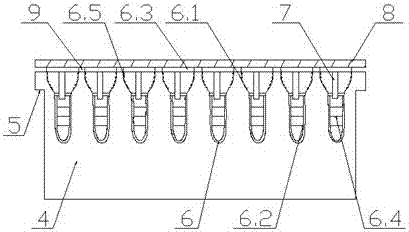
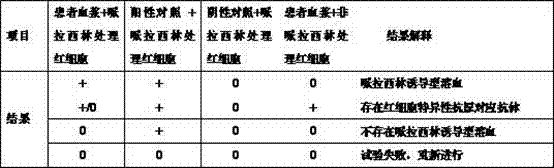
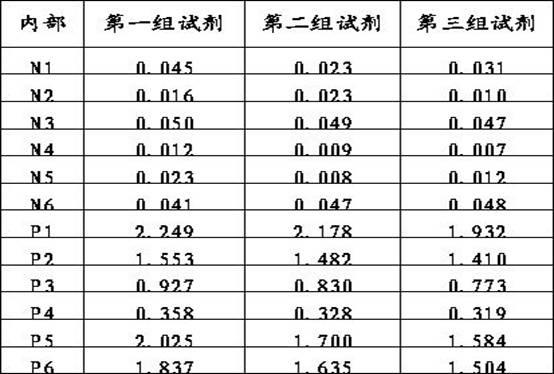
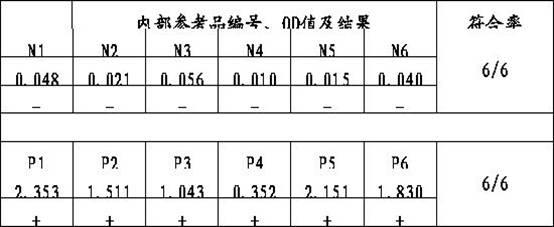
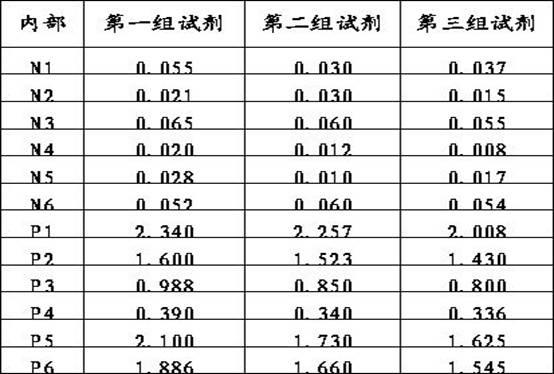
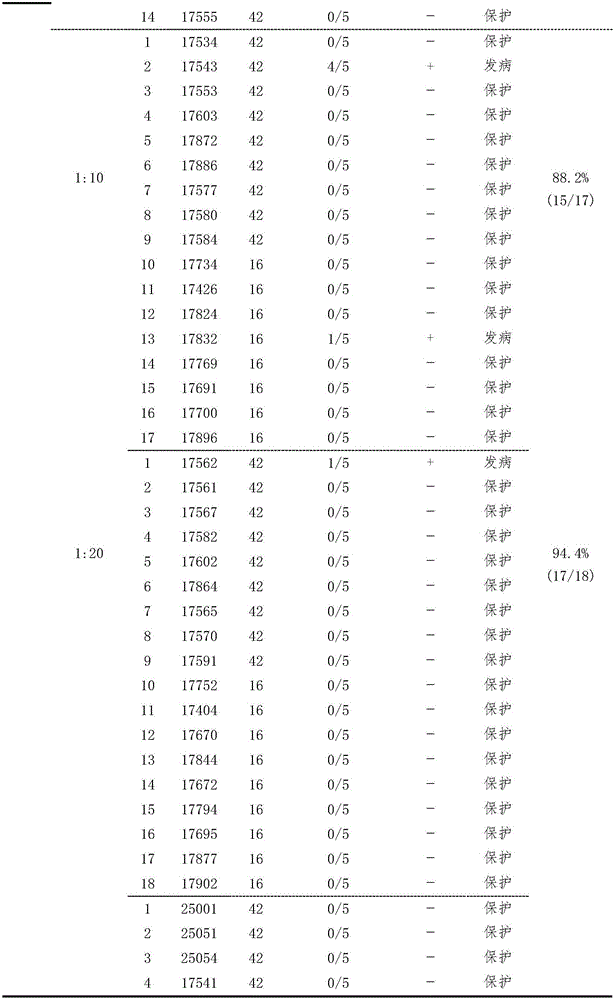
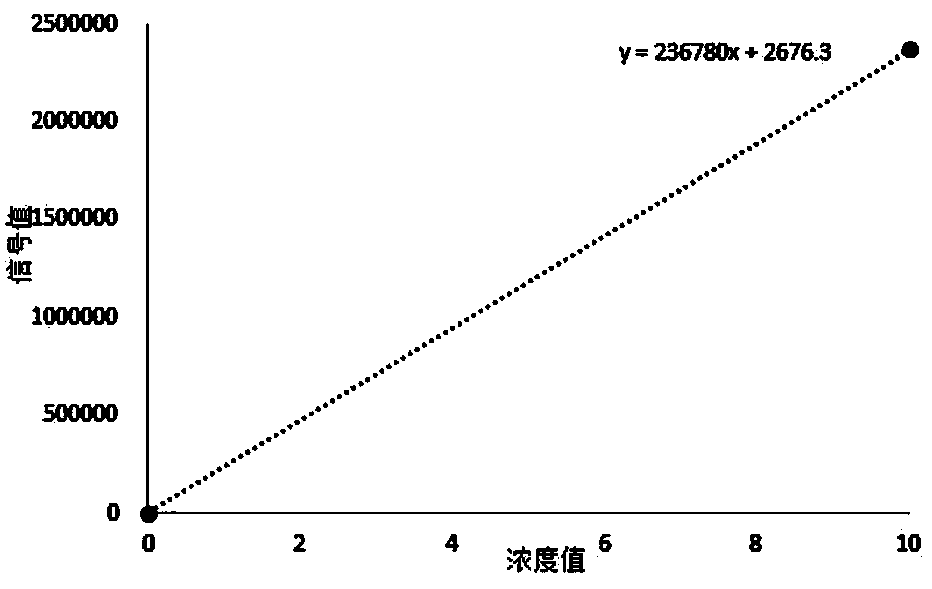
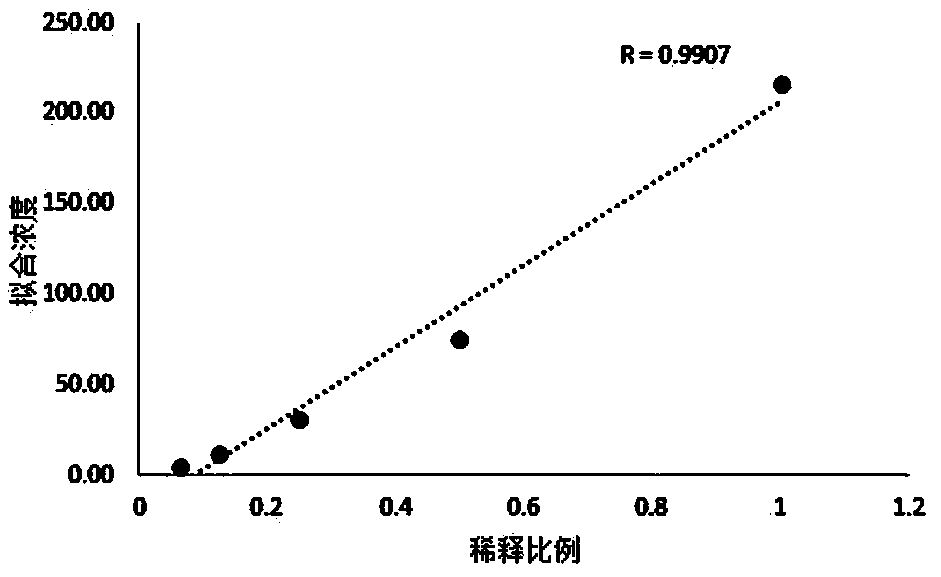
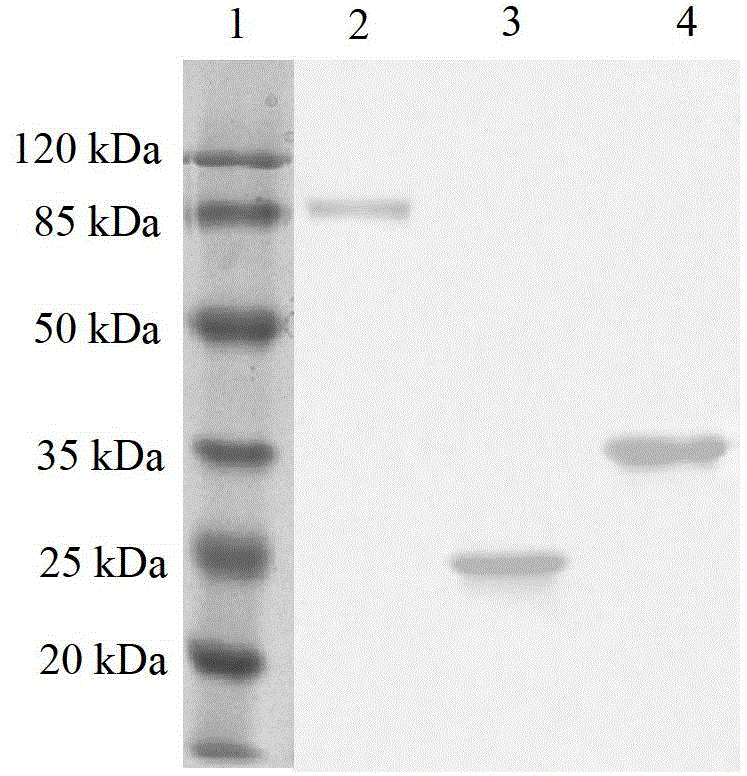
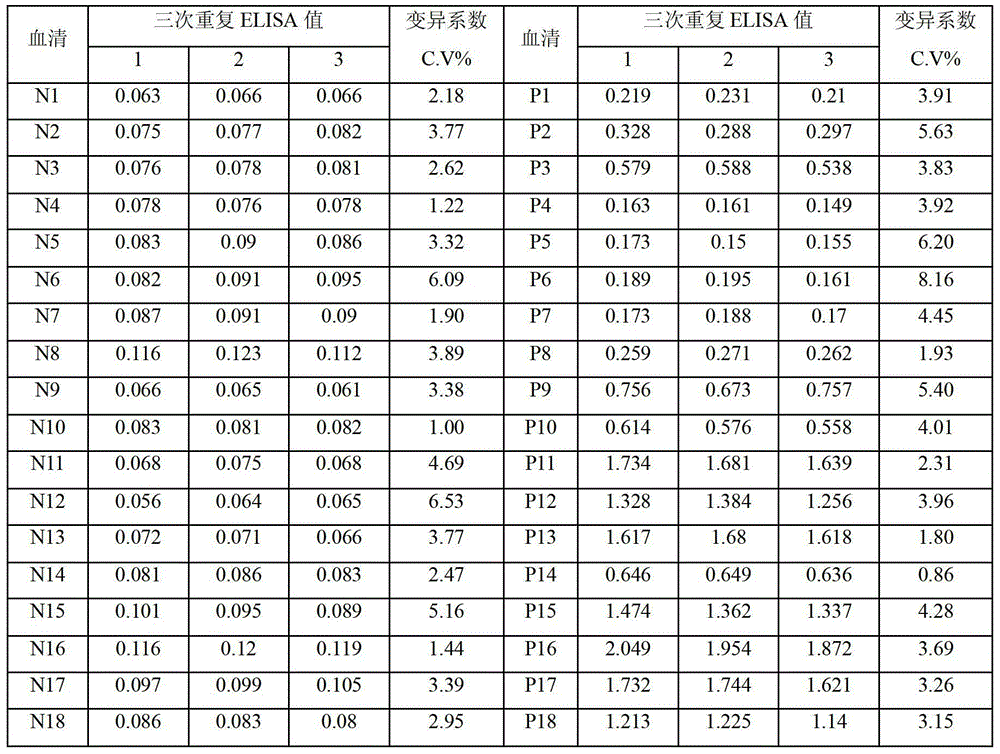
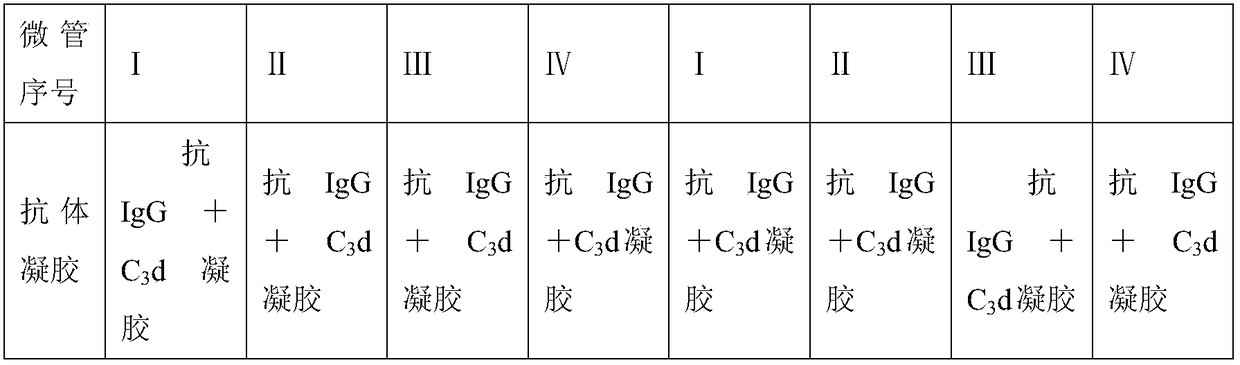
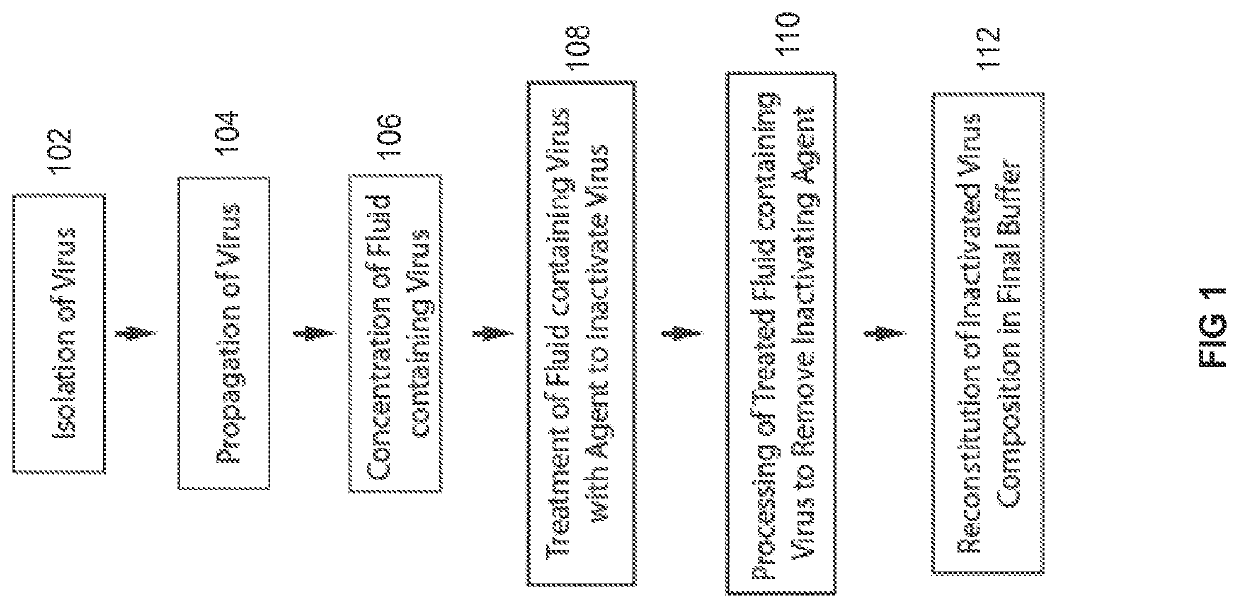
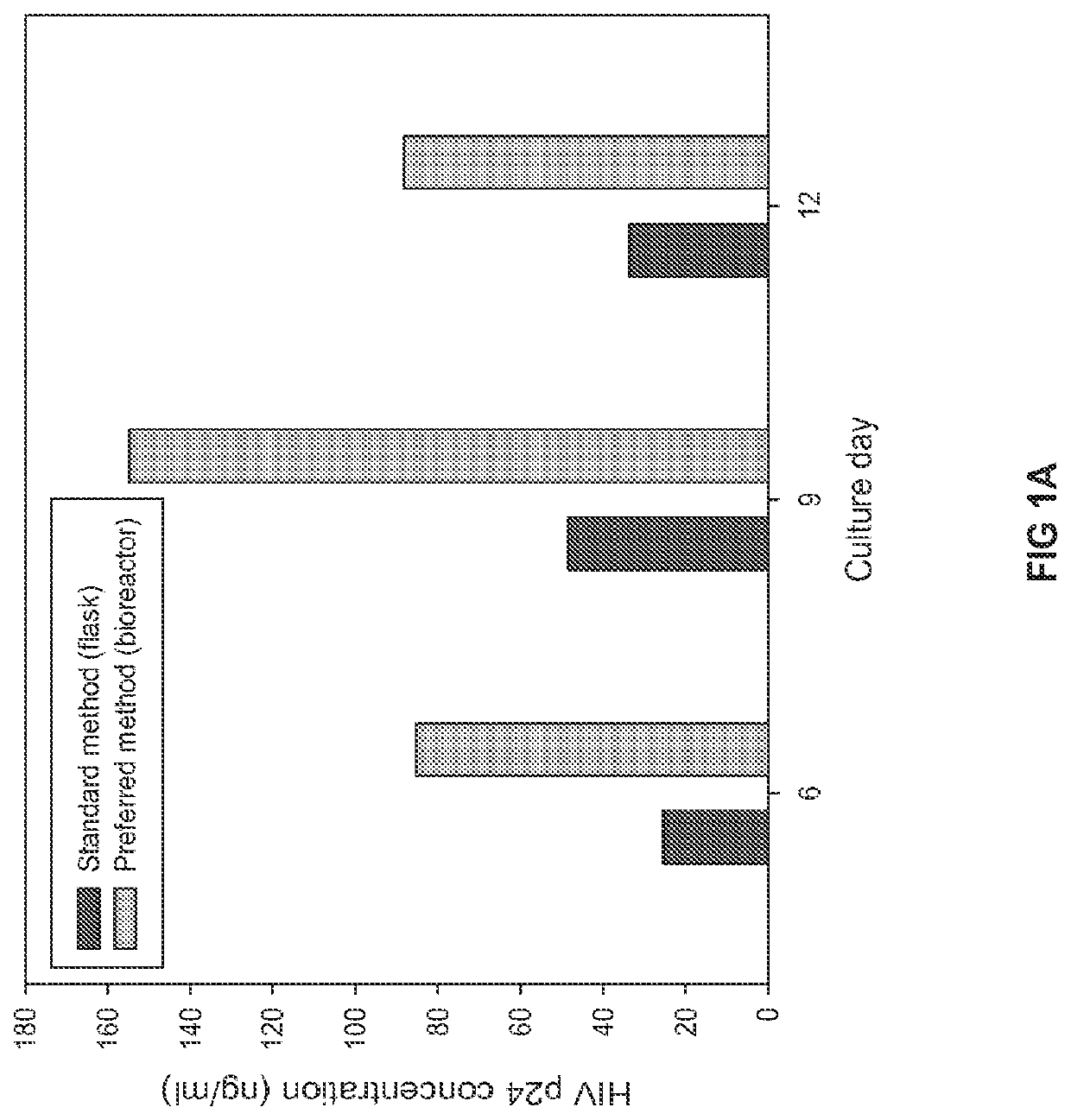
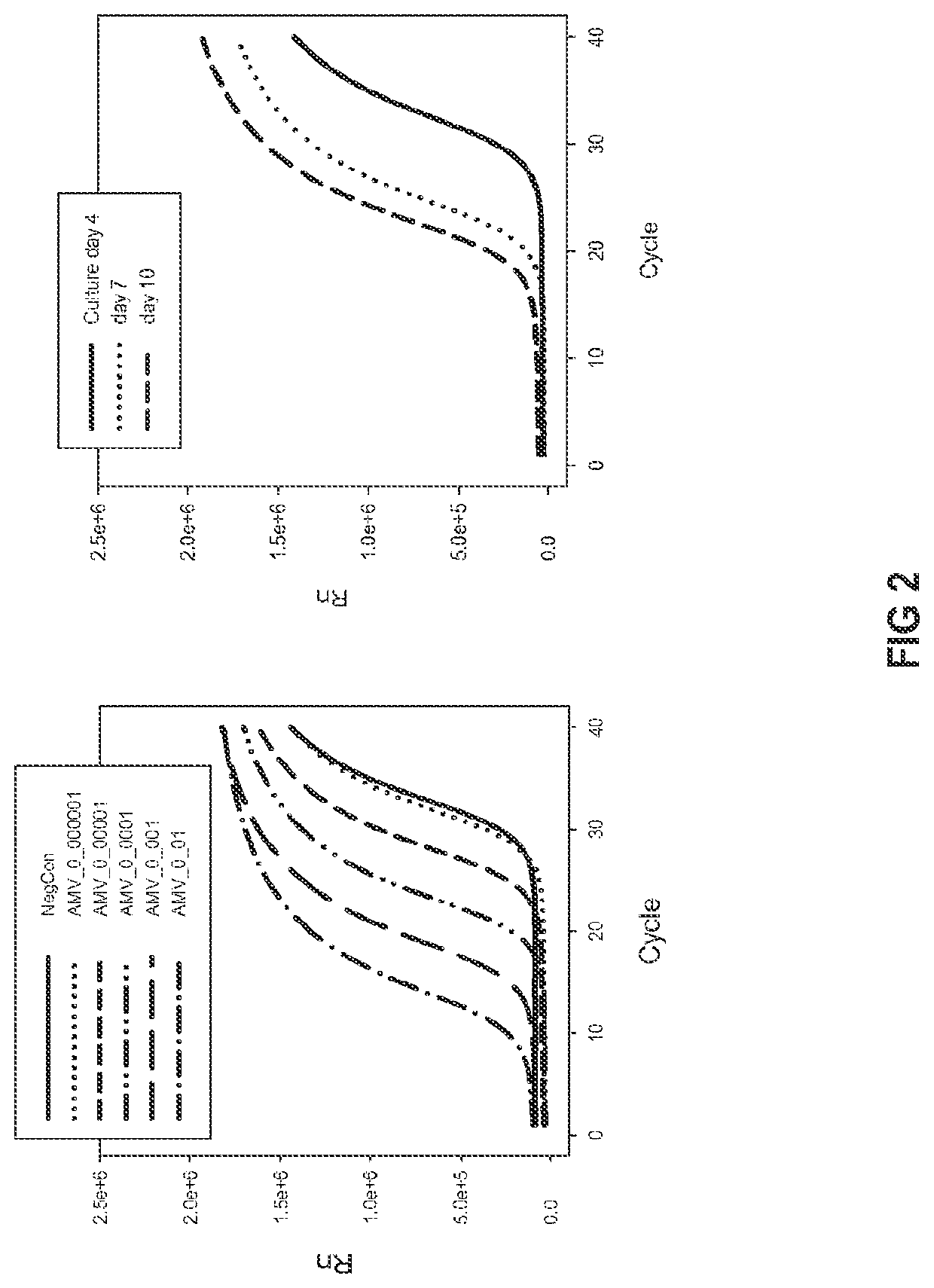
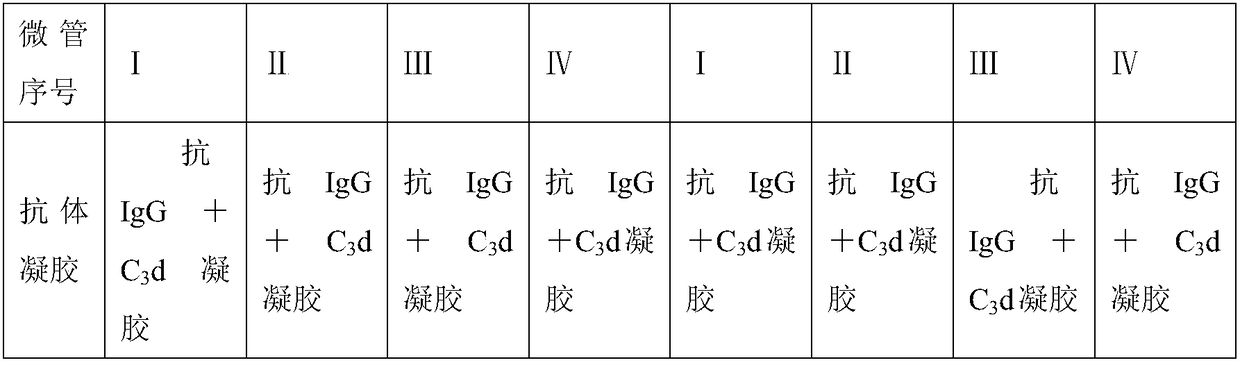
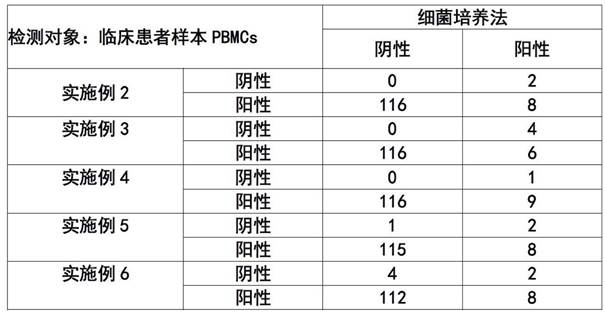
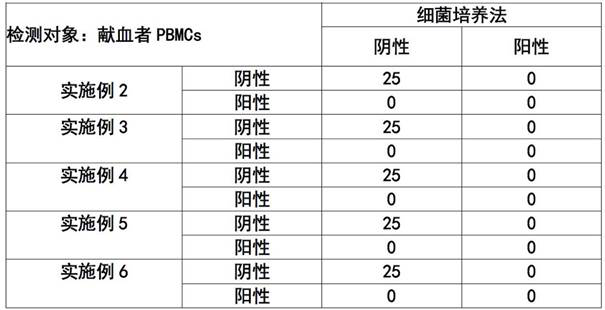
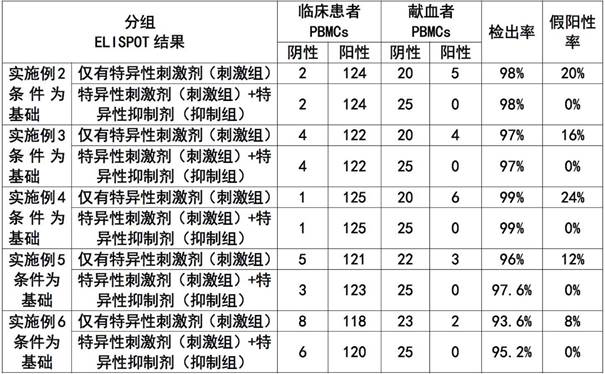
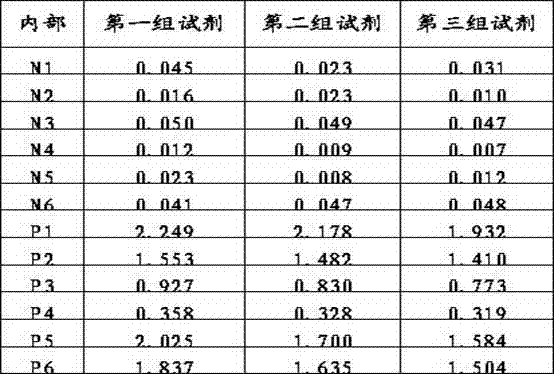
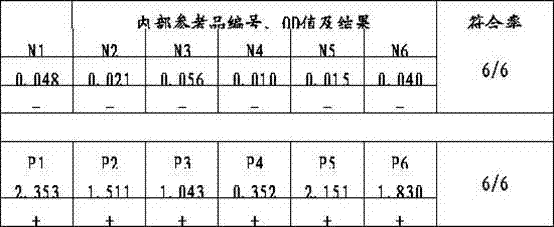
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Antibody negative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
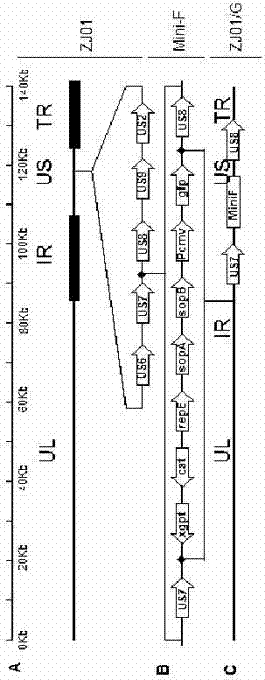
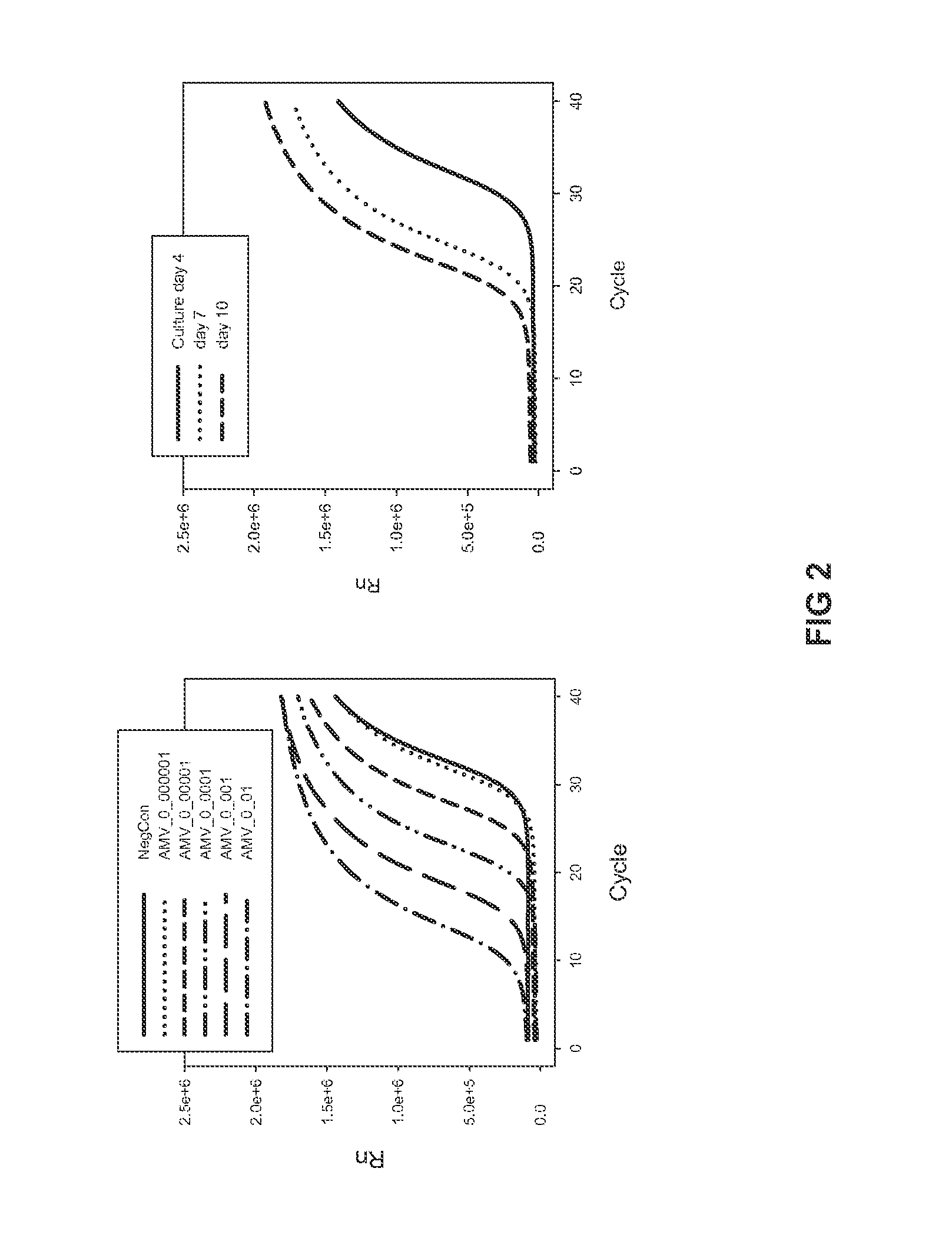
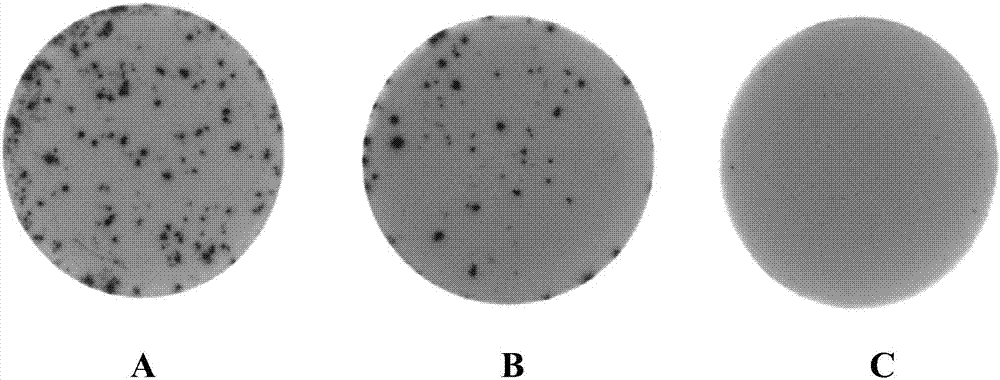
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
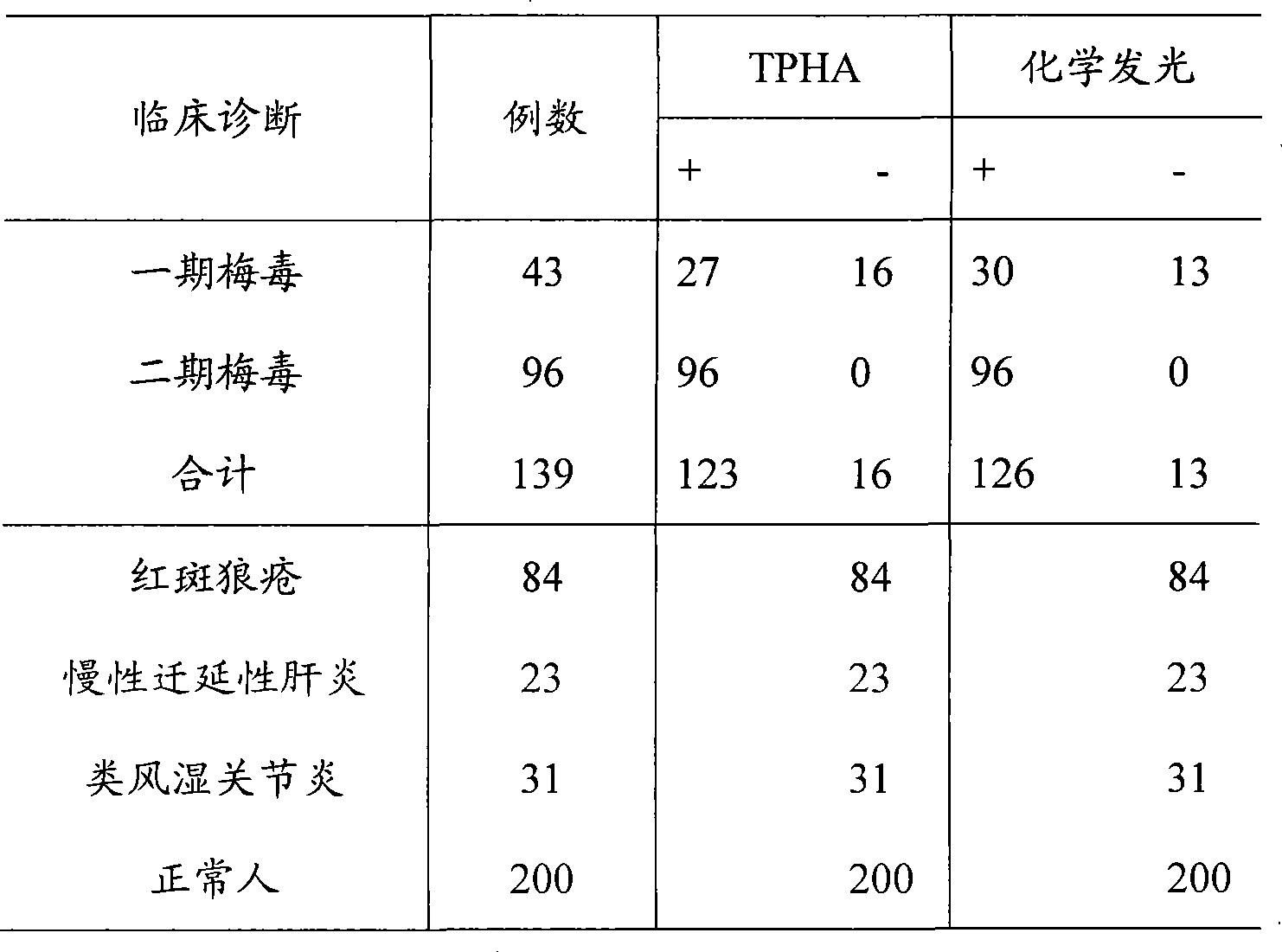
Syphilis helicoid antibody chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363860AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescencePositive controlNon toxicity
