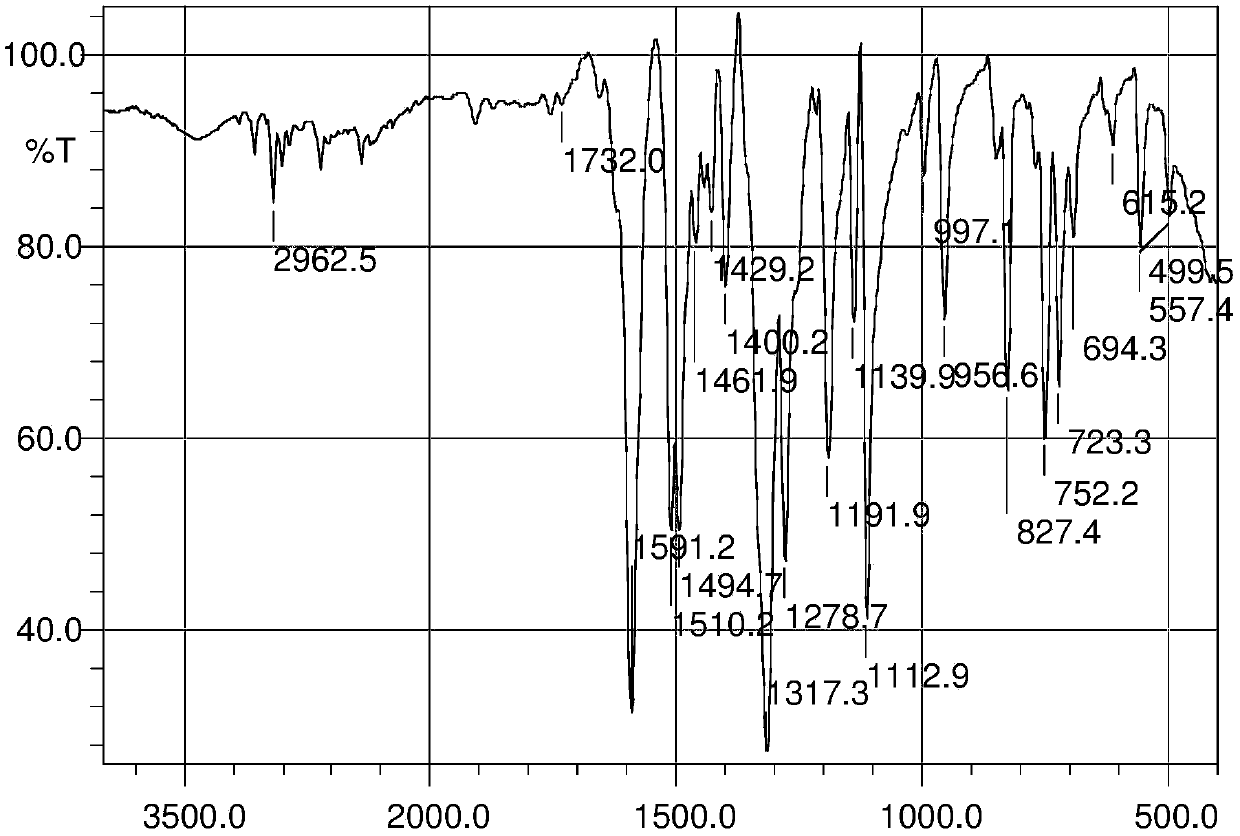
3-position derivatives of beta-carboline as well as preparation method and application of 3-position derivatives
ActiveCN113563330ATest biological activityGood anti-tumor cell proliferation effectOrganic active ingredientsOrganic chemistryPharmaceutical drugPharmaceutical medicine
The invention discloses 3-position spliced nitrogen mustard derivatives of beta-carboline as well as a preparation method and application of the 3-position spliced nitrogen mustard derivatives, and belongs to the field of natural medicines and medicinal chemistry. The invention particularly relates to a preparation method of a series of 3-position spliced nitrogen mustard derivatives of beta-carboline with antitumor activity and novel application of the derivatives in antitumor drugs. The3-position spliced nitrogen mustard derivatives of beta-carboline and the pharmaceutically acceptable salt of the derivatives disclosed by the invention are shown as a general formula I defined in the description, wherein R1 and n are described in the claims and the description.
