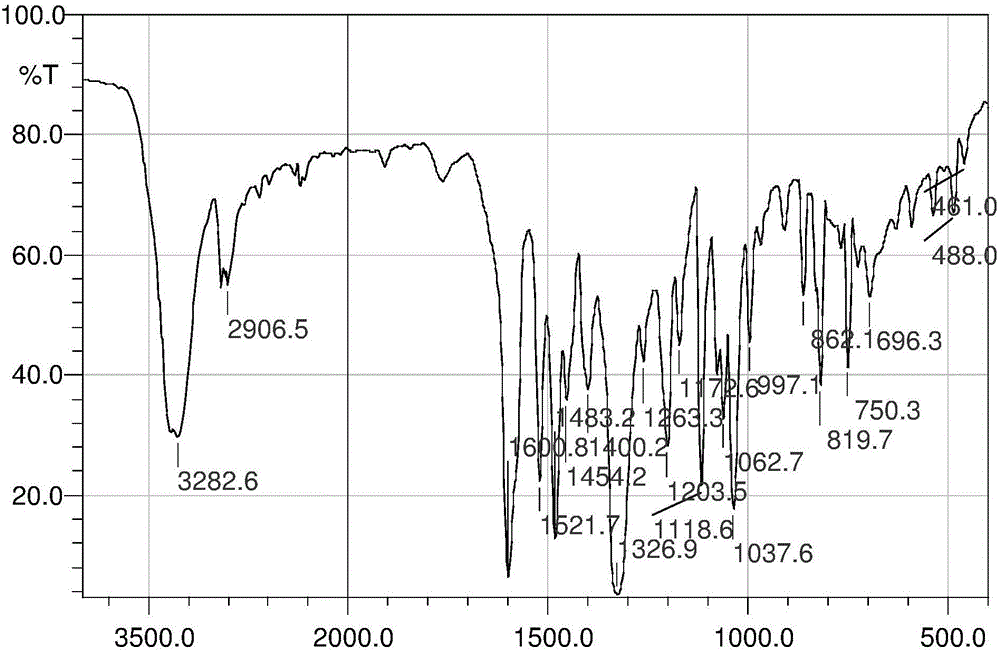
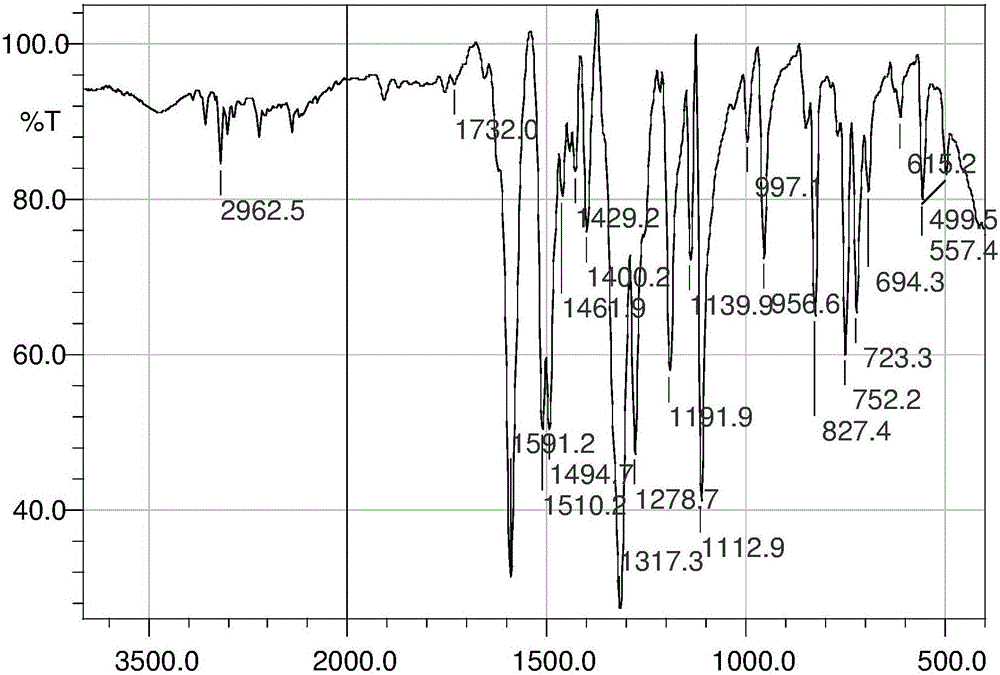
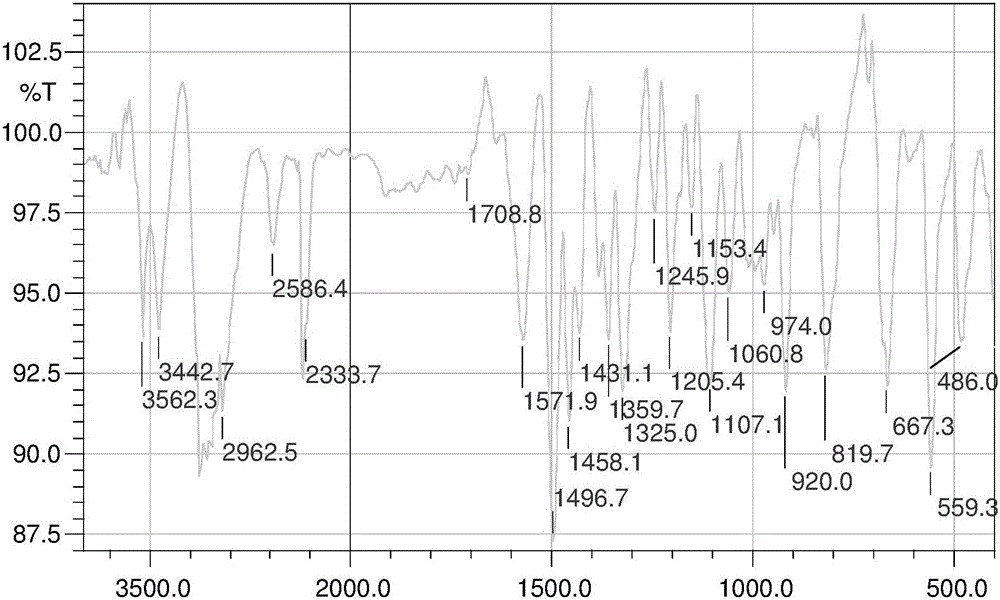
Aryl nitrogen mustard derivative N,N-di(2-chloroethyl)-N'-propionyl-1,4-phenylenediamine and preparation method thereof
A technology of arylamine nitrogen mustard and chloroethyl, which is applied in the preparation of carboxylic acid amide, amino compound, organic compound, etc., can solve the problems of reducing the body's defense function, blocking and killing, and achieve the goal of reducing complications risk, enhanced therapeutic index, and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the above-mentioned arylamine nitrogen mustard derivative N,N-bis(2-chloroethyl)-N'-propionyl-1,4-phenylenediamine comprises the following steps:
[0040] Step 1, preparing N,N-bis(2-chloroethyl)-1,4-phenylenediamine;
[0041] Substep 1a, first prepare N,N-di(2-hydroxyethyl)-4-nitroaniline according to the following steps: 4-chloronitrobenzene, diethanolamine, potassium carbonate and 1% CuSO 4Add the solution into the reactor one by one, stir evenly, when the temperature rises to 82°C, add toluene into the reactor, continue to heat up to 115-120°C, keep stirring at a constant temperature for 6-9 hours, after the reaction is complete, remove the toluene by distillation, distill The final mother liquor was poured into hot water at 40°C-60°C, stirred, and after standing overnight, the water layer was suction filtered to obtain the crude product, which was recrystallized with absolute ethanol to obtain yellow needle-shaped crystals N,N-di( 2-Hydro...
Embodiment 1
[0046] 1.1 Synthesis of N,N-bis(2-hydroxyethyl)-4-nitroaniline
[0047]