The invention discloses a kit of chemiluminescent immunological analysis measurement of a Treponema pallidum antibody, and preparation method thereof, which belongs to the technical field of immunological analysis medical diagnosis. The kit comprises (1) a carrier coated with a treponema pallidum specific recombination protein antigen; (2) treponema pallidum antibody negative and positive reference substances; (3) an enzyme labeled treponema pallidum specific recombination protein antigen; and (4) an enzyme acted chemiluminescent primer. Furthermore, the preparation method of the kit based on the invention comprises the following steps of (1) preparing TP antibody negative and positive reference substances; (2) labeling the TP specific recombination protein antigen with an enzyme; (3) coating with the carrier; (4) sub-packaging; and (5) assembling. The kit of the detection of the Treponema pallidum antibody has the advantages of easy and simple operation, easy popularization, high sensitivity, strong specificity, good repeatability, safety, non toxicity, and no pollution.
Owner:北京科美东雅生物技术有限公司
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Multi-antigen ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting African swine fever virus antibody
The invention provides a multi-antigen enzyme linked immunosorbent assay (ELISA) kit for detecting an African swine fever virus (ASFV) antibody, belonging to the field of a biotechnology and diagnosis and research of animal-borne diseases. The multi-antigen enzyme linked immunosorbent assay kit comprises expression and purification of three types of ASFV recombined antigens, preparation of positive and negative control blood serum of an ASFV antibody, optimal envelope antigen combination and concentration determination, optimization of multi-antigen ELISA (MA-ELISA (Microalbumin-Enzyme Linked Immunosorbent Assay)) reaction parameters, determination of an ASFV antibody negative blood serum critical value, MA-ELISA detection artificial infection and determination of sensitivity, specificity and repeatability of field blood serum samples. By detecting and testifying a large quantity of known blood serum samples, the sensitivity, the specificity and the repeatability of detecting the ASFV antibody by the MA-ELISA are obviously higher than those of an ELISA method recommended by World Organization for Animal Health and oversea similar kits; and the multi-antigen enzyme linked immunosorbent assay kit can be used for ASFV serological diagnosis, epidemiological investigation and live pig import and export quarantine inspection.
