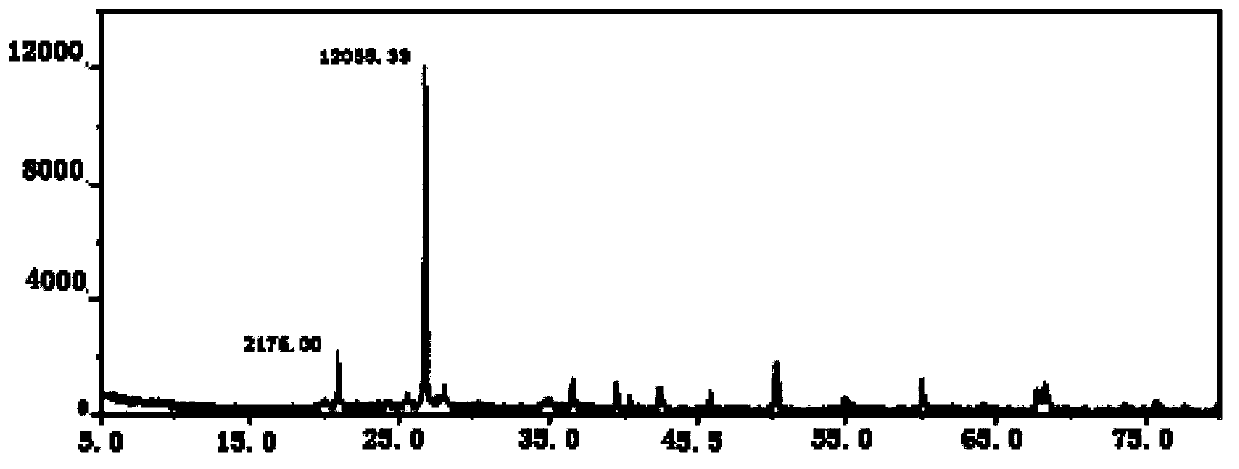
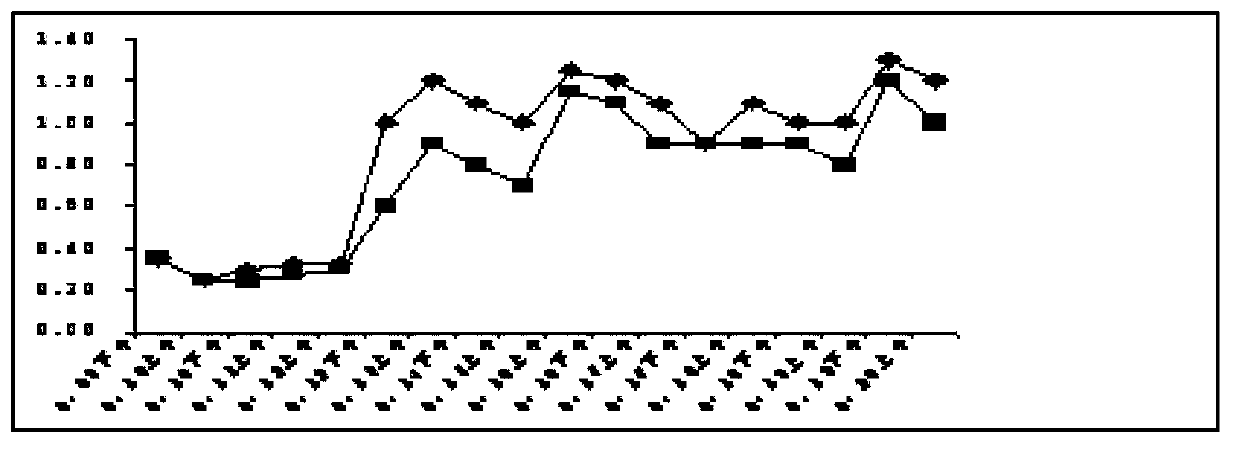
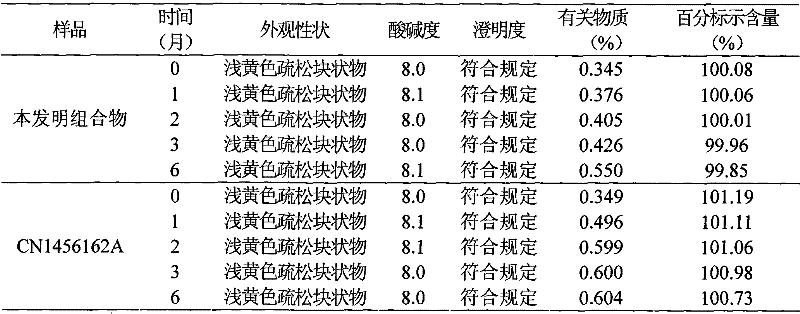
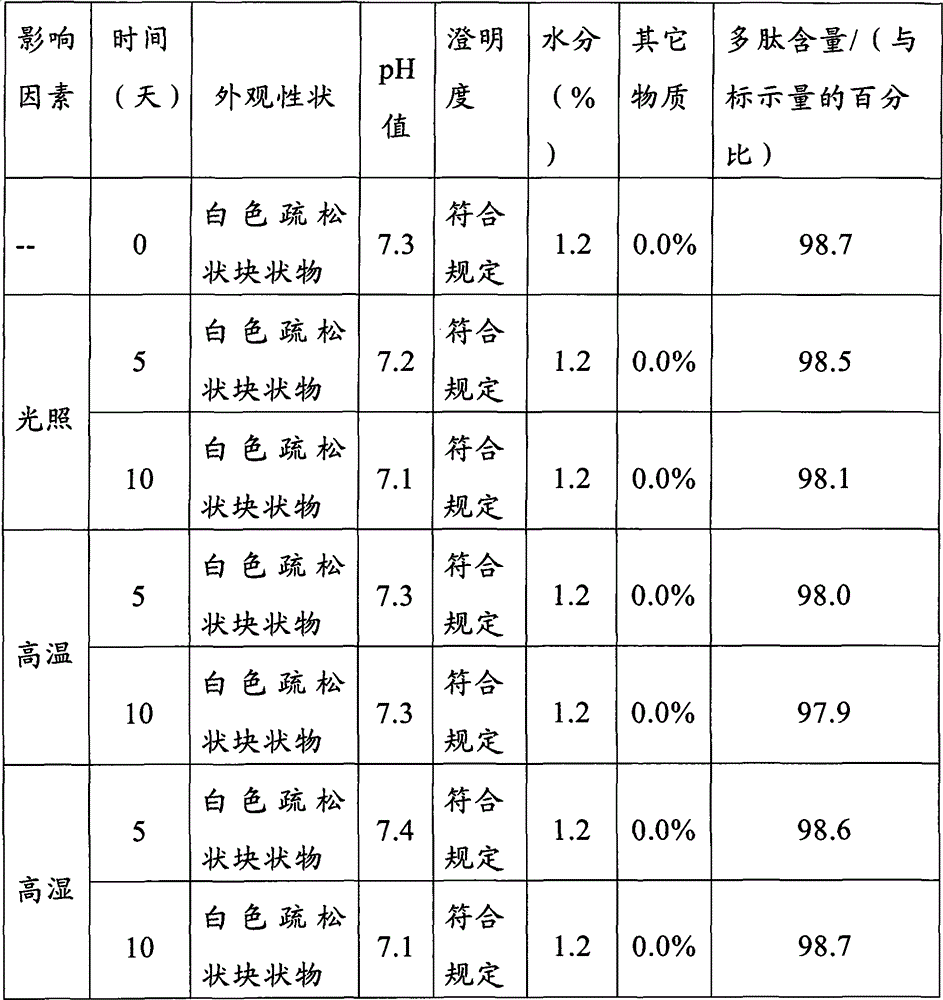
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42results about How to "Recovery properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
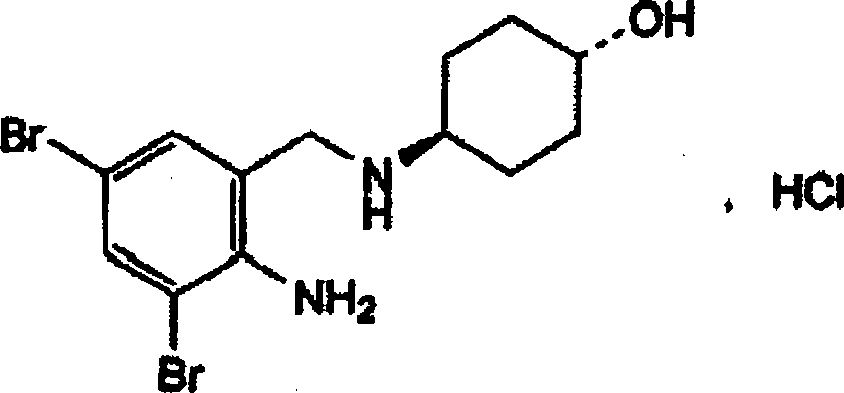
Ambroxol hydrochloride freeze-dried powder injection and preparing method thereof
ActiveCN101224196AAvoid interactionImprove stabilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The invention relates to an ambroxol hydrochloride freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises ambroxol hydrochloride and mannitol. The ambroxol hydrochloride freeze-dried powder injection prepared by a freeze-drying technology has good stability and high dissolution velocity while all other indexes accord with regulation.
