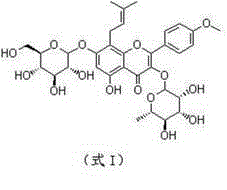
Method for obtaining anhydroicaritin from icariin by adopting naringinase
A technology of icariin and icariin, which is applied in the field of obtaining icariin by naringinase reaction, can solve the problems of low aglycone content and low yield, and achieve the improvement of pharmacological activity, Improve the conversion rate and reduce the effect of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
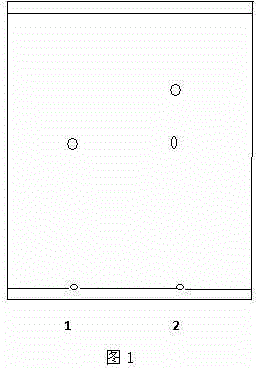
[0021] Example 1: Into a 1000mL conical flask, add 10mg of icariin standard substance and 400ml of 30% ethanol aqueous solution, adjust the pH value to 4.0 with 1M NaOH solution, and the system reaches a certain temperature of 50°C, then add 0.5g of naringinase (standard activity 475AGUPg), and finally, at 50°C and 200 rpm, the reaction was stirred for 30 hours. After the reaction, extract with the same volume of ethyl acetate to remove sugar and enzyme protein impurities. The extract was developed on a G silica gel plate (100mm×25mm), chloroform:methanol=8:2 as the mobile phase, and was developed under 245nm ultraviolet light. Observed below and carried out qualitative analysis with TLC, the results are as follows figure 1 shown in (1.R f淫羊藿苷标准品 =0.50,
[0022] 2. R f反应产物 =0.8), the reaction product R f The value 0.8 is much larger than the standard R f A value of 0.5 shows that the polarity of icariin less than icariin has been produced by the enzymatic reaction, and th...
example 2
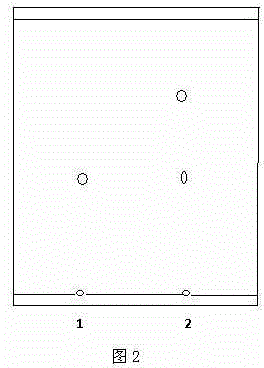
[0025] Example 2: In a 1000ml Erlenmeyer flask, add 10mg of icariin standard substance and 400ml of 40% ethanol aqueous solution, adjust the pH value to 4.0 with 1M NaOH solution, and the system reaches a certain temperature of 50°C, then add 0.5g of naringinase (standard activity 475AGUPg), and finally, at 50°C and 200 rpm, the reaction was stirred for 30 hours. After the reaction, extract with the same volume of ethyl acetate to remove sugar and enzyme protein impurities. The extract is developed on a G silica gel plate (100mm×25mm), chloroform:methanol:water=7.5:2.5:0.25 as the mobile phase, Observe under 245nm ultraviolet light and carry out qualitative analysis with TLC, the result is as follows figure 2 shown (R f淫羊藿苷标准品 =0.42, 2.R f反应产物 ==0.8) Reaction product R f The value of 0.8 is much larger than the standard R f A value of 0.42 indicates that icariin, which is less polar than icariin, is produced by the enzymatic reaction, and the reaction mechanism is the sam...
example 3
[0026] Example 3: In a 1000ml Erlenmeyer flask, add 10mg of icariin standard substance and 400ml of 30% ethanol aqueous solution, adjust the pH value to 6.0 with 1M NaOH solution, and the system reaches a certain temperature of 60°C, then add 0.5g of naringinase (standard activity 475AGUPg), and finally, at 60°C and 200 rpm, the reaction was stirred for 30 hours. After the reaction, extract with the same volume of ethyl acetate to remove sugar and enzyme protein impurities. The extract is developed on a G silica gel plate (100mm×25mm), chloroform:methanol:water=7.5:2.5:0.25 as the mobile phase, Observe under 245nm ultraviolet light and carry out qualitative analysis with TLC, the result is as follows figure 2 shown (R f淫羊藿苷标准品 =0.42, 2.R f反应产物 = 0.8) Reaction product R f The value of 0.8 is much larger than the standard R f A value of 0.42 indicates that icariin, which is less polar than icariin, is produced by the enzymatic reaction, and the reaction mechanism is the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com