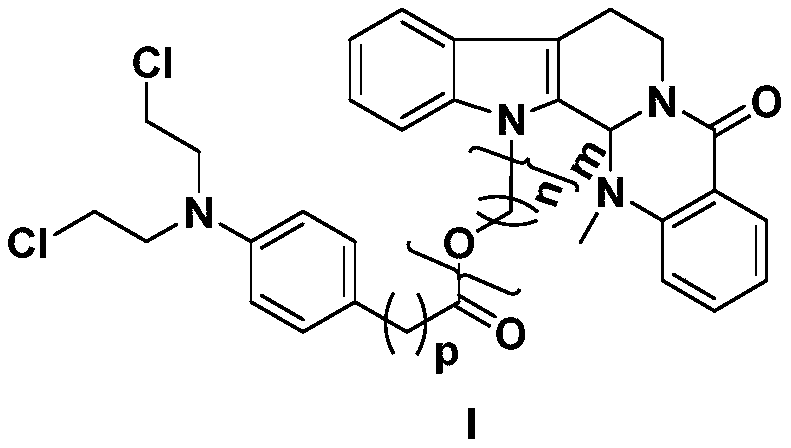
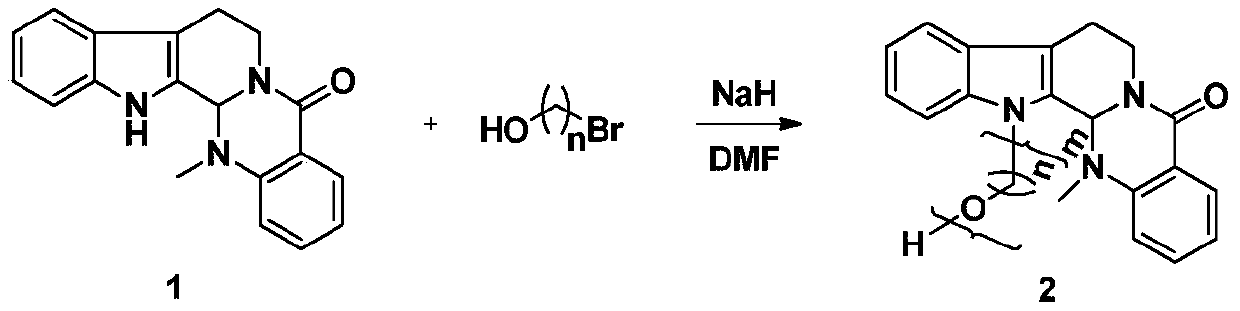
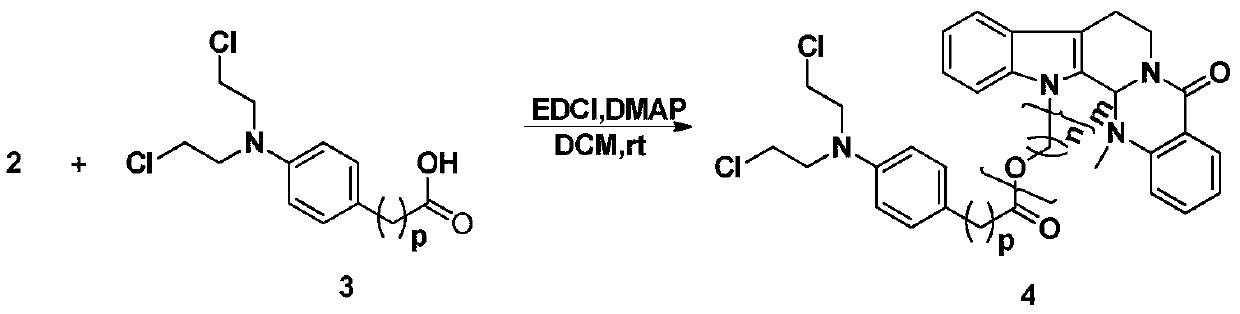
Preparation method and application of a class of evodiamine and nitrogen mustard derivatives with antitumor activity
A technology of evodiamine and evodiamine hydroxyl, which is applied in the field of natural medicine and medicinal chemistry, and can solve the problems of lack of specificity in cell action, large toxic and side effects, and unsatisfactory therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Take evodiamine intermediate 2 (n is 2, m is 1), 65mg, 0.19mmol, dissolve in dichloromethane (15ml), add chlorambucil (60mg, 0.19mmol), EDCI (108mg, 0.56 mmol), DMAP (7mg, 0.06mmol), the reaction was stirred at room temperature, the reaction progress was monitored by TCL, and the reaction was terminated after 24h. The reaction solution was poured into 20ml of ice-water mixture, extracted with dichloromethane (30ml×3), washed with saturated saline solution, dried over anhydrous sodium sulfate, recovered dichloromethane to obtain crude product 8a, passed through a silica gel column (petroleum ether: acetic acid Ethyl ester=5:1), separated to obtain a yellow oil with a yield of 66%. HR-MS(ESI,M+H)m / z calcdfor C 35 h 38 Cl 2 N 4 o 3 H:633.2394,found:633.2385. 1 H NMR (CDCl 3 ,400MHz), δ(ppm)8.15(1H,dd,J=7.8,1.4Hz,Ar-H),7.60(1H,d,J=7.9Hz,Ar-H),7.50(1H,td,J= 7.9,1.43Hz,Ar-H),7.43(1H,d,J=7.9Hz,Ar-H),7.30(1H,d,J=7.9Hz,Ar-H),7.27(1H,m,Ar-H H),7.23(1H,m,Ar-H)...