Owner:SHANDONG YUXIN PHARMA CO LTD
Method for restoring totipotency of mesenchymal stem cell
ActiveCN103667186ARecovery propertiesAchieve maintenanceSkeletal/connective tissue cellsMesenchymal stem cellCultured cell
The invention discloses a culture method for restoring totipotency of a mesenchymal stem cell. The method for restoring totipotency of a mesenchymal stem cell, provided by the invention, comprises the following steps: 1) performing hanging-drop culture on an in-vitro mesenchymal stem cell with reduced totipotency, thus obtaining a hanging-drop cultured cell; and 2) further performing suspension culture on the hanging-drop cultured cell, thus realizing totipotency restoration of the mesenchymal stem cell. The experiment of the invention proves that, according to the method for restoring totipotency of a mesenchymal stem cell (specifically 3D culture), after the mesenchymal stem cell (in-vitro mesenchymal stem cell with reduced totipotency) subcultured multiple times is subjected to 3D culture treatment, the increased volume is gradually restored, the cell viability is enhanced, the cloning capability and differentiation capability are enhanced, and the paracrine action is also improved, thereby activating the aged mesenchymal stem cell and restoring the characteristics of the stem cell.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Hydrochloric acid diltiazem freeze-dried powder injection for injections and preparation method thereof
ActiveCN101327194AImprove stabilityReduced stabilityOrganic active ingredientsPowder deliveryFreeze-dryingMannitol
The invention provides a diltiazem hydrochloride freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection comprises diltiazem hydrochloride and mannite, the proportion by weight of diltiazem hydrochloride and mannite is 1: 1 to 8. The freeze-dried powder injection has favorable clarity and stability and high content of main drug, thereby being freeze-dried powder injection with good quality.
Owner:HAINAN JINRUI PHARMA
Adenine arabinoside monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN101642440ASimple operation processLow technical requirementsPowder deliveryOrganic active ingredientsEthylene diamineDrugs solution
The invention relates to an adenine arabinoside monophosphate freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is formed by freeze-drying an adenine arabinoside monophosphate drug solution. The adenine arabinoside monophosphate drug solution comprises 100g of adenine arabinoside monophosphate, 25g of mannitol, 3g of disodium hydrogen phosphate, 0.3gof monosodium orthophosphate, 1g of disodium ethylene diamine tetraacetate and 2,000ml of water for injection. The freeze-drying process in the preparation method comprises pre-freezing, sublimationand heating for drying. Compared with the common water injections in the prior art, the freeze-dried powder injection and the preparation method thereof have obvious advantages on storage and transportation.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Novel sodium houttuynin lyophilized powder for injection and its preparing method
InactiveCN1640390APlump appearanceDelicate appearanceAntibacterial agentsOrganic active ingredientsHouttuyninAcetic acid
The present invention relates to a freeze-dried composition of new houttuynin sodium. It includes new houttuynin sodium with therapeutic effective dose and additive agent which can be freeze-dried, in which the dose of new houttuynin sodium is 0.05-0.5% of prescription solution dose, and the additive agent which can be freeze-dried includes tween-80 and dilutent agent, in which the dose of tween-80 is 0.5-2.5 % of prescription solution dose, and the dilutent agent is one kind selected from mannitol, lactose, dextran, dextrose, amino acetic acid, hydrolysed gelatin and polyvidone, etc. or mixture of them. Said invention also provides its preparation process and concrete steps.
