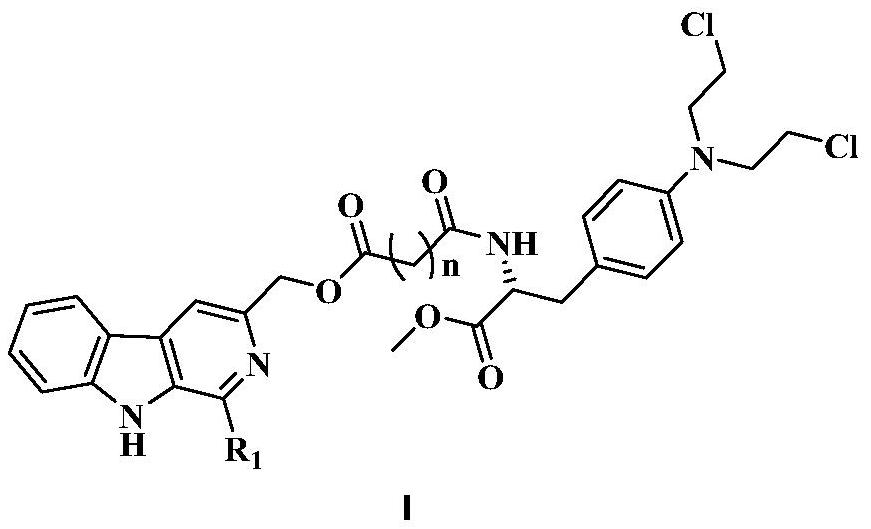
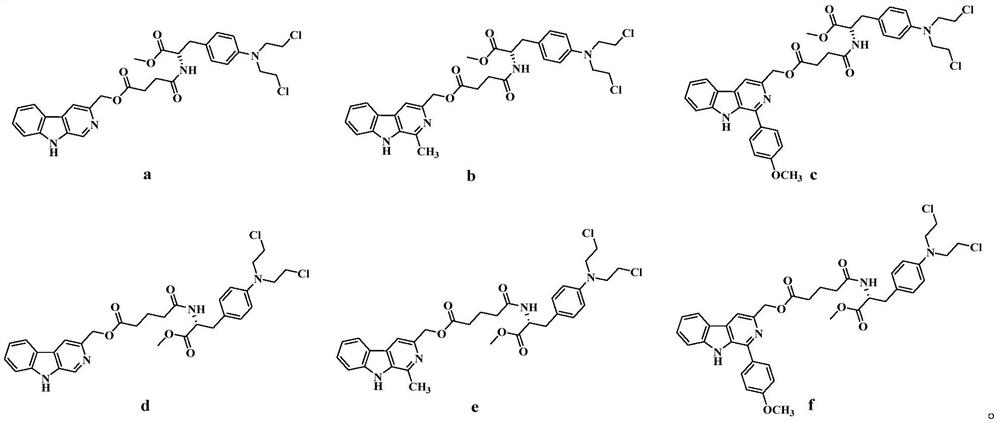
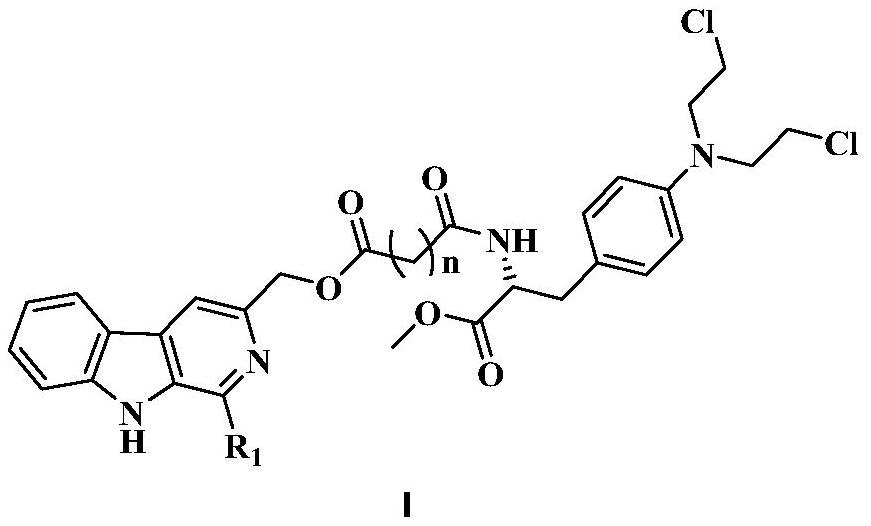
3-position derivatives of beta-carboline as well as preparation method and application of 3-position derivatives
A technology of carbolin and its derivatives, which is applied in the field of natural medicine and medicinal chemistry, can solve the problems of side effects and drug resistance, and achieve the effect of anti-tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] (1) Dissolve 5 g of compound L-tryptophan 1 (24.51 mmol) in 60 mL of 0.4 N NaOH solution, then add 3 mL of 37% formaldehyde solution (36.97 mmol), and react at 37° C. for three days. TLC monitors, reaction is substantially complete, cools down, then adds glacial acetic acid, has precipitation generation, suction filtration, drying, obtains intermediate 2a (R 1 =-H) 4.64 g. Dissolve 4.64g of intermediate 2a (21.48mmol) in 50mL of anhydrous methanol, and add 3.68mL of SOCl dropwise under ice-bath conditions 2 (50.67mmol), and then reflux at 65°C for 6h. Monitored by TLC, the reaction was complete, cooled, concentrated the reaction liquid, then added 50mL saturated sodium bicarbonate solution, extracted 3 times with ethyl acetate, washed 1 time with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain the intermediate 3a3.5g. 3.5g of intermediate 3a (15.22mmol) was dissolved in 65mL of DMF, 7g of potassium permanganate (...
Embodiment 2
[0034]
[0035] The step of synthesizing 2a in step (1) of Example 1 is replaced by: dissolving 5g compound L-tryptophan 1 (24.51mmol) in 80mL 1,2-dichloroethane, then adding 1.52mL acetaldehyde (26.96mmol ), 3.64mL trifluoroacetic acid (49.02mmol), reflux at 110°C for 