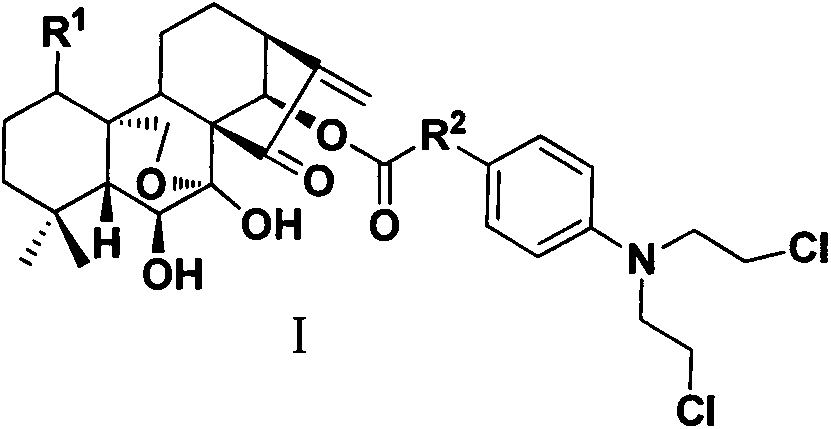
Oridonin A 14-o-substituted nitrogen mustard derivative, preparation method and use
A technology of Rubescensine A and its derivatives, which can be applied in pharmaceutical formulations, drug combinations, and medical preparations containing active ingredients, etc., can solve problems such as large toxic and side effects, low antitumor activity, and drug resistance failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
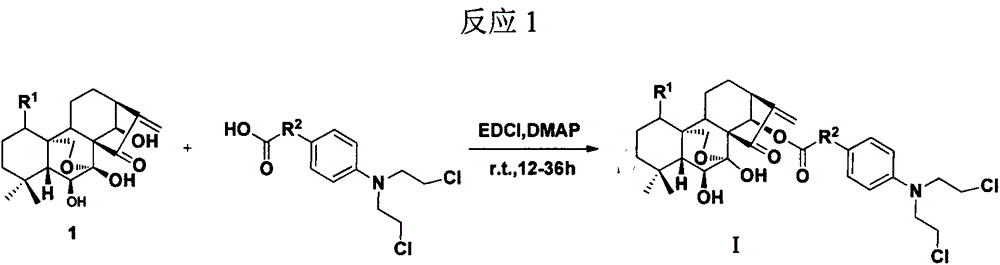
[0081] Dissolve oridonin (0.2mmol) in 15ml of dichloromethane, add benzoic acid mustard (0.22mmol), stir at room temperature for 18 hours, add appropriate amount of water (about 15ml), extract with ethyl acetate (10ml×3 times ), washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and subjected to column chromatography (dichloromethane:methanol=300:1) to obtain a white powdery solid.
[0082] ent-1,6,7-trihydroxy-(14-O-(4-(bis(2-chloroethyl)amino)benzoyl))-15-oxo-7,20-oxo-16- Kaurene. 1 H NMR (CDCl 3 , 300MHz): δ (ppm) 7.78 (d, J = 9.0Hz, 2H, Ar-H), 6.62 (d, J = 9.0Hz, 2H, Ar-H), 6.17 (m, 2H), 6.03 (s , 1H), 5.47(s, 1H), 4.30(s, 1H), 4.08, 4.33(dd, J A =J B =10.2Hz, each1H), 3.78(m, 4H), 3.65(m, 4H), 3.57(m, 1H), 3.28(d, J=9.6Hz, 1H), 2.67(m, 1H), 2.39(m , 1H), 2.04(m, 1H), 1.78(m, 2H), 1.68(m, 3H), 1.49(m, 2H), 1.36(m, 2H), 1.13(s, 3H), 1.12(s, 3H); ESI-MS m / z608.4[M+H] + .
Embodiment 2
[0084]
[0085] ent-1,6,7-trihydroxy-(14-O-(4-(bis(2-chloroethyl)amino)phenylacetyl))-15-oxo-7,20-oxo-16- Kaurene. Referring to the synthetic method of Example 1. 1 H NMR (CDCl 3 , 300MHz): δ (ppm) 7.78 (d, J = 9.0Hz, 2H, Ar-H), 6.62 (d, J = 9.0Hz, 2H, Ar-H), 6.17 (m, 2H), 6.03 (s , 1H), 5.47(s, 1H), 4.30(s, 1H), 4.08, 4.33(dd, J A =J B =10.2Hz, each1H), 4.12(s, 2H), 3.78(m, 4H), 3.65(m, 4H), 3.57(m, 1H), 3.28(d, J=9.6Hz, 1H), 2.67(m , 1H), 2.39(m, 1H), 2.04(m, 3II), 1.78(m, 2H), 1.68(m, 3H), 1.49(m, 2H), 1.36(m, 2H), 1.13(s, 3H), 1.12(s, 3H); ESI-MS m / z622.2[M+H] + .
Embodiment 3
[0087]
[0088] ent-1,6,7-trihydroxy-(14-O-(4-(bis(2-chloroethyl)amino)phenylbutyryl))-15-oxo-7,20-oxo-16- Kaurene. Referring to the synthetic method of Example 1. 1 H NMR (CDCl 3 , 300M Hz), δ(ppm): 6.92(d, J=8.4Hz, 2H, Ar-H), 6.53(d, J=8.4Hz, 2H, Ar-H), 6.07(s, 1H), 6.06 (d, J=6.0Hz, 1H), 5.75(s, 1H), 5.41(s, 1H), 4.25(s, 1H), 4.23, 4.01(dd, J A =J B =10.2Hz, each1H, 20-CH 2 ), 3.69(m, 1H), 3.63(m, 4H), 3.55(m, 4H), 3.43(m, 1H), 3.12(d, J=9.6 Hz, 1H), 2.65(m, 1H), 2.43 (t, J=7.2Hz, 2H), 2.20(t, J=7.2Hz, 2H), 1.91(m, 1H), 1.81(m, 2H), 1.73(m, 2H), 1.70(m, 2H) , 1.65(m, 3H), 1.37(m, 2H), 1.18(s, 3H, -CH 3 ), 1.05(s, 3H, -CH 3 ); ESI-MS m / z650.5[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com