Owner:BEIJING BOERDA BIO TECH DEV
Freeze dried monophosphate adenine arabinoside powder injection for injection and preparation method thereof
ActiveCN102389403ASimple operation processLow technical requirementsOrganic active ingredientsPowder deliveryDrugChemistry
The invention relates to freeze dried monophosphate adenine arabinoside powder injection for injection and a preparation method thereof. The freeze dried powder injection is prepared by freeze-drying a monophosphate adenine arabinoside liquid, wherein, the monophosphate adenine arabinoside liquid comprises 100 g of monophosphate adenine arabinoside, 25 g of mannitol, 3 g of disodium hydrogen phosphate, 0.3 g of sodium dihydrogen phosphate, 1 g of ethylenediaminetetraacetic acid, and 2000 mL water for injection. The preparation method comprises the following three steps: prefreezing, sublimating, and heating drying. Compared with common water injection in the prior art, the freeze dried powder injection and its preparation method disclosed herein have remarkable advantages in storage and transportation.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
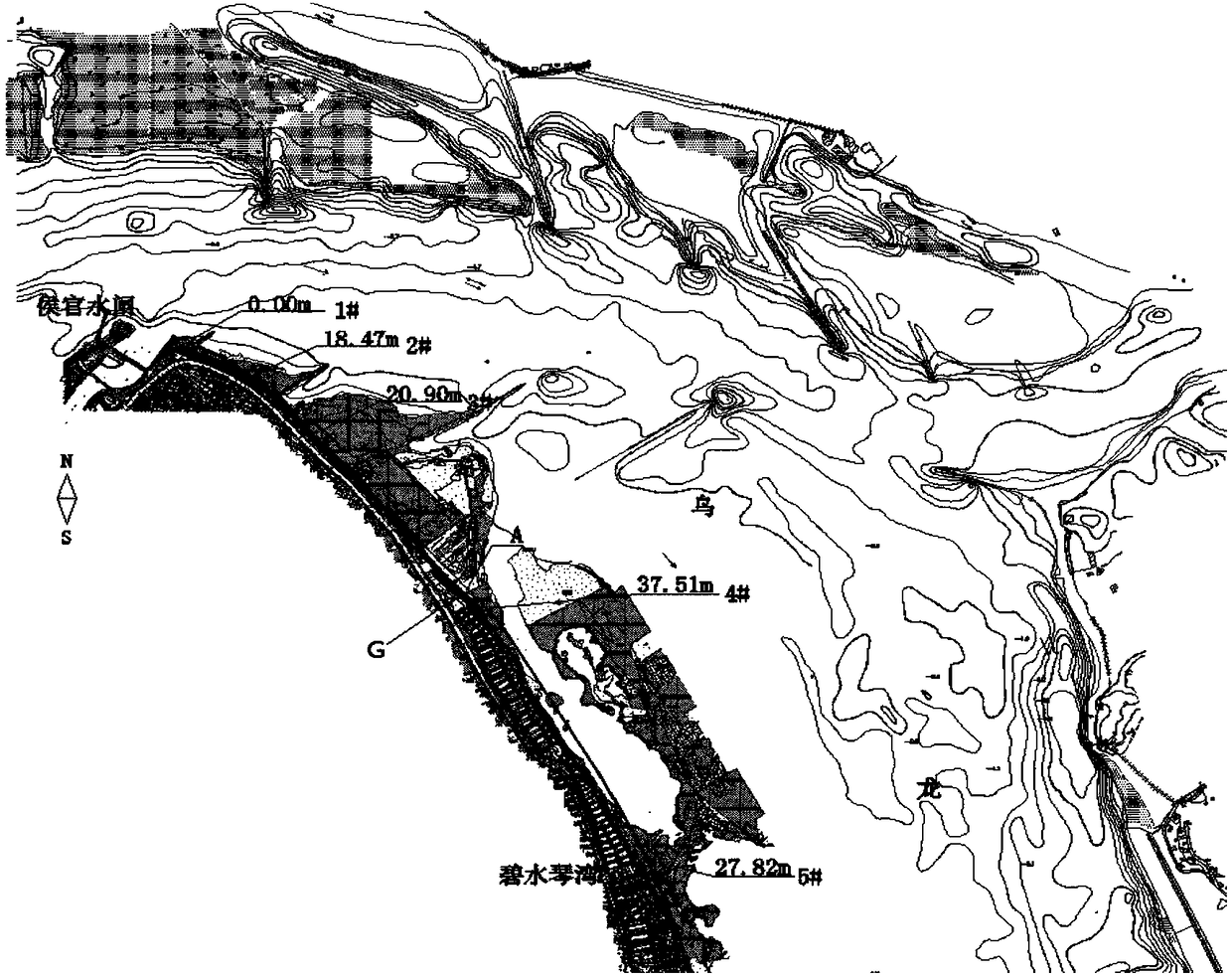
Tide-sensing river channel flood prevention and moisture prevention shoreline making method
ActiveCN108532532ATroubleshoot authentication issuesEffective simulationHydraulic modelsTerrainMathematical model
The invention discloses a tide-sensing river channel flood prevention and moisture prevention shoreline making method, and belongs to the technical field of flood prevention and moisture prevention foundation water conservancy facilities. The method is based on actually measured river channel terrain and hydrology sediment materials, a river channel physical model and a mathematical model are established, the mathematical model comprises a two-dimensional mathematical model and a three-dimensional mathematical model, and the river channel physical model, the two-dimensional mathematical modeland the three-dimensional mathematical model are carrying out digital-to-analogue coupling; and according to hydrodynamic characteristics under different regional tidal currents and different workingconditions, water force of a river channel field, change rule of flow velocity gradient, distribution rule a water power curve, erosion and silting process of a riverbed and a form of ultimate erosionand silting are under the long-time action of the river flood and the outer sea boundary at different frequencies are achieved, and a shoreline conforming to a natural river tidal flooding intersection movement rule is formulated. The tide-sensing river channel flood prevention and moisture prevention shoreline making method has the advantages that the defect that in traditional shoreline making,the flood power function is considered independently and the long-term effect of the tidal power is ignored are broken through, and the adaptability of a combined action area river channel shorelineof a runoff and a tide is enhanced.