Embodiment 2
[0022]
[0023] Compound 8b was prepared according to the synthesis method of Example 1. Yellow oil, 80% yield. HR-MS(ESI,M+H)m / z: calcd for C 36 h 40 Cl 2 N 4 o 3 H:647.2550,found:647.2572. 1 H NMR (CDCl 3 ,400MHz),δ(ppm)8.14(1H,dd,J=7.8,1.0Hz,Ar-H),7.60(1H,d,J=7.8Hz,Ar-H),7.48(1H,m,Ar-H H),7.37(1H,d,J=8.2Hz,Ar-H),7.28(1H,m,Ar-H),7.24(1H,m,Ar-H),7.19(1H,m,Ar-H ),7.16(1H,m,Ar-H),7.02(2H,d,J=8.5Hz,Ar-H),6.62(2H,d,J=8.5Hz,Ar-H),5.96(1H,s ,NCH),4.02-4.53(4H,m,-CH 2 ),3.69(4H,m,NCH 2 CH 2 Cl),3.61(4H,m,NCH 2 CH 2 Cl),2.45-3.18(6H,m,-CH 2 ),2.39(3H,s,NCH 3 ),1.79-2.18(6H,m,-CH 2 ); 13 C NMR (CDCl 3 ,100MHz)δ(ppm)173.42,164.62,150.02,144.37,137.25,133.01,130.77,129.78(×2),129.14,128.45,125.97,124.49,124.34,123.26,122.92,119.90,119.27,113.53,112.48(× 2),109.63,68.09,61.64,53.80(×2),40.58(×2),39.39,36.54,34.07,33.56,29.82,29.33,26.69,20.46.
Embodiment 3
[0025]
[0026] Compound 8c was prepared according to the synthesis method of Example 1. Yellow oil, yield 34%. HR-MS(ESI,M+H)m / z: calcd for C 39 h 46 Cl 2 N 4 o 4 H:705.2969,found:705.2986. 1 H NMR (CDCl 3,400MHz), δ(ppm)8.12(1H,dd,J=7.8,1.0Hz,Ar-H),7.60(1H,d,J=7.8Hz,Ar-H),7.47(1H,m,Ar-H H),7.42(1H,d,J=8.2Hz,Ar-H),7.29(1H,m,Ar-H),7.22(1H,m,Ar-H),7.19(1H,m,Ar-H ),7.16(1H,m,Ar-H),7.07(2H,d,J=8.5Hz,Ar-H),6.64(2H,d,J=8.5Hz,Ar-H),5.99(1H,s ,NCH),3.99-4.90(6H,m,-CH 2 ),3.69(4H,m,NCH 2 CH 2 Cl),3.61(4H,m,NCH 2 CH 2 Cl),2.45-3.18(8H,m,-CH 2 ),2.40(3H,s,NCH 3 ),1.74-2.31(8H,m,-CH 2 ); 13 C NMR (CDCl 3 ,100MHz)δ(ppm)173.62,164.72,151.07,144.25,137.40,132.97,131.05,129.85(×2),129.04,128.67,125.84,124.23,124.19,123.16,122.72,119.71,119.09,113.22,112.53(× 2),109.93,68.00,67.69,67.44,61.52,53.85(×2),40.72,40.53(×2),39.44,36.44,34.15,33.76,30.34,29.12,26.90,20.52
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com