Owner:YANGZHOU UNIV
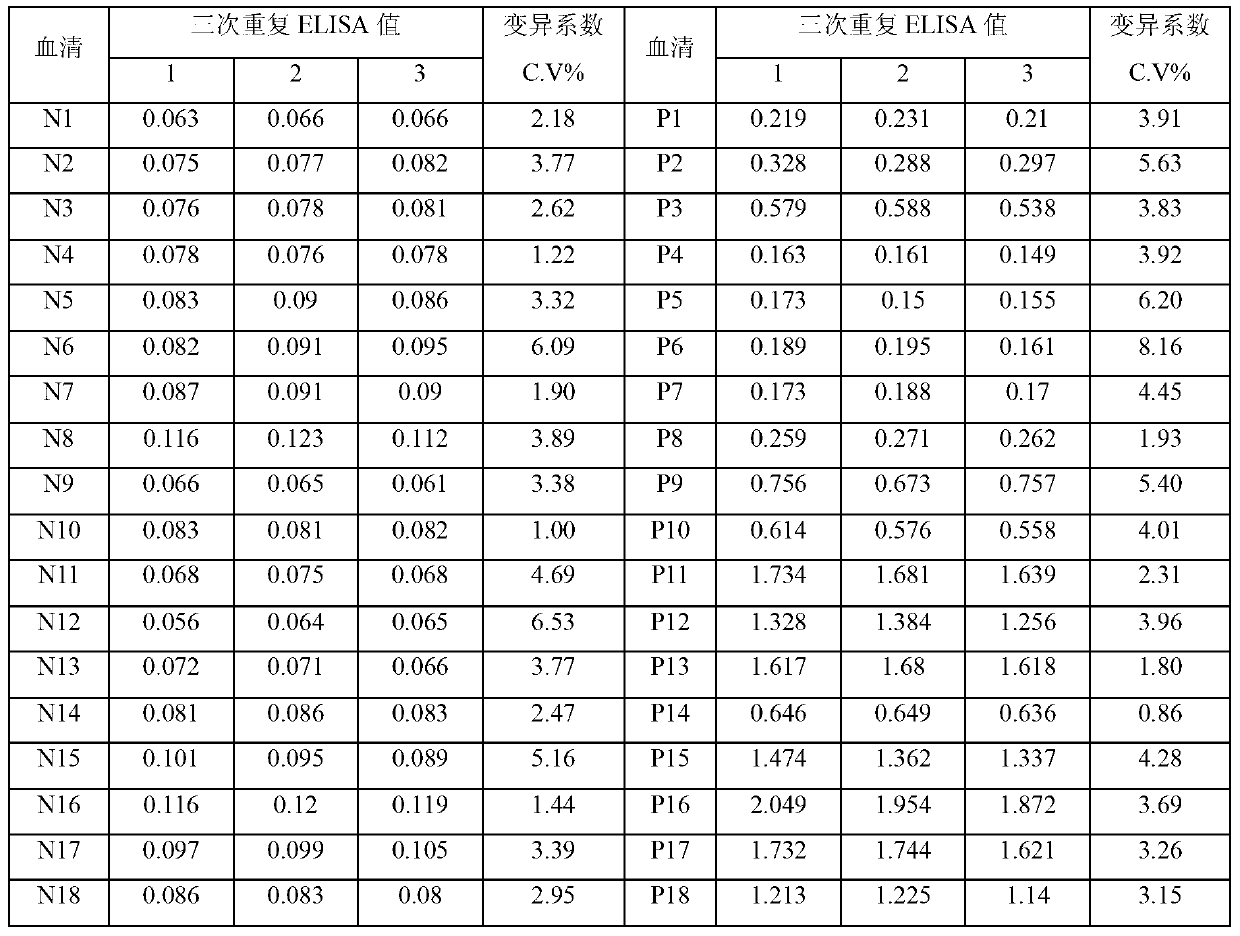
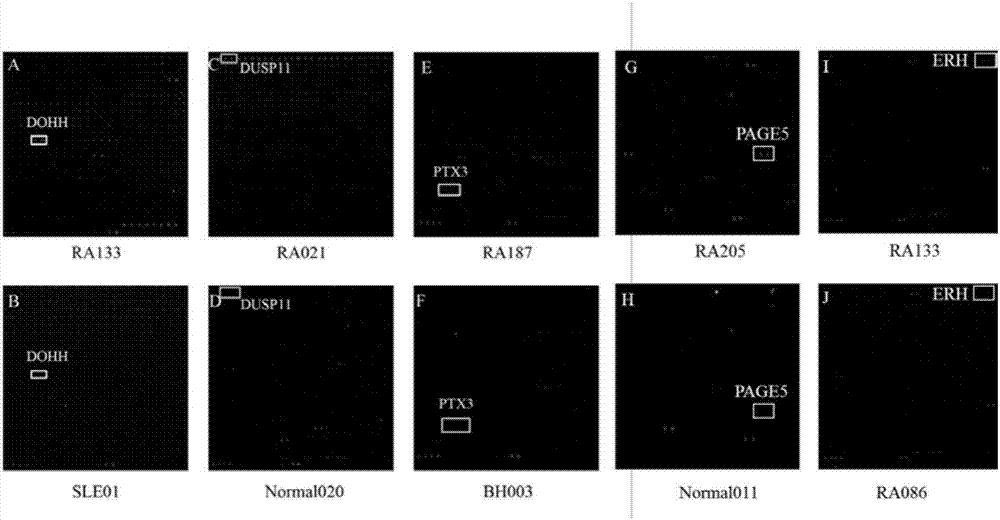
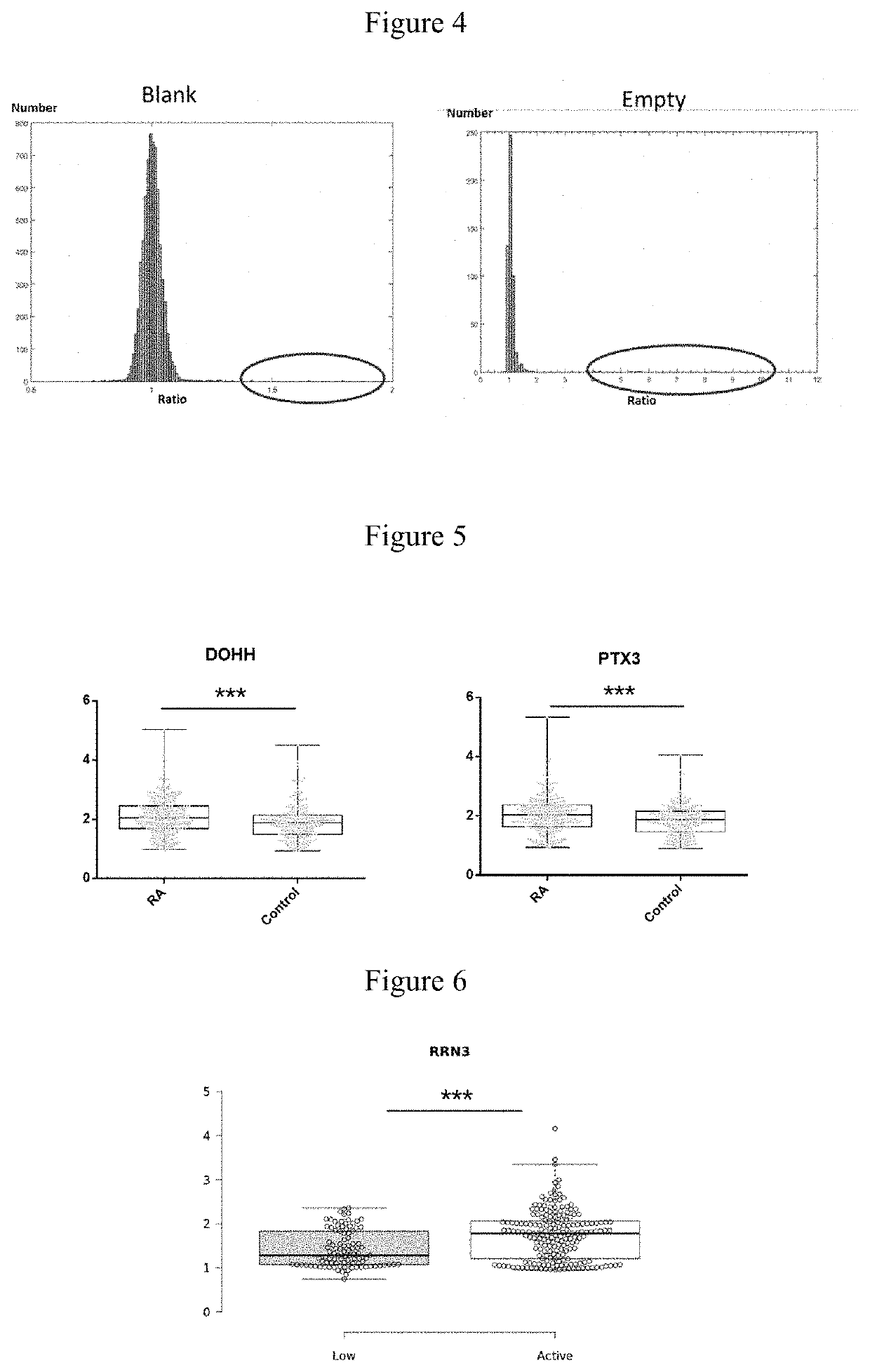
ACPA-negative RA diagnosis marker and application thereof
ActiveCN106950365AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiseaseAuto antigen
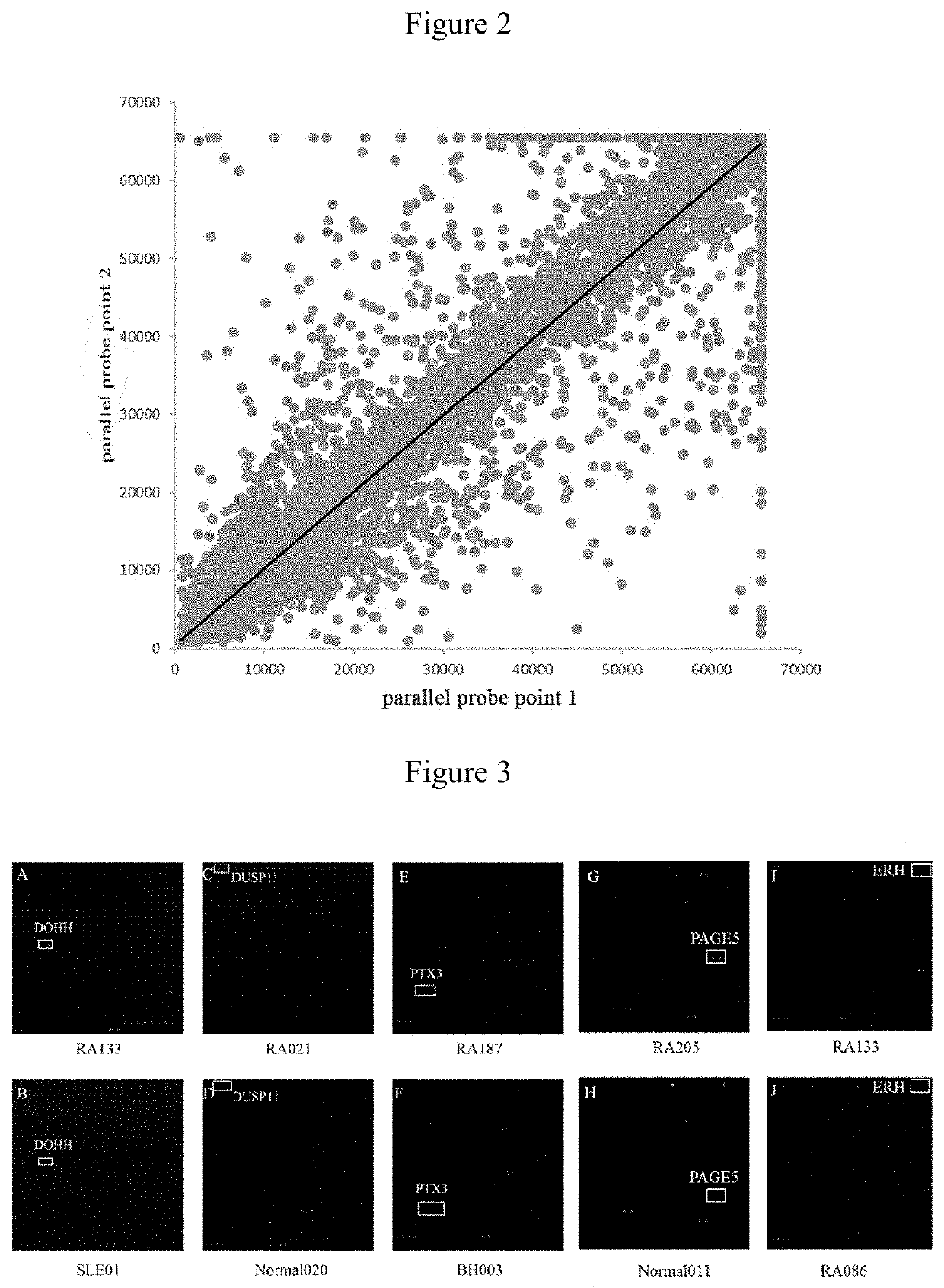
The invention provides applications of deoxyhypusine dioxygenase, namely, DOHH, or a fragment thereof in preparation of a reagent for diagnosis of anti-citrullinated protein antibody negative (ACPA-negative) rheumatoid arthritis (RA). In the method, a high-density protein chip is hybridized with RA serum to screen 35 proteins, which are candidate auto-antigens of the ACPA-negative RA. It is identified that four protein antigens, DOHH, DUSP11, PTX3 and PAGE5, have high sensitivity and specificity in ACPA-negative serum of the RA, wherein the four protein antigens are all novel auto-antigens. The method shows that the DOHH, DUSP11, PTX3 and PAGE5 can be used as diagnosis markers for the ACPA-negative RA.