Owner:SHENYANG PHARMA UNIVERSITY
Nitrogen mustard derivative n,n-bis(2-chloroethyl)-n'-benzoyl-1,4-phenylenediamine and its preparation method
InactiveCN106631856BImprove therapeutic indexSmall toxicityOrganic compound preparationAntipyreticPhenacylSide effect
Owner:CHANGAN UNIV
Preparation method and application of a class of evodiamine and nitrogen mustard derivatives with antitumor activity
ActiveCN107674076BImprove pharmacological activityOrganic chemistryAntineoplastic agentsEvodiamineMedicinal chemistry
The invention relates to the fields of natural drugs and medicinal chemistry, particularly a preparation method of evodiamine N-13 combined nitrogen mustard derivatives and application of the evodiamine N-13 combined nitrogen mustard derivatives in preparing antineoplastic drugs. The structure of the evodiamine combined nitrogen mustard derivatives and pharmaceutically acceptable salts thereof isdisclosed as a general formula I, wherein n, m and p are as described in the claims and specification. The evodiamine derivatives provided by the invention have better antineoplastic effects, and canbe used for further preparing antineoplastic drugs.
Owner:SHENYANG PHARMA UNIVERSITY
Nitrogen mustard derivative n,n-di(2-chloroethyl)-n'-tetradecanoyl-1,4-phenylenediamine and its preparation method
InactiveCN106631876BImprove therapeutic indexSmall toxicityAntipyreticOrganic compound preparationSide effectIce water
The invention specifically discloses the structural formula of a nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-tetradecanoyl-1,4-phenylenediamine; and a preparation method thereof: firstly prepare N, N-bis(2-chloroethyl)-1,4-phenylenediamine; then N,N-bis(2-chloroethyl)-1,4-phenylenediamine, dichloromethane and triethylamine into the reactor, cooled in an ice-water bath, stirred, and added dropwise a mixed solution of myristyl chloride and dichloromethane to the reactor, after the dropwise addition was completed, the ice-water bath was removed, and the room temperature was reacted for 10-14 hours. After the reaction was complete, The reaction solution is washed, dried with anhydrous copper sulfate, and distilled at normal pressure, and the distilled filter cake is purified to obtain the obtained product. Under the premise of enhancing the nitrogen mustard therapeutic index, the nitrogen mustard derivatives of the present invention can effectively reduce the toxic and side effects of the nitrogen mustard, and at the same time have the curative effects of sterilization and anti-inflammation, so as to reduce the symptoms caused by the decreased immunity of patients after chemotherapy. risk of complications.