Owner:FUJIAN PROVINCIAL INVESTIGATION DESIGN & RES INST OF WATER CONSERVANCY & HYDROPOWER
Fleraxacin for injection and preparation method thereof
InactiveCN101327192AFreeze-drying Process ImprovementFreeze-drying process looseAntibacterial agentsOrganic active ingredientsFleroxacinFreeze-drying
The invention provides injection fleroxacin which is composed of fleroxacin, mannite and lactic acid; the invention also provides a preparation method of the fleroxacin freeze-dried powder: fleroxacin and mannite are added into water, then lactic acid is also dropped into the water, and the solution is stirred until the fleroxacin is dissolved completely; active carbon is added into the mixed solution which then is filtered; the filtrate is placed in a material disc of a freezedryer to be cooled slowly to 30DEG below zero to 60DEG below zero and be kept the prelimitary freezing for 2.5 to 3 hours at 30DEG below zero to 60DEG below zero, then is rapidly cooled to about 45DEG below zero to 55DEG below zero to be pumped to vacuum, is heated to 5DEG below zero to 5DEG for 12 to 15 hours and is kept at 5DEG below zero to 5DEG for 4 to 6 hours, is heated to 25DEG to 30DEG within 3 to 5 hours and is kept at 25DEG to 30DEG for 5 to 7 hours so that the fleroxacin freeze-dried powder is obtained. The obtained fleroxacin freeze-dried powder has simple components, favorable stability and resolubity and low water content.
Owner:HAINAN JINRUI PHARMA
Isosorbide mononitrate freeze-dried power injection and preparation method thereof
ActiveCN101816636ARecovery propertiesLoose texturePowder deliveryPharmaceutical non-active ingredientsAlcoholClinical efficacy
The invention belongs to the technical field of pharmaceutical preparation and provides an isosorbide mononitrate freeze-dried power injection and a preparation method thereof. In the preparation process of the isosorbide mononitrate freeze-dried power injection of the invention, sorbic alcohol is used as a propping agent and the pH value of the solution of a middle body is adjusted to between 6.5 and 7.5 by acidic or alkaline buffer solution, so the hydrolysis of a main medicament is restrained and the stability of the main medicament is improved; and a pre-freezing initial temperature is controlled to be below 50 DEG C below zero, and the second sublimation temperature in a free-drying process is controlled to be between 5 and 15 DEG C, so the medicament content and the stability of related substances are improved. The stable isosorbide mononitrate freeze-dried power injection prepared by the method has reduced toxicity and good clinical effects.