Owner:北京埃克帕生物医学科技有限公司
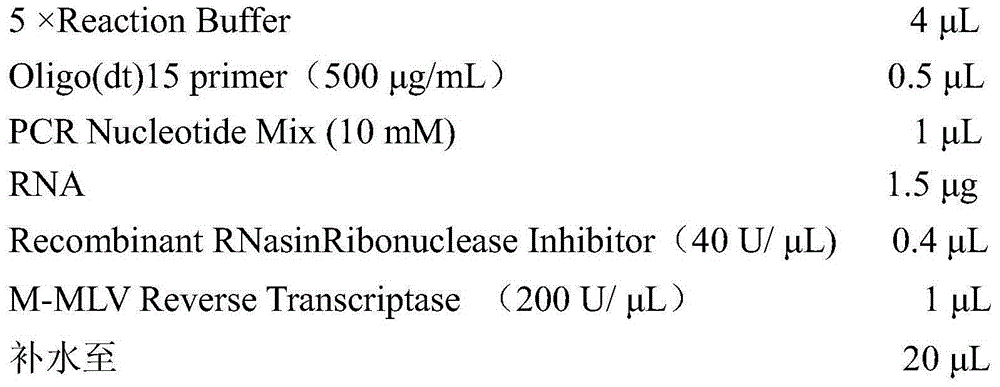
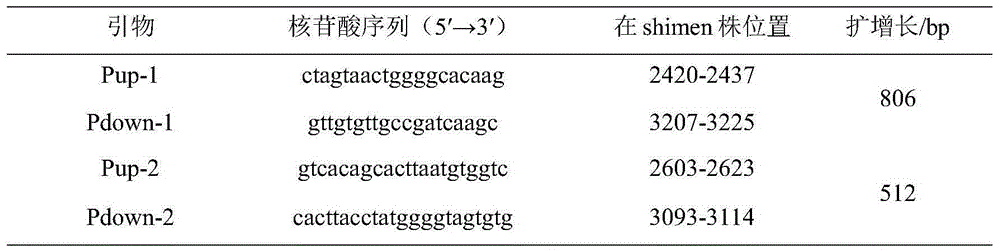
Improved method for identifying target RNA sequences of RNA-binding protein in cell sample
InactiveCN107674870ASimplify the experimental processLibrary creationDNA preparationCDNA libraryPhosphorylation
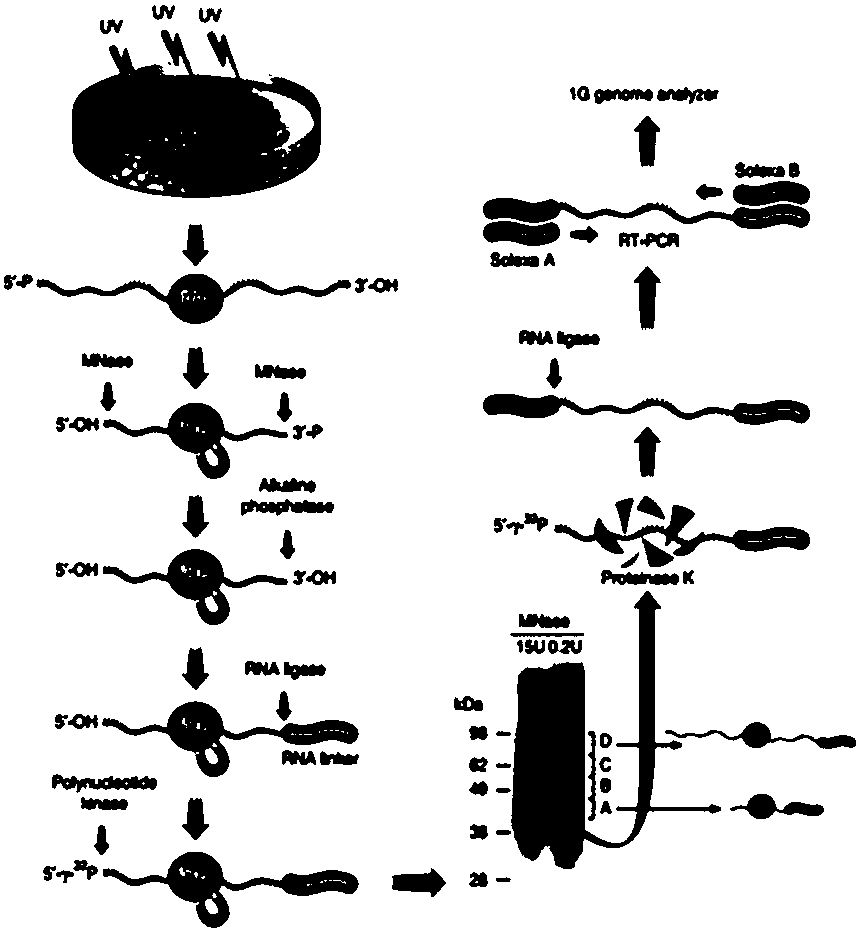
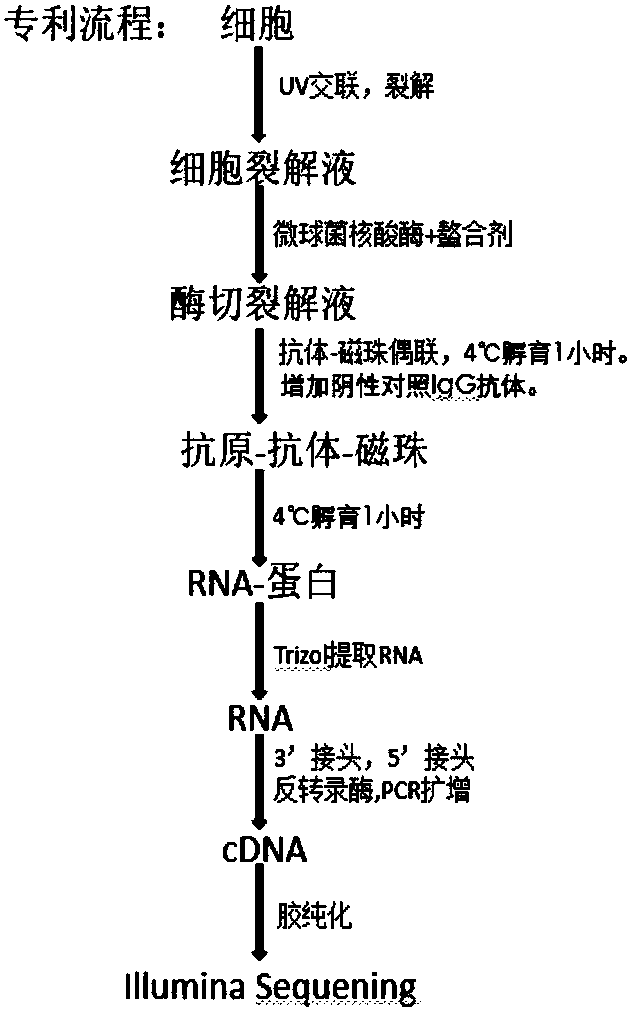
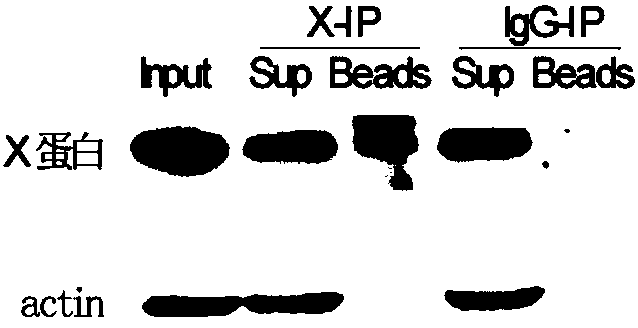
The invention relates to the field of biotechnology, and provides improved identification for target RNA sequences bound to RNA-binding protein in cells. Through UV crosslinking-immunopricipitation, target RNA of RNA-binding protein is obtained, micrococcal nuclease is adopted for carrying out incomplete enzymolysis on the target RNA after ultraviolet crosslinking, after enzymolysis, a chelating agent capable of removing Ca<2+> is adopted for inactivating the enzyme, the 3' terminal of the target RNA obtained through the method is phosphorylated, the phosphorylated 3' terminal is ligated witha 3' RNA linker, then, the ligation product is phosphorylated, the phosphorylated ligation product is separated, the separated phosphorylated ligation product is recycled, then the 3' terminal is ligated with a 5' RNA linker, RT-PCR amplification is carried out, so that a cDNA library corresponding to the target RNA is obtained. With the technical scheme provided by the invention, the experiment flow is simplified, the step of isotope labelling is eliminated, due to the adding of an IgG antibody negative control sample, the influences of non-specific binding and background on the experimentalresult are eliminated, and the data result with the quality equal to that of Clip-seq is obtained.
Owner:武汉生命之美科技有限公司
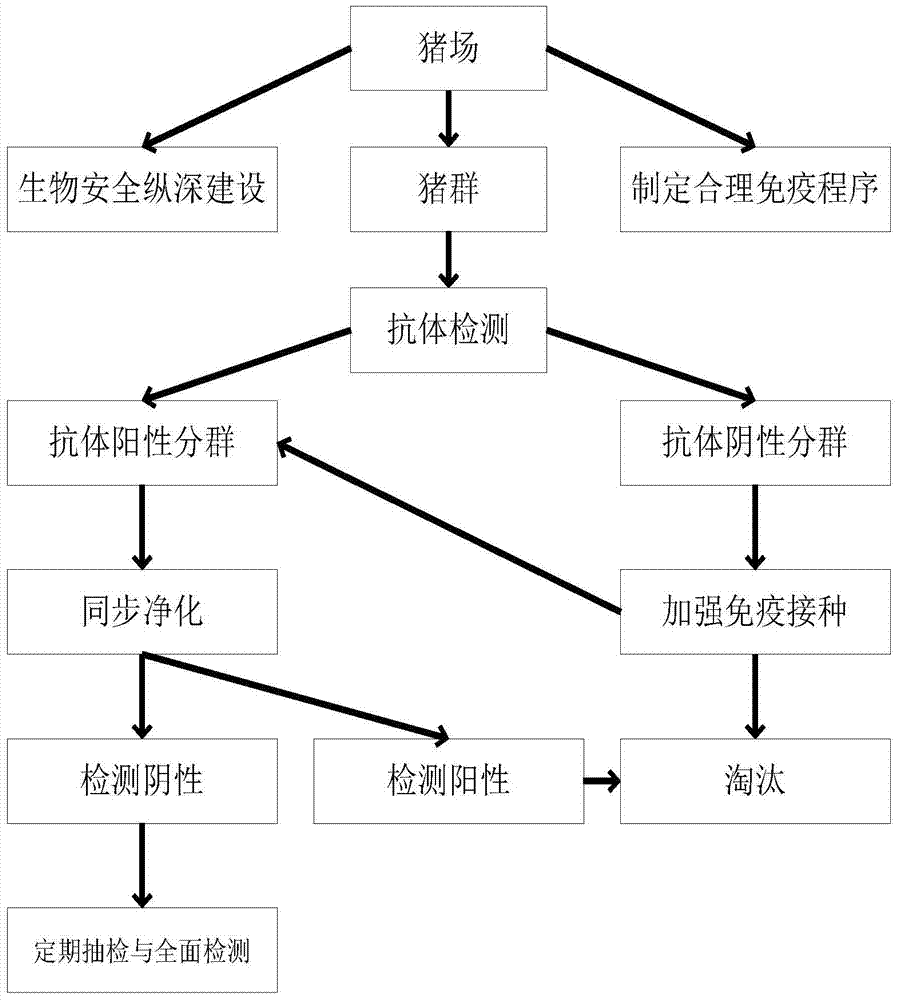
Method for cleaning pig farm to prevent diseases
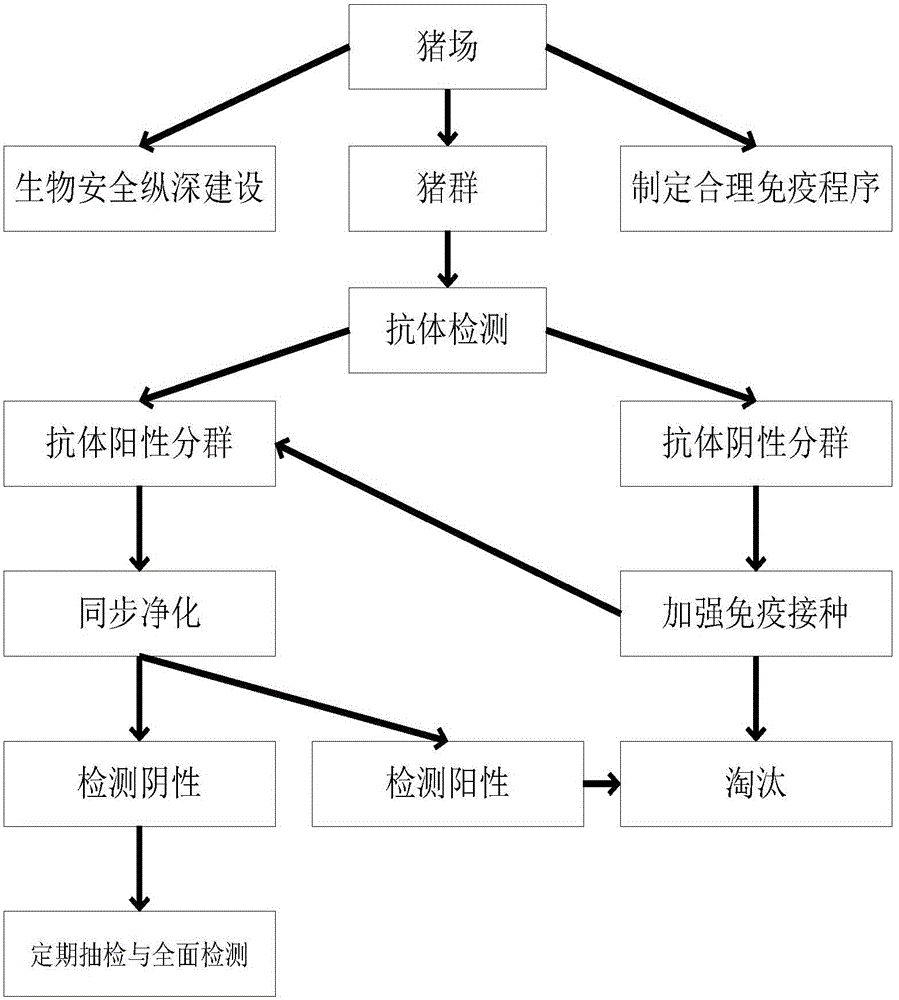
The invention discloses a method for cleaning a pig farm to prevent diseases. Pig groups are divided into antibody negative subgroups and antibody positive subgroups according to immunity antibody detection; strengthen immunization is conducted on the antibody negative subgroups, immunity antibodies are detected again after immunization, and antibody positive pigs are brought into the antibody positive subgroups; fluid of umbilical cords of piglets is collected as detection samples when sows in the antibody positive subgroups give birth to the piglets, hog cholera virus and porcine pseudorabies virus are synchronously detected through a PCR method, and the pigs negative for both hog cholera virus and porcine pseudorabies virus are kept; regular casual inspection and comprehensive detection are conducted on the kept pigs, the pigs positive for both hog cholera virus and porcine pseudorabies virus or positive for one of hog cholera virus and porcine pseudorabies virus are weeded out, and then hog cholera virus and porcine pseudorabies virus are synchronously cleaned away. By synchronously cleaning away hog cholera virus and porcine pseudorabies virus, the method is high in efficiency, small in stress and easy and convenient to operate.