Owner:CHANGAN UNIV
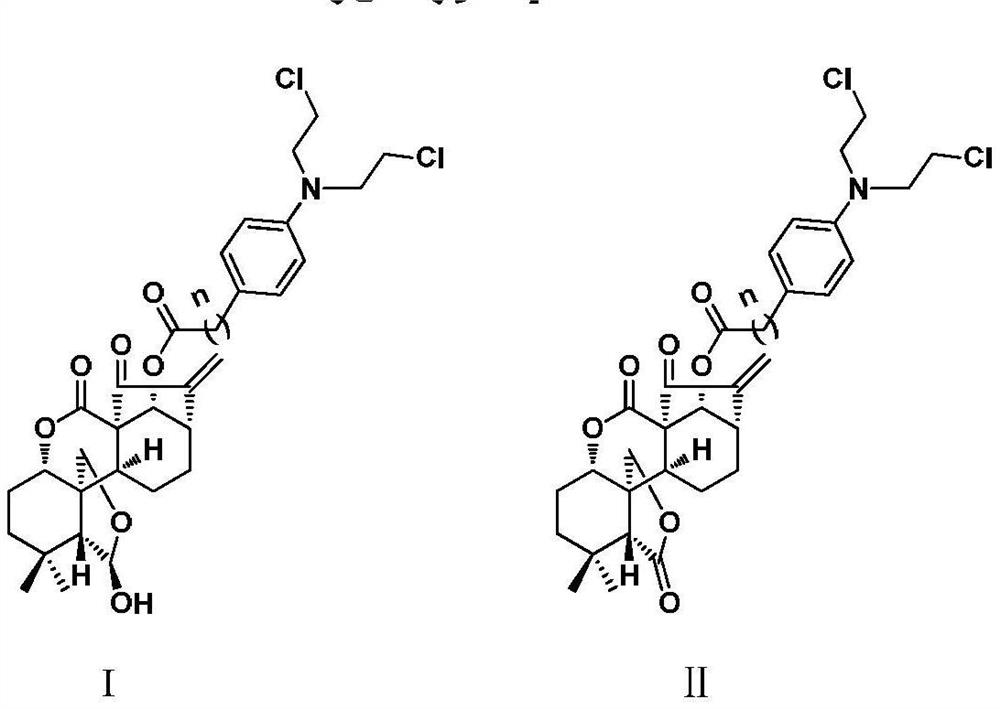
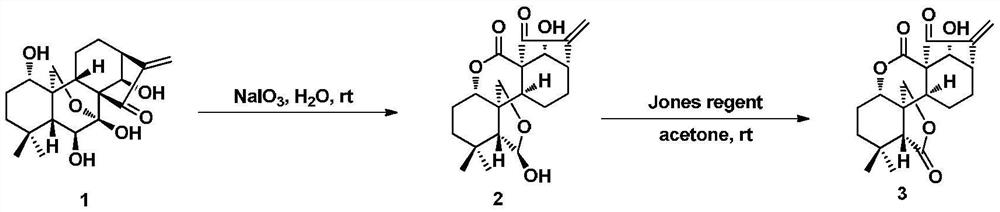
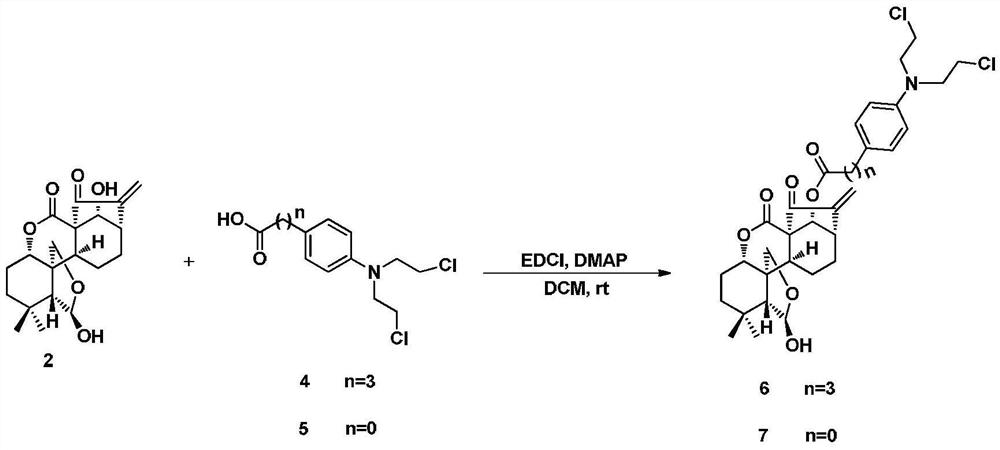
4-position spliced melphalan nitrogen mustard derivative of brefeldin a and its preparation method and application
ActiveCN110028482BImprove pharmacological activityOrganic active ingredientsOrganic chemistryAlkylating antineoplastic agentPharmaceutical drug
Owner:SHENYANG PHARMA UNIVERSITY
Oridonin A 14-o-substituted nitrogen mustard derivative, preparation method and use
The invention relates to the field of pharmaceutical chemistry, specifically to novel oridonin 14-0-sustituted nitrogen mustard derivatives with antineoplastic activity. The invention further discloses a preparation method for the oridonin 14-0-sustituted nitrogen mustard derivatives, a pharmaceutical composition containing the derivatives and application of the derivatives in treatment of neoplastic diseases and other related diseases or illness.
Owner:CHINA PHARM UNIV
Two nitrogen mustard derivatives, as well as preparation method and application therefore in tumor treatment
InactiveCN103450096BSynthetic steps with high yieldsEasy to makeOrganic active ingredientsOrganic chemistryAlkyl transferTumor therapy
The novel nitrogen mustard derivative is characterized in that: one end has a nitrogen mustard alkylating group; the other end has a 6,7-substituted quinazoline structure, and the substituent R1 is located at the 4-position of the quinazoline parent, which is 2- or 3 - the nitrogen mustard group; the 6 and 7 positions of the quinazoline parent are morpholine propoxy and methoxy. The structure is as formula A. Experiments show that this kind of compound can inhibit cell cycle in G2 / M phase, and is a kind of bifunctional alkylating agent. Anti-tumor activity experiments in vivo show that the compound has good activity. Not only that, this type of compound also has the advantage that nitrogen mustards do not have, namely less toxicity. Simultaneously, the compounds are easy to synthesize, and the total yield is high. Various advantages show that this kind of compound has great potential to be a tumor treatment drug.