Owner:鲁南新时代生物技术有限公司
Itraconazole freeze-dried powder injection and preparing method
InactiveCN1615870APlump appearanceFast redissolutionOrganic active ingredientsAntimycoticsWater contentChemistry
The freeze-dried Itraconazole powder for injection is prepared through preparing solution with Itraconazole, hydroxypropyl-beta-cyclodextrin, propylene glycol, hydrochloric acid and sodium hydroxide; adding proper amount of diluent and freeze drying. Compared with Itraconazole injection, the present invention has the advantages of raised physical stability, no high temperature decomposing degradation of Itraconazole, loose product and fast recovering of the original solution characteristic after adding water, low water content and drying in vacuum resulting in less oxidation and high stability for long term storing, and less pollution and particle matter introduced during production.
Owner:BEIJING BOERDA BIO TECH DEV
Preparation method for cerebroprotein hydrolysate freeze-dried injection for injection
ActiveCN101637457ASimple operation processThe technical content is not highPowder deliveryNervous disorderFreeze dryPhotochemistry
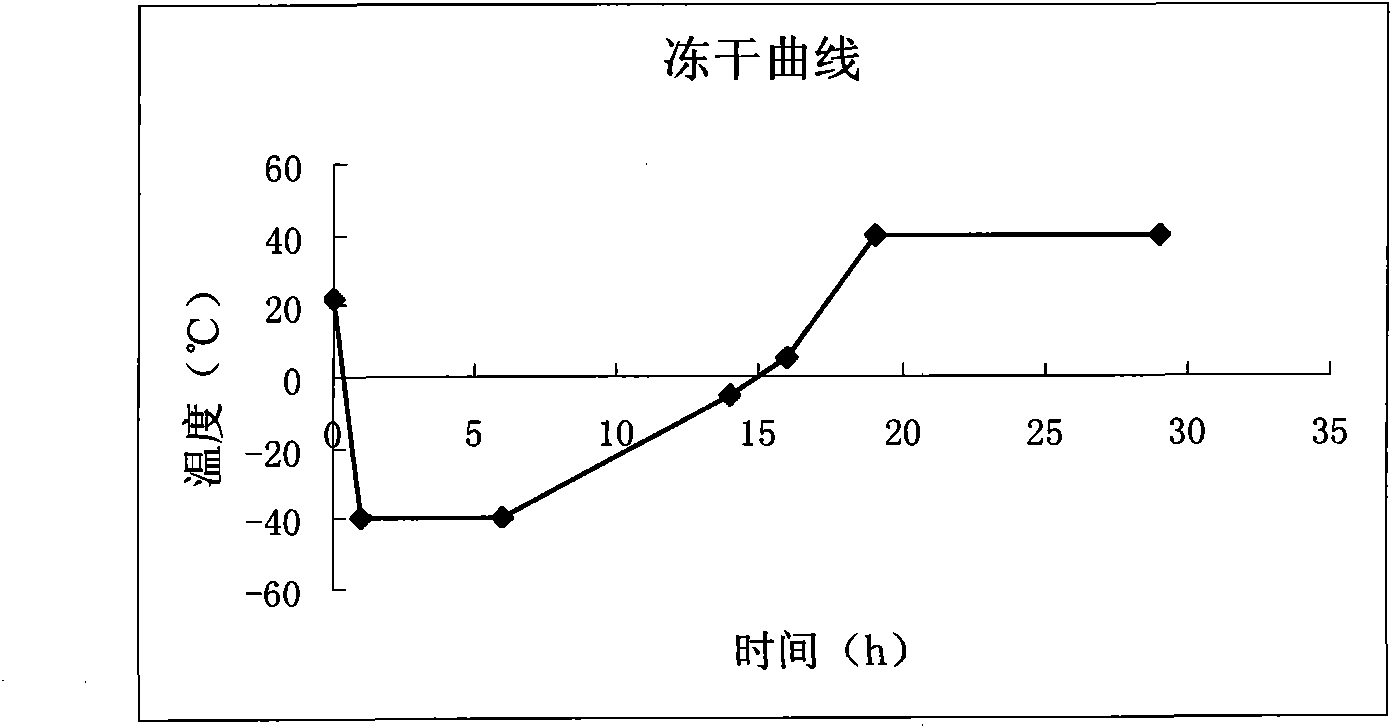
The invention relates to a preparation method for cerebroprotein hydrolysate freeze-dried injection for injection, which comprises the following steps: weighing 5000 volume of cerebroprotein hydrolysate solution; adding 4-10 mass / volume% of skeleton agent into the solution in the aseptic condition, and adjusting the range of Ph to be 6.9-7.5; collecting filter liquor after 0.45mum bacilli-eliminated filtration; filing and drying, wherein the drying time is sequentially 5 hours in the temperature of minus 40 DEG C, 4 hours in the temperature of minus 40 DEG C to minus 15 DEG C, 4 hours in the temperature of minus 15 DEG C to 5 DEG C, 2 hours in the temperature of minus 5 DEG C to 5 DEG C, 3 hours in the temperature of 5 DEG C to 40 DEG C, and 10 hours in the temperature of 40 DEG C; and thedrying time is totally 28 hours. The product prepared by the preparation method is stable and controllable; and the operational technique is simple.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Officinal composition of vidarabine monophosphate for injection
ActiveCN103599080AEasy to controlSpray bottlePowder deliveryOrganic active ingredientsActive componentVidarabine Monophosphate
The invention relates to an officinal composition of vidarabine monophosphate for injection, wherein the main active components of the composition comprise vidarabine monophosphate and lysine.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
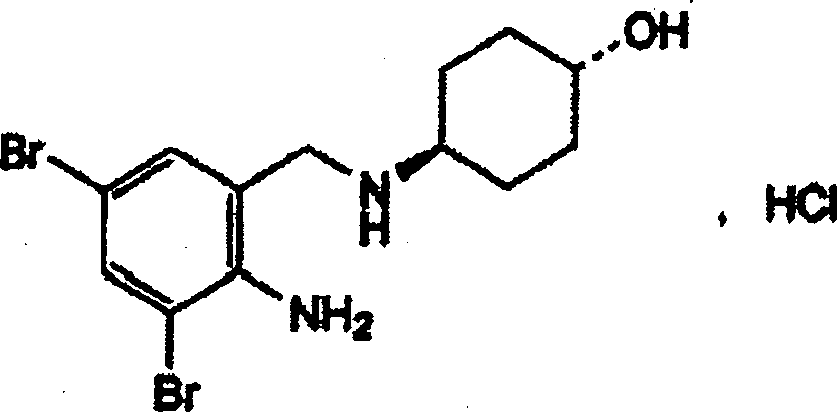