Owner:HUNAN XINNANFANG CULTURE SERVICE CO LTD
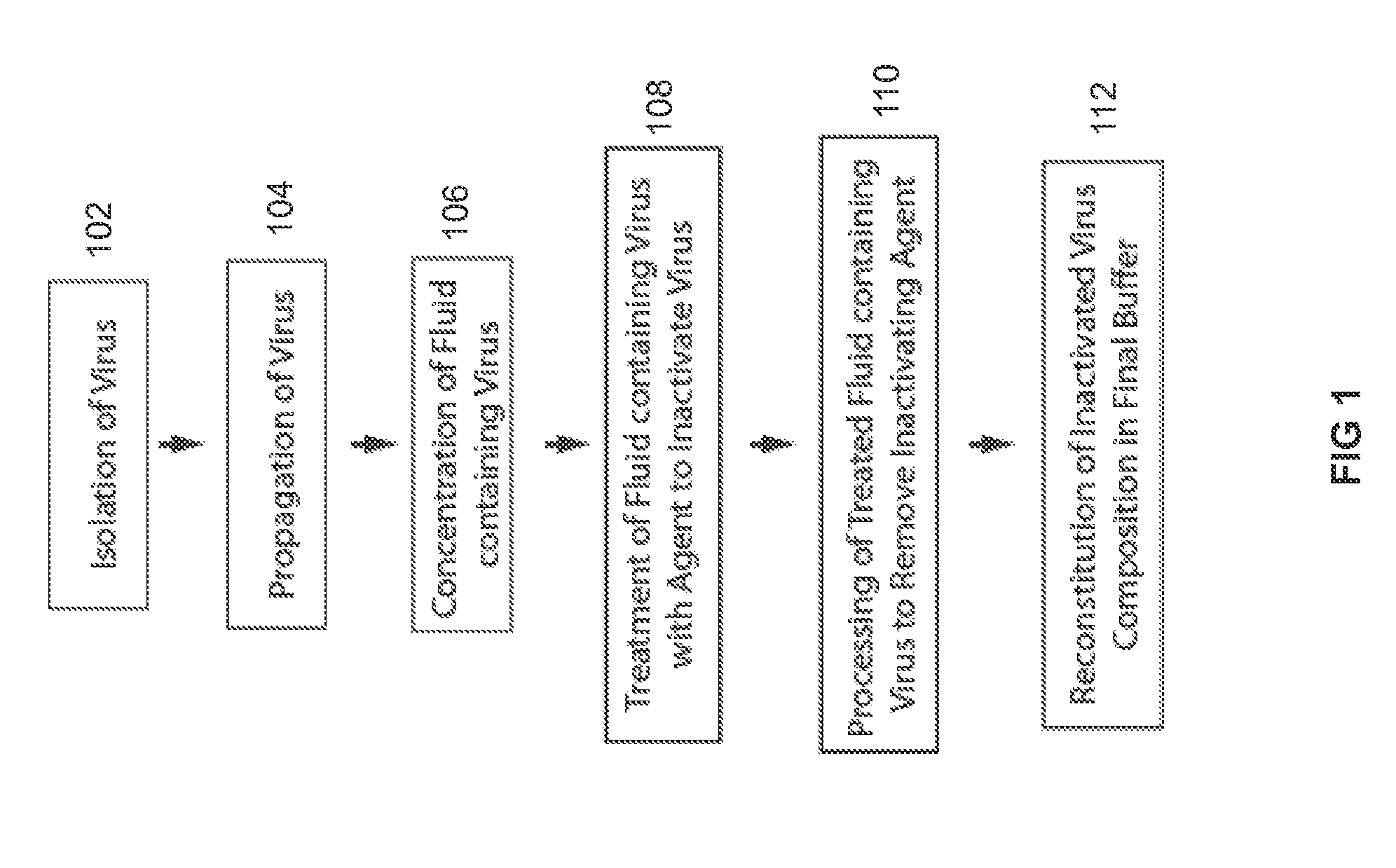
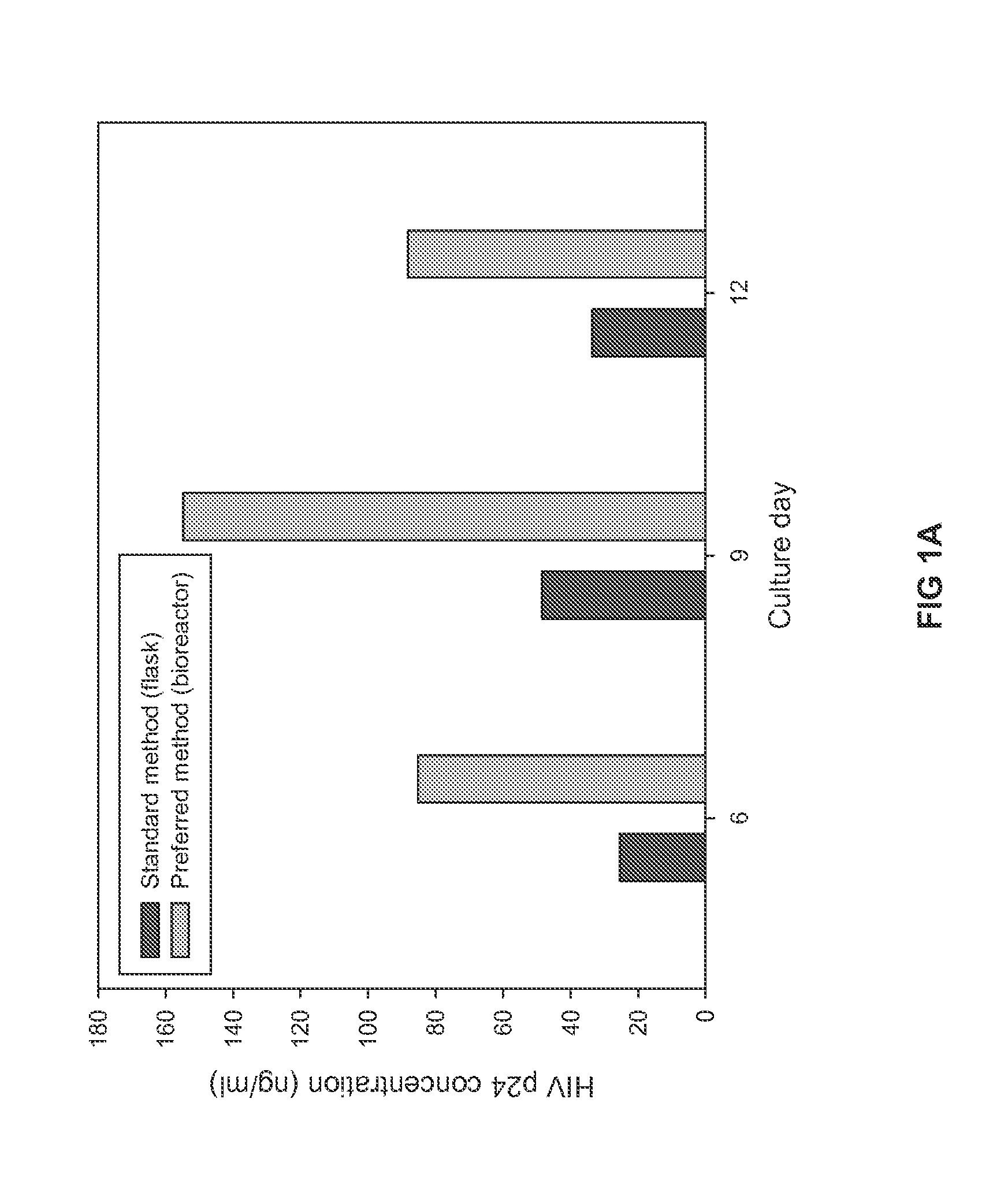
HIV Antigens and Antibodies
InactiveUS20160002319A1Reducing disease progressionViral antigen ingredientsVirus peptidesAnti virusProtein C
The present invention relates to a method for reducing the occurance and / or severity of viral infections. The method embodies procedures for expanding HIV from the blood of HIV antibody negative donors and deriving a non-infectious virus particle product that is antigenic. The procedures for deriving the antigenic, non-infectious virus particle product are optimally designed to maintain the integrity of the envelope proteins while maximizing the depletion of capsid proteins and RNA. The resulting virus particle product, when introduced into humans or non-human animals, enables the production of antibodies that target the natural envelope macromolecular structure that is required for infectivity. The present invention can be applied to producing virus stocks from the blood of HIV-seronegative donors, for deriving non-infectious virus particles that retain intact envelope proteins, for producing anti-viral antibodies, and for administering anti-virus antibodies to patients.
Owner:THERABIOL
Prevention and control method of porcine pseudorabies
InactiveCN104721837AReally protectConsolidate the results of purificationMicrobiological testing/measurementGenetic material ingredientsPig farmsSocial benefits
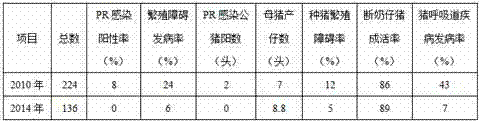
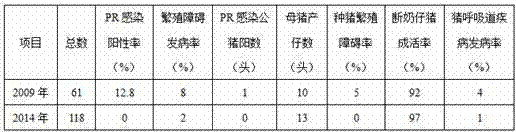
The invention discloses a prevention and control method of porcine pseudorabies. Porcine pseudorabies gene-deleted vaccine immunity is carried out on all pigs in a pig breeding farm, then, purification treatment is carried out, breeding pigs with the positive wild virus infection test result are eliminated, then, remaining breeding pigs are divided into an antibody positive group and an antibody negative group, and then the antibody positive group is divided into a high antibody group and a low antibody group; vaccines are rebred into the antibody negative group and the low antibody group, then, purification treatment is carried out on breeding sows at a time every year, and purification treatment is carried out on breeding boars at a time every 6 months. The method is adopted to comprehensively prevent and control the porcine pseudorabies, the infection positive rate of the porcine pseudorabies is reduced year by year, through implementation of three years to four years, the virus detection result of the breeding boars and the breeding sows shows zero, and the pig farm obtains the obvious economic benefits and the social benefits. The method is simple and easy to implement, animals prone to infection are truly protected, the porcine pseudorabies of the pig farm can be completely and effectively purified and controlled, the PR purification effect is consolidated, and the prevention and control cost is greatly reduced.
Owner:YUNNAN AGRICULTURAL UNIVERSITY
Limbal epithelial stem cell isolated culture method
InactiveCN111560348AReduce the probability of contaminationMild and fast digestionCell dissociation methodsNervous system cellsCorneal fibroblastSurface marker
The invention provides a limbal epithelial stem cell isolated culture method, the limbal epithelial stem cells obtained by the isolated culture method have higher cell activity and stronger cell proliferation ability, and the culture method is simple, effective and low in rejection reaction. Wherein the number of cells subjected to primary culture is 9.5-105, the positive expression rate of a surface marker is detected by flow cytometry, and a result is negative expression of a surface symbolic antibody of an antibody AE5 corneal fibroblast, negative expression of CX43 corneal stem cells, positive expression of a PCNA antibody and positive expression of a BrdU antibody.
Owner:BEIJING YULONG SHENGSHI BIOTECH CO LTD
Piperacillin induced hemolysis detection reagent kit and method for preparing same
PendingCN107132323AHigh sensitivityGood repeatabilityDisease diagnosisTesting medicinal preparationsHemolysisRed blood cell
The invention relates to a piperacillin induced hemolysis detection reagent kit and a method for preparing the same. The piperacillin induced hemolysis detection reagent kit comprises piperacillin inducible hemolysis detection reagent cards I, piperacillin inducible hemolysis detection reagent cards II, piperacillin treated red blood cells, non-piperacillin treated red blood cells, antibody positive control liquid and antibody negative control liquid. The piperacillin treated red blood cells are normal O Rh (rhesus) negative red blood cells treated by piperacillin medicine solution; the non-piperacillin treated red blood cells are normal O Rh negative red blood cells with the concentration of 2-2.5%; the piperacillin antibody positive control liquid is piperacillin antibody positive serum; the piperacillin antibody negative control liquid is piperacillin antibody negative serum. The piperacillin induced hemolysis detection reagent kit and the method have the advantages that the piperacillin inducible hemolysis detection reagent kit is high in sensitivity, good in repeatability and stable in quality, detection procedures are short in time consuming, the method is simple, convenient and feasible, and results are easy to judge.