30min. TLC monitoring showed that the reaction was almost complete, cooled, then added saturated sodium bicarbonate solution and saturated brine to wash the reaction solution once, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain intermediate 2b (R 1 =-CH 3 ).
[0036] The remaining steps were prepared according to the synthetic method of Example 1 to obtain compound 9b, an orange solid, with a yield of 13%. 1 HNMR (400MHz, CDCl 3 )δ:8.40(s,1H),8.11(d,J=7.80Hz,1H),7.89(s,1H),7.54(m,2H),7.29(m,1H),6.94(d,J=8.72 Hz,2H),6.55(d,J=8.76Hz,2H),6.10(d,J=7.76Hz,1H),5.38(s,2H),4.82(m,1H),3.71(s,3H), 3.67(m,4H),3.59(m,4H),2.99(m,2H),2.82(s,3H),2.76(m,2H),2.55(m,2H); 13 C NMR (100MHz, CDCl ...
Embodiment 3
[0038]
[0039] The step of synthesizing 2a in step (1) of Example 1 was replaced by: dissolving 5 g of compound L-tryptophan 1 (24.51 mmol) in 80 mL of 1,2-dichloroethane, and then adding 3.27 mL of p-methoxybenzene Formaldehyde (26.96mmol), 3.64mL trifluoroacetic acid (49.02mmol), reflux at 110°C for 30min. TLC monitoring showed that the reaction was almost complete, cooled, then added saturated sodium bicarbonate solution and saturated brine to wash the reaction solution once, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain intermediate 2c (R 1 =p-CH 3 OPh-).
[0040] The remaining steps were prepared according to the synthetic method of Example 1 to obtain compound 9c, a yellow-white solid, with a yield of 8%. 1 H NMR (400MHz, CDCl 3 )δ: 8.69(s, 1H), 8.15(d, J=7.92Hz, 1H), 7.97(s, 1H), 7.87(d, J=8.76Hz, 2H), 7.53(m, 1H), 7.48( d,J=8.08Hz,1H),7.29(m,1H),7.06(d,J=8.76Hz,2H),6.92(d,J=8.68Hz,2H),6.53(d,J=8.76Hz, 2H), 6.10(d, J=7.76Hz, 1H), 5.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com