Owner:BEIJING NORMAL UNIVERSITY
Diosgenin combined nitrogen mustard derivative with antitumor activity and its preparation method and use
ActiveCN112979744BHigh activityStrong cytotoxicityOrganic active ingredientsSteroidsBenzoic acidParenchyma
The invention belongs to the field of natural medicine and medicinal chemistry, and relates to a class of diosgenin combined nitrogen mustard derivatives and a preparation method and application thereof. It specifically relates to a preparation method for introducing a benzoic mustard derivative into the 3-OH or 26-OH site of the diosgenin core structure and its use in the preparation of antitumor drugs. The diosgenin and nitrogen mustard derivatives of the invention have good antitumor effects and can be used for further preparation of antitumor drugs.
Owner:QIQIHAR MEDICAL UNIVERSITY
Process for making chiral 1,4-disubstituted piperazines
InactiveUS20050228181A1Organic active ingredientsNervous disorderPiperazineNitrogen mustard derivative
A process for a stereoselective preparation of novel chiral nitrogen mustard derivatives useful in synthesizing optically active 1,4-disubstituted piperazines of formula: wherein R, Ar, and Q are defined as set forth herein, and intermediate compounds therefor. The 1,4-disubstituted piperazines act as 5HT1A receptor binding agents useful in the treatment of Central Nervous System (CNS) disorders.
Owner:WYETH
Nitrogen mustard derivative n,n-bis(2-chloroethyl)-n'-hexadecanoyl-1,4-phenylenediamine and its preparation method
InactiveCN106631875BImprove therapeutic indexSmall toxicityAntipyreticOrganic compound preparationSide effectIce water
The invention discloses a structural formula of a nitrogen mustard derivative N,N-bis(2-chloroethyl)-N'-hexadecanoyl-1,4-phenylenediamine; and a preparation method thereof: first preparing N,N ‑bis(2‑chloroethyl)‑1,4‑phenylenediamine; then the reaction starting materials N,N‑bis(2‑chloroethyl)‑1,4‑phenylenediamine, dichloromethane and triethylamine Put it into the reactor, cool it in an ice-water bath, stir, and add dropwise the mixed solution of hexadecanoyl chloride and dichloromethane into the reactor. After the dropwise addition is completed, remove the ice-water bath and react at room temperature for 12-14 hours. , wash the reaction solution, dry, and distill at atmospheric pressure, and purify the distilled filter cake to obtain. The arylamine nitrogen mustard derivative of the present invention can effectively reduce the toxic and side effects of the nitrogen mustard under the premise of enhancing the therapeutic index of the nitrogen mustard, and at the same time have the effects of sterilization and anti-inflammation, so as to reduce the symptoms of the patient due to decreased immunity after chemotherapy. risk of complications.
Owner:CHANGAN UNIV