Owner:江苏中济万泰生物医药有限公司
Enzyme linked immunosorbent assay (ELISA) kit for detecting encephalomyocarditis virus (EMCV) antibodies and application of kit
The invention discloses an enzyme linked immunosorbent assay (ELISA) kit for detecting encephalomyocarditis virus (EMCV) antibodies and application of the kit. The kit comprises an ELISA plate coating EMCV recombinant antigens, enzyme labeled EMCV antigen working solution, EMCV antibody positive control, EMCV antibody negative control, lotion, a color-developing agent A, a color-developing agent B and stop solution. The invention also relates to application of the kit to detecting EMCV antibodies of human and animals. The kit can detect human and animals, has high sensitivity, strong specificity, good thermal stability, low detection cost and short detection time and is suitable for detecting mass EMCV antibodies. Through tests, positivity and negativity of 99.6% of serums can be directly judged, so the kit has good practical effect.
Owner:NORTHWEST UNIVERSITY FOR NATIONALITIES
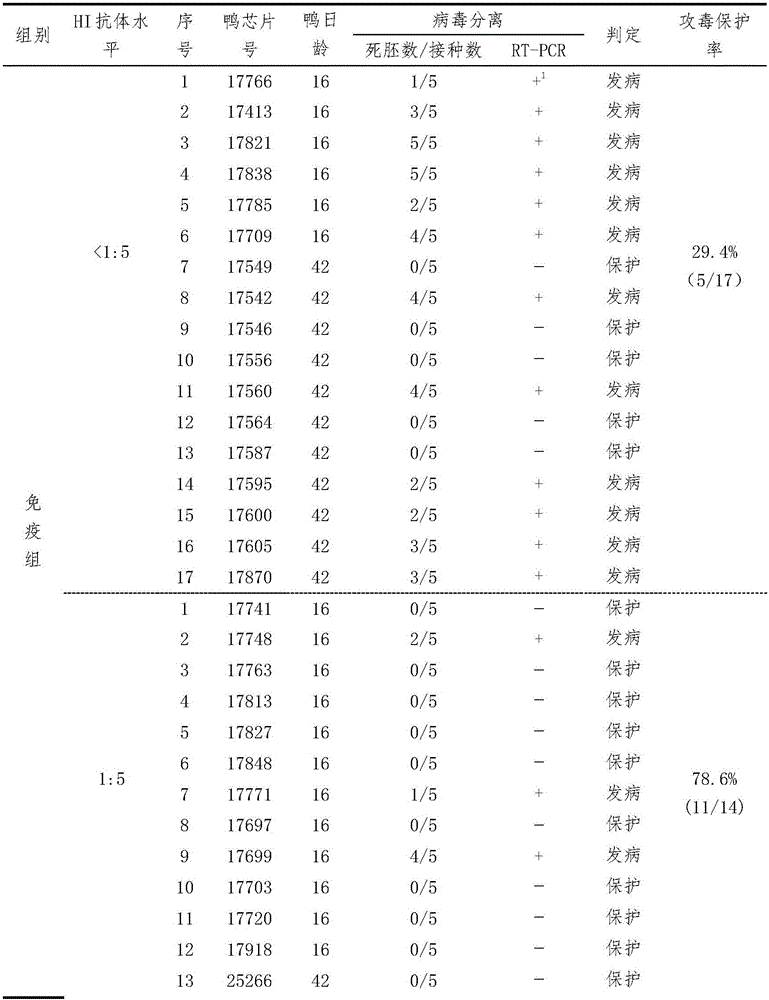
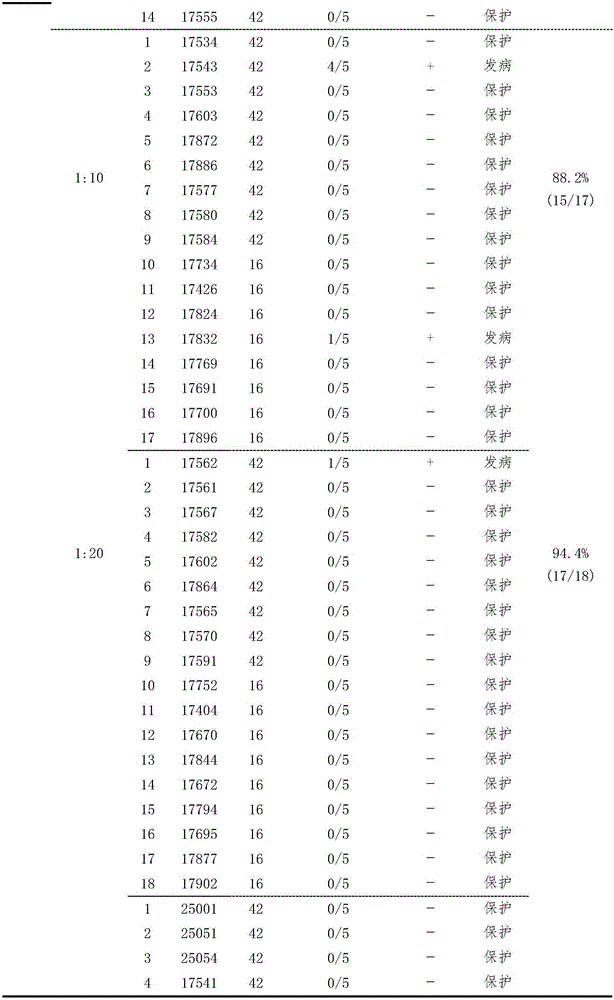
Testing method for potency of inactivated vaccine against duck Tembusu viral diseases
ActiveCN105866424ASolutionResolving Gaps in Judgment CriteriaCompounds screening/testingBiological testingTembusuPrimary immunization
The invention provides a serological testing method for the potency of an inactivated vaccine against duck Tembusu viral diseases. According to the method, HI antibody-negative ducks are used and divided into two groups, i.e., an immunized group consisting of ten ducks and a control group consisting of five ducks; each duck in the immunized group receives hypodermic or intramuscular injection of a vaccine against duck Tembusu viral diseases; immunization with an inactivated vaccine is carried out twice, a dosage of 0.5 ml or 1.0 ml per duck is used each time, and secondary immunization is carried out in two weeks after primary immunization; for immunization with a live vaccine, each duck is immunized according to a dosage of a standard vaccine usage amount for poultry once; in 3 to 4 weeks after inoculation, blood is acquired, serum is separated and the titer of an HI antibody is determined; and when the titer of the HI antibody in the ducks of the control group is less than 1: 5 and the titer of the HI antibody in serum of at least seven ducks of the immunized group is no less than 1: 10, it is determined that the potency of the vaccine is qualified in testing. The serological testing method provided by the invention is convenient to operate and accurate in results and can be extensively applied to potency evaluation of vaccines against duck Tembusu viral diseases and formulation of immune procedure in primary-level organizations and vaccine development and examination units.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Kit for quantificationally and qualitatively detecting smooth muscle antibodies
InactiveCN109633169AEasy to detectHigh sensitivityChemiluminescene/bioluminescenceDisease diagnosisAntigenBiotin-streptavidin complex
The invention discloses a kit for quantificationally and qualitatively detecting smooth muscle antibodies. The kit comprises a magnetic microparticle reagent marked with streptavidin, a goat anti-human IgG storage buffer solution containing HRP marks, a biotin antigen reagent, a smooth muscle antibody calibration product, a smooth muscle antibody critical value quality control product and a smoothmuscle antibody negative quality control product, wherein the surfaces of the magnetic microparticles contain carboxyl groups; and biotin antigens in the biotin antigen reagent are marked with actin.The kit has the advantages that the detection is convenient and fast; the quantificational and qualitative detection on the smooth muscle antibodies can be realized in a short time, so that the typeand the serious degree of autoimmune liver diseases can be accurately diagnosed; and the clinic medication of a doctor is guided. The prepared kit has the advantages of high sensitivity, high specificity and high accuracy, and is applicable to a semi-automatic or fully-automatic detection system. The kit combines a chemiluminescence technology with a magnetic microparticle technology, has a high use value on clinics, and is suitable for being popularized and applied.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Tunneling magnetoresistive biosensor and preparation method and application thereof
PendingCN113189193AImprove the detection rateMagnetic signal stabilizationMagnetic sensor arraysMaterial magnetic variablesIgm antibodyEngineering
The invention discloses a tunneling magnetoresistive biosensor and a preparation method and application thereof. The biosensor comprises a tunneling magnetoresistance multilayer film structure, a reaction area of the tunneling magnetoresistance biosensor, a biological sensitive film in the reaction area and magnetic beads for generating signals. The reaction area of the tunneling magnetoresistive biosensor is divided into a novel coronavirus IgM antibody detection area, a negative control area and a novel coronavirus IgG antibody detection area, the novel coronavirus IgM antibody detection area fixes an anti-human IgM antibody, the negative control area modifies bovine serum albumin, and the novel coronavirus IgG antibody detection area fixes an anti-human IgG antibody. The tunneling magnetoresistive biosensor is more accurate in result, high in sensitivity, simple and convenient to operate, free of operation by professionals, low in detection cost and suitable for field detection of communities, airports, families and the like, and an effective means is provided for rapid screening of novel coronaviruses.
Owner:XI AN JIAOTONG UNIV
A kind of elispot detection kit for detecting bovine brucellosis
ActiveCN105759054BHigh potencyStrong specificityBiological material analysisBiological testingBrucella antibodyAntibodies monoclonal
Owner:YANGZHOU UNIV
Porcine pseudorabies mutant strain ge and gi gene deletion virus strain and its application
ActiveCN103756977BImprove securityImproving immunogenicityAntiviralsViruses/bacteriophagesDiseaseImmunogenicity
The present invention involves the field of pseudo-rabies virus technology. It specially involves a pseudo-pseudo-rabies disease variable and GI gene lack of virus strain PRV-ZJ011g. The preservation number is CGMCC?No.7957.Its application in the preparation of vaccines.Will 106.0?The TCID50 reorganization virus inocus the New Zealand big white rabbit, which did not cause itching and other clinical symptoms.Made into an oily vaccine, 4 weeks of GB after the piglets are immunized?ELISA antibody is positive, but GE antibody is negative, and immune protection efficiency is 100%. After the sow immunization, its offspring can obtain immune protection, which is 100%of PRV mutant poison and traditional toxic immune protection efficiency;Can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
A multi-antigen ELISA kit for detecting antibodies against African swine fever virus
Owner:YANGZHOU UNIV
Amoxicillin antibody detection kit and preparation and application method thereof
The invention relates to an amoxicillin antibody detection kit and a preparation and application method thereof. The amoxicillin antibody detection kit is mainly composed of an amoxicillin antibody detection reagent card, amoxicillin processing erythrocyte, non-amoxicillin processing erythrocyte, an amoxicillin antibody positive contrast liquid, and an amoxicillin antibody negative contrast liquid. The kit has simple in operation, is strong in sensitivity and stability, and is more convenient in a clinical application, and can rapidly detect an amoxicillin antibody for a patient.
Owner:江苏中济万泰生物医药有限公司
HIV Antigens and Antibodies
The present invention relates to a method for reducing the occurrence and / or severity of viral infections. The method embodies procedures for expanding HIV from the blood of HIV antibody negative donors and deriving a non-infectious virus particle product that is antigenic. The procedures for deriving the antigenic, non-infectious virus particle product are optimally designed to maintain the integrity of the envelope proteins while maximizing the depletion of capsid proteins and RNA. The resulting virus particle product, when introduced into humans or non-human animals, enables the production of antibodies that target the natural envelope macromolecular structure that is required for infectivity. The present invention can be applied to producing virus stocks from the blood of HIV-seronegative donors, for deriving non-infectious virus particles that retain intact envelope proteins, for producing anti-viral antibodies, and for administering anti-virus antibodies to patients.
Owner:GNVIE LLC
Ampicillin antibody detection kit and preparation and use methods thereof
The invention relates to an ampicillin antibody detection kit and preparation and use methods thereof. The ampicillin antibody detection kit mainly comprises an ampicillin antibody detection reagent card, ampicillin-treated erythrocyte, non-ampicillin-treated erythrocyte, ampicillin antibody positive control solution and ampicillin antibody negative control solution. The kit has the advantages ofsimplicity for operation and high sensitivity and stability, is more convenient in clinical application and can carry out rapid detection of an ampicillin antibody on a patient.