[0048] Add 12.64g (0.080mol) of 4-chloronitrobenzene, 10.99g (0.104mol) of diethanolamine and 7.18g (0.052mol) of potassium carbonate in a 250mL three-necked flask, and finally add 1.2mL of CuSO with a mass percentage concentration of 1%. 4 well mixed. Heat to raise the temperature. When the temperature reaches 82°C, add 5.0mL of toluene to the reaction liquid, continue to raise the temperature to control the reaction temperature to 117°C, use thin-layer chromatography to track the reaction progress, and stir at constant temperature for 6h. After the reaction is complete, toluene is removed by distillation, and the mother liquor after distillation is poured into hot water at 60°C, stirred vigorously, left to stand overnight, and then filtered to remove the water layer to obtain the crude product, which is recrystallized with absolute ethanol to obtain yellow needle-shaped crystals N,N-bis(2-hydroxyet...
Embodiment 2
[0059] 1.1 Synthesis of N,N-bis(2-hydroxyethyl)-4-nitroaniline
[0060]
[0061] Add 4-chloronitrobenzene, diethanolamine and potassium carbonate at a molar ratio of 1:1.2:0.6 to a 250mL three-necked flask, and finally add CuSO with a mass concentration of 1%. 4 solution, and the mass concentration is 1% CuSO 4 The dosage ratio of the solution to 4-chloronitrobenzene is 0.5mL:3g, mix well; heat up the temperature, when the temperature reaches 82°C, add toluene to the reaction solution, and the dosage of toluene and 4-chloronitrobenzene The ratio was 1mL:2g; continue to increase the temperature to control the reaction temperature to 120°C, use thin-layer chromatography to track the reaction progress, and stir the reaction at constant temperature for 9h. After the reaction is complete, toluene is removed by distillation, and the mother liquor after distillation is poured into hot water at 60°C, stirred vigorously, left to stand overnight, and the water layer is removed by su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com