Preparation method and application of a class of 7-position nitrogen mustard derivatives of brefeldin a
ActiveCN110028479BImprove pharmacological activityOrganic active ingredientsOrganic chemistryPharmaceutical medicinePerylene derivatives
The invention relates to the fields of natural medicine and medicinal chemistry, in particular to a preparation method and application of a 7-position spliced nitrogen mustard derivative of brefeldin A with antitumor activity. The structure of the nitrogen mustard derivative at the 7-position of brefeldin A according to the present invention and its pharmaceutically acceptable salt is shown in the following general formula I, wherein, n is as described in the claims and description.
Owner:SHENYANG PHARMA UNIVERSITY
A kind of samiculin-type kaurane diterpene combined nitrogen mustard derivative and its preparation method and application
The present invention relates to the technical field of medicine, and relates to a kind of sliverin-type kaurane diterpene combined nitrogen mustard derivative and its preparation method and application, in particular to the 14-OH combination nitrogen mustard derivative of sliverin-type kaurane diterpene Its preparation method and its application in the preparation of antitumor drugs. The structures of the samiculin-type kaurane diterpene nitrogen mustard derivatives and their pharmaceutically acceptable salts according to the present invention are shown in the following general formula I or II, wherein n is as described in the claims and description.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
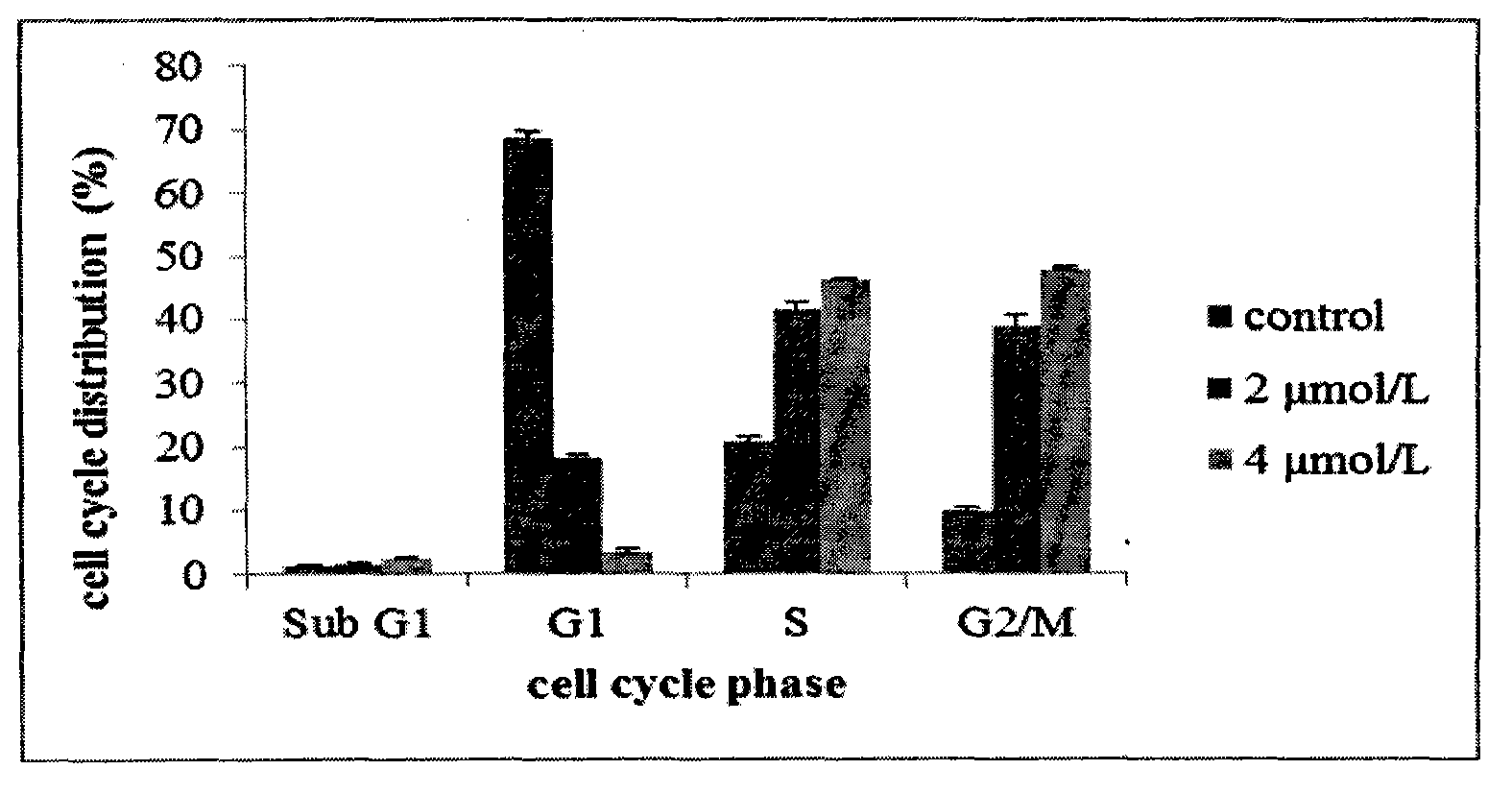
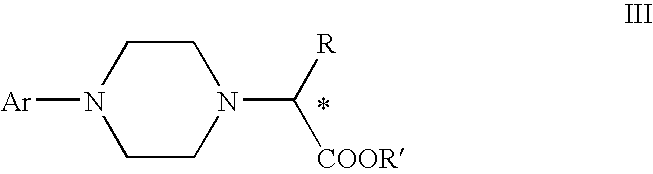

![Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/218652e6-673e-4751-a17d-748a9d98484f/BSA0000098504170000021.PNG)
![Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/218652e6-673e-4751-a17d-748a9d98484f/BSA0000098504170000022.PNG)
![Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy Pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, preparation methods of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives, and application of pyrazolo[1,5-alpha] pyrimidine nitrogen mustard derivatives in oncotherapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/218652e6-673e-4751-a17d-748a9d98484f/BSA0000098504170000023.PNG)
![Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df9612a-30ab-4b95-b74c-4bbce1e25936/BSA0000098504170000021.png)
![Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df9612a-30ab-4b95-b74c-4bbce1e25936/BSA0000098504170000022.png)
![Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application Pyrazolo[1,5‑a] pyrimidine mustard derivatives, preparation method and tumor treatment application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0df9612a-30ab-4b95-b74c-4bbce1e25936/BSA0000098504170000023.png)