Owner:江苏中济万泰生物医药有限公司
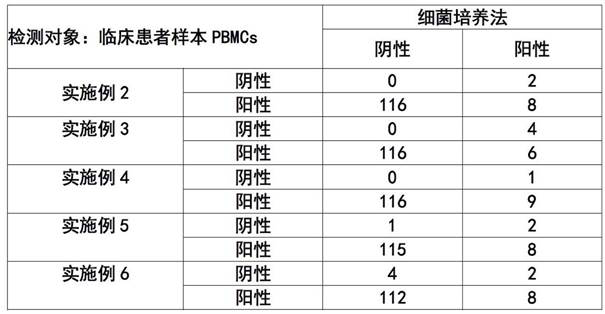
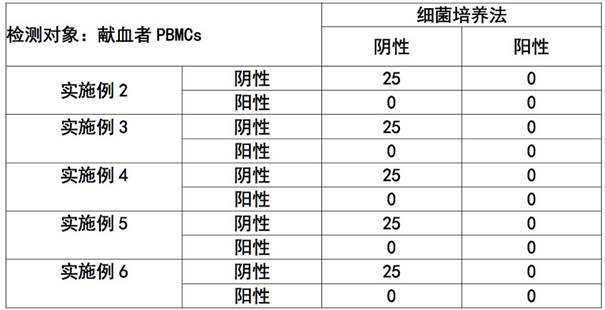
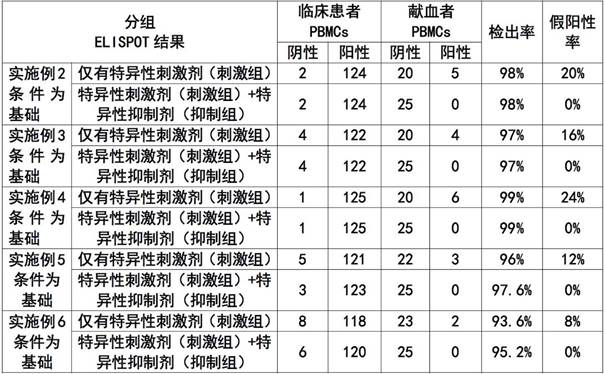
A kind of elispot detection kit for detecting brucellosis and application thereof
ActiveCN111638329BImprove featuresIncreased sensitivityMaterial analysisAntiendomysial antibodiesCapture antibody
The invention discloses an ELISPOT detection kit for detecting brucellosis and an application thereof. An ELISPOT detection kit for detecting brucellosis, said ELISPOT detection kit comprising: support medium, capture antibody, detection antibody, negative control and positive control and specific stimulator, said ELISPOT detection kit Specific inhibitors are also included to detect the presence or absence of Brucella infection bidirectionally by specific stimulators and specific inhibitors. The ELISPOT detection kit of the present invention determines whether there is Brucella infection through positive and negative experiments, and the detection result is more objective and accurate, and can truly reflect the immune level in the body, so as to solve the accuracy rate existing in the current detection of Brucella infection Low, long cycle and other issues.
Owner:SOUTHERN MEDICAL UNIVERSITY
Enzyme linked immunosorbent assay (ELISA) kit for detecting encephalomyocarditis virus (EMCV) antibodies and application of kit
The invention discloses an enzyme linked immunosorbent assay (ELISA) kit for detecting encephalomyocarditis virus (EMCV) antibodies and application of the kit. The kit comprises an ELISA plate coating EMCV recombinant antigens, enzyme labeled EMCV antigen working solution, EMCV antibody positive control, EMCV antibody negative control, lotion, a color-developing agent A, a color-developing agent B and stop solution. The invention also relates to application of the kit to detecting EMCV antibodies of human and animals. The kit can detect human and animals, has high sensitivity, strong specificity, good thermal stability, low detection cost and short detection time and is suitable for detecting mass EMCV antibodies. Through tests, positivity and negativity of 99.6% of serums can be directly judged, so the kit has good practical effect.
Owner:NORTHWEST UNIVERSITY FOR NATIONALITIES
IPMA antibody detection method of Lawsonia intracellularis
The invention discloses an IPMA antibody detection method of Lawsonia intracellularis. The method comprises the following steps: (1) culturing the Lawsonia intracellularis by using McCoy cells; (2) preparing an IPMA reaction plate: adding the Lawsonia intracellularis cultured in the step (1) into a cell plate containing McCoy cells for culturing, continuously culturing for 4-5 days, and then fixing, permeating and sealing in sequence; (3) adding to-be-detected serum into the IPMA reaction plate obtained in the step (2), respectively adding positive control serum and negative control serum at the same time, and incubating; adding a peroxidase-labeled secondary antibody, and incubating; and adding a developing solution, reacting and drying; and (4) observing under a microscope, and when the to-be-detected serum sample is positive of a lawsonia intracellularis antibody, staining the cytoplasm of the positive cell infected by the lawsonia intracellularis to be brownish red; and when a to-be-detected pig serum sample is negative in the lawsonia intracellularis antibody, enabling the cytoplasm of the McCoy cells infected by the lawsonia intracellularis to be not colored. The method has the advantages of high specificity, high sensitivity, simplicity in operation and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
ELISPOT detection kit for detecting brucellosis and application of ELISPOT detection kit
ActiveCN111638329AImprove featuresIncreased sensitivityMaterial analysisAntiendomysial antibodiesCapture antibody
The invention discloses an ELISPOT detection kit for detecting brucellosis and application of the ELISPOT detection kit. The ELISPOT detection kit comprises a support medium, a capture antibody, a detection antibody, a negative control, a positive control, a specific stimulant, and a specific inhibitor; whether brucella infection exists or not can be tested and determined through two-way detectionof the specific stimulant and the specific inhibitor. According to the ELISPOT detection kit, whether brucella infection exists or not is determined through positive and negative experiments, a detection result is objective and accurate, and an in-vivo immune level can be truly reflected; problem that existing brucella infection detection is low in accuracy rate and long in period.
Owner:SOUTHERN MEDICAL UNIVERSITY
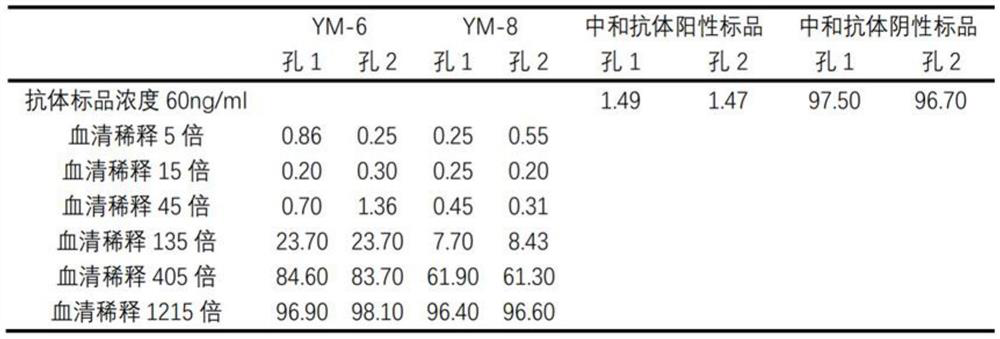
Novel coronavirus neutralizing antibody magnetic bead fluorescence detection kit and detection method
ActiveCN113791212AIncreased Sensitivity of Serum DetectionIncreased sensitivitySsRNA viruses positive-senseAntibody mimetics/scaffoldsImmune profilingMagnetic bead
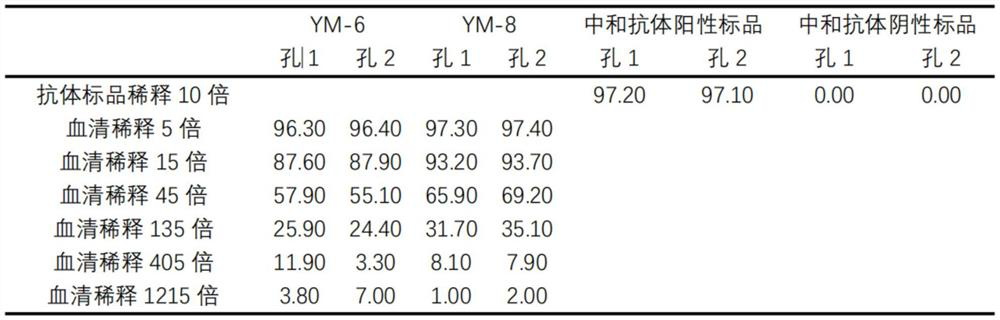
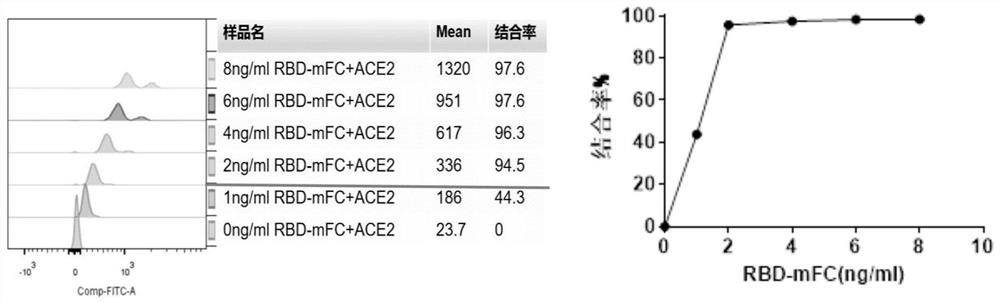
The invention relates to the technical field of immunoassay detection, and discloses a novel coronavirus neutralizing antibody magnetic bead fluorescence detection kit and detection method. The kit comprises ACE2 protein magnetic beads, novel coronavirus S1 protein RBD structural domain-mouse IgG Fc fragment fusion protein, a fluorescent substance labelled animal anti-mouse IgG antibody, a novel coronavirus neutralizing antibody positive standard substance, a novel coronavirus neutralizing antibody negative standard substance, a mouse IgG homotype control antibody, a 10x concentrated washing solution and a sample diluent. The detection method implemented by the kit has relatively high sensitivity on the detection of the novel coronavirus neutralizing antibody. The kit is low in cost and high in cost performance when used for detecting the novel coronavirus neutralizing antibody, and the kit can fill the gap of high-sensitivity products in the current rapid detection kit market for the novel coronavirus neutralizing antibody.
Owner:SHANXI UNIV
Acpa-negative ra diagnostic marker and application thereof
InactiveUS20190369123A1Validate sensitivityValidate specificityDisease diagnosisBiological testingDiseaseCitrulline
The invention provides a use of a deoxyhypusine dioxygenase DOHH or a fragment thereof in the preparation of a reagent for diagnosing an anti-citrulline polypeptide antibody-negative rheumatoid arthritis disease. 35 proteins as candidate ACPA-negative RA autoantigens were screened by hybridizing high density protein chips with RA serum. 4 protein antigens (DOHH, DUSP11, PTX3 and PAGE5) were identified as having high sensitivity and specificity in the ACPA-negative serum of RA, wherein DOHH can be used as the ACPA-negative RA diagnostic markers.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Treponema pallidum specific antibody chemical light emitting detection kit and preparation method thereof
InactiveCN104316681AHigh sensitivityShorten detection timeChemiluminescene/bioluminescenceSolid phasesNegative control
The invention discloses a treponema pallidum specific antibody chemical light emitting detection kit and a preparation method thereof, and relates to treponema pallidum. The kit comprises an outer package box, an alkaline phosphatase marked recombinant antigen bottle, a light emitting substrate bottle, a treponema pallidum specific antibody negative control bottle, a treponema pallidum specific antibody positive control bottle, a washing liquid bottle and a recombinant antigen coated micropore plate. The preparation method comprises the following steps: firstly, preparing reponema pallidum specific recombinant antigen and the recombinant antigen coated micropore plate, marking alkaline phosphatase of the recombinant antigen, further preparing a light emitting substrate, a washing liquid and a control group, and finally assembling the treponema pallidum specific antibody chemical light emitting detection kit. The treponema pallidum specific antibody chemical light emitting detection kit can be used for detecting syphilis specificity specific antibodies in clinical specimens, specific solid phase is adopted, immunity and light emitting reaction can be rapidly completed within a short time, the light emitting signals are greatly intensified, the sensitivity is improved, the detection time can be shortened, and the precision is improved.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV +1
A method for testing the efficacy of duck Tembusu virus disease vaccine
ActiveCN105866424BAccurate methodSensitive methodCompounds screening/testingBiological testingSerum igeIntramuscular injection
The invention provides a serological testing method for the potency of an inactivated vaccine against duck Tembusu viral diseases. According to the method, HI antibody-negative ducks are used and divided into two groups, i.e., an immunized group consisting of ten ducks and a control group consisting of five ducks; each duck in the immunized group receives hypodermic or intramuscular injection of a vaccine against duck Tembusu viral diseases; immunization with an inactivated vaccine is carried out twice, a dosage of 0.5 ml or 1.0 ml per duck is used each time, and secondary immunization is carried out in two weeks after primary immunization; for immunization with a live vaccine, each duck is immunized according to a dosage of a standard vaccine usage amount for poultry once; in 3 to 4 weeks after inoculation, blood is acquired, serum is separated and the titer of an HI antibody is determined; and when the titer of the HI antibody in the ducks of the control group is less than 1: 5 and the titer of the HI antibody in serum of at least seven ducks of the immunized group is no less than 1: 10, it is determined that the potency of the vaccine is qualified in testing. The serological testing method provided by the invention is convenient to operate and accurate in results and can be extensively applied to potency evaluation of vaccines against duck Tembusu viral diseases and formulation of immune procedure in primary-level organizations and vaccine development and examination units.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Methods of purifying epidemic diseases in pig farms
The invention discloses a method for cleaning a pig farm to prevent diseases. Pig groups are divided into antibody negative subgroups and antibody positive subgroups according to immunity antibody detection; strengthen immunization is conducted on the antibody negative subgroups, immunity antibodies are detected again after immunization, and antibody positive pigs are brought into the antibody positive subgroups; fluid of umbilical cords of piglets is collected as detection samples when sows in the antibody positive subgroups give birth to the piglets, hog cholera virus and porcine pseudorabies virus are synchronously detected through a PCR method, and the pigs negative for both hog cholera virus and porcine pseudorabies virus are kept; regular casual inspection and comprehensive detection are conducted on the kept pigs, the pigs positive for both hog cholera virus and porcine pseudorabies virus or positive for one of hog cholera virus and porcine pseudorabies virus are weeded out, and then hog cholera virus and porcine pseudorabies virus are synchronously cleaned away. By synchronously cleaning away hog cholera virus and porcine pseudorabies virus, the method is high in efficiency, small in stress and easy and convenient to operate.
Owner:HUNAN XINNANFANG CULTURE SERVICE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com