Novel coronavirus neutralizing antibody magnetic bead fluorescence detection kit and detection method
A coronavirus and fluorescence detection technology, which is applied in the direction of virus/bacteriophage, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of high detection cost, insufficient sensitivity, and no cost-effective advantages, so as to achieve good detection accuracy Sexuality and the effect of reducing the cost of testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
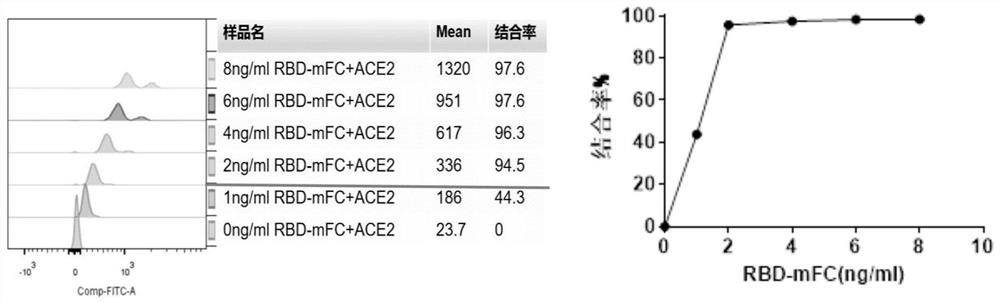
[0060] The critical concentration at which the RBD-mFc protein binds to most of the ACE2 protein magnetic beads at a high level The specific operation steps are as follows:
[0061] (1) Dilute the RBD-mFc recombinant protein into a series of concentration gradients with the sample diluent, the concentrations are 2ng / mL, 4ng / mL, 8ng / mL, 12ng / mL and 16ng / mL. Dilute the mouse IgG1 isotype control antibody to a concentration of 16 ng / mL with sample diluent.
[0062] (2) Add RBD-mFc and mouse IgG isotype control antibody at various concentrations diluted in step (1) to a round-bottom 96-well plate at 50 μL / well, and conduct experiments in duplicate wells for each concentration sample.
[0063] (3) Resuspend the ACE2 magnetic beads, absorb the ACE2 magnetic bead solution at 1 μL / reaction, wash the ACE2 magnetic beads in a round-bottom 96-well plate with 200 μL of sample diluent, and wash twice. When the cleaning solution is sucked and discarded, the magnetic beads are retained in t...
Embodiment 2
[0070] The influence of different concentrations of RBD-mFc on the sensitivity of the kit of the present invention to detect the neutralizing antibody of the new coronavirus
[0071] The program operation steps of this embodiment are as follows:
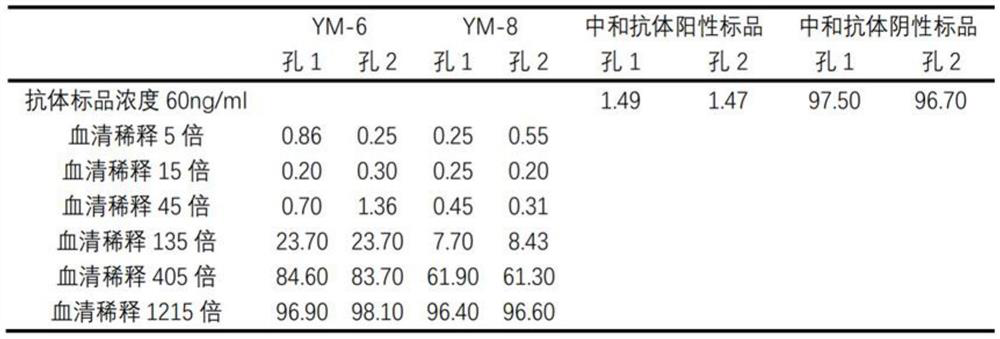
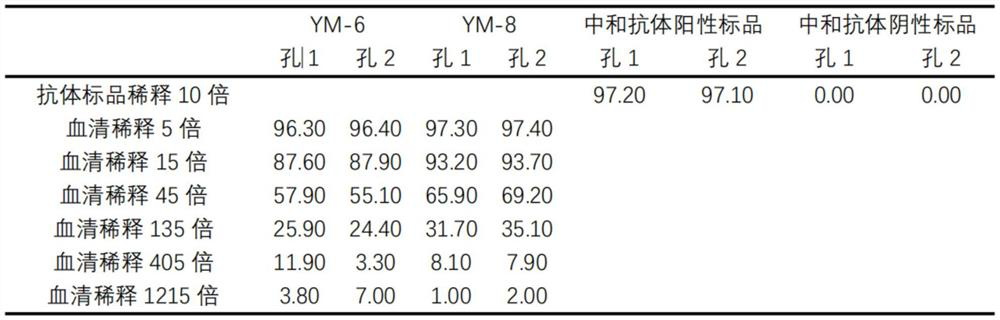
[0072] (1) Use the sample diluent to dilute the positive standard and negative standard of the new coronavirus neutralizing antibody respectively to a concentration of 5ng / mL, 15ng / mL, 45ng / mL, 135ng / mL, 400ng / mL, 1200ng / mL and For 3600ng / mL antibody diluent, add 50 μL / well of the positive standard diluent and negative standard diluent of each concentration of the new coronavirus neutralizing antibody to the round-bottom 96-well plate, and add duplicate wells for each antibody concentration;
[0073] (2) Dilute RBD-mFc and mouse IgG isotype control antibodies with sample diluent to concentrations of 4 ng / mL, 100 ng / mL, 200 ng / mL and 2000 ng / mL respectively, and dilute each concentration of RBD-mFc and mouse IgG isotype Add 50 μL / wel...
Embodiment 3
[0083] Sensitivity evaluation of ACE2 magnetic beads / RBD-mFc novel coronavirus neutralizing antibody detection kit
[0084] In this example, a commercially available novel coronavirus neutralizing antibody function ELISA detection kit (SARS-CoV-2 Surrogate Virus Neutralization Test Kit product number: L00847-A produced by GenScript Biotechnology Co., Ltd. Item No.: L00847A) is used as a reference to evaluate the detection sensitivity of the ACE2 magnetic beads / RBD-mFc new coronavirus neutralizing antibody detection kit established by the present invention. In this example, a humanized monoclonal antibody (Acrobiosystems, Cat. No.: SAD-S35) with neutralizing activity of the new coronavirus and a human IgG1 isotype control antibody "Human IgG 1, K Isotype Control" ( BioLegend, Cat. No. Cat403502) as a positive standard for neutralizing antibodies to the new coronavirus and neutralizing antibodies to the new coronavirus in the ACE2 magnetic beads / RBD-mFc neutralizing antibody det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com