Composition of ambroxol hydrochloride and arginine and preparation method thereof
InactiveCN101756948AUniform colorImprove water solubilityPowder deliveryOrganic active ingredientsDrugChemistry
The invention belongs to the technical field of medicine, in particular to a composition of ambroxol hydrochloride and arginine and a preparation method thereof. The weight ratio of the ambroxol hydrochloride and the arginine in the composition is 1:0.01 to 0.8. The invention also provides a preparation method of freeze-dried powder preparation of the composition, and a use of the composition in the preparation of expectorant drugs.
Owner:HAINAN SIHUAN PHARMA +1
Magnolia biondii pamp volatile oil nanoliposome freeze-dried powder, temperature-sensitive magnolia biondii pamp nanogel and preparation method thereof
InactiveCN101849991AEasy to manufactureFor long-term storagePowder deliveryAntipyreticMANNITOL/SORBITOLMagnolia biondii
The invention relates to a magnolia biondii pamp volatile oil nanoliposome freeze-dried powder. The magnolia biondii pamp volatile oil nanoliposome freeze-dried powder is prepared by the following steps of: A, preparing a magnolia biondii pamp volatile oil nanoliposome from magnolia biondii pamp volatile oil by a high-pressure homogenization method; and B, preparing the magnolia biondii pamp volatile oil nanoliposome freeze-dried powder from the magnolia biondii pamp volatile oil nanoliposome obtained in the step A by a freeze-drying method, wherein a selected liposome freeze-drying protective agent in the step B is 10 to 15 percent mannitol. The invention further provides a temperature-sensitive magnolia biondii pamp nanogel and a preparation method thereof. In the invention, the magnolia biondii pamp volatile oil nanoliposome is prepared into the freeze-dried powder by the freeze-drying technique creatively so as to improve the stability of a medicament and facilitate large-scale preparation and long-term preservation of the liposome.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Depth domain velocity modeling method for igneous rock
InactiveCN110967745ADelicately portrayedPortray realitySeismic signal processingWell loggingImage resolution
The invention discloses a depth domain velocity modeling method for igneous rock. The method comprises: acquiring an initial velocity field and logging stratification data of the igneous rock; inverting a background velocity field based on the initial velocity field and the logging stratification data; based on the background velocity field, improving the resolution and carrying out network chromatography inversion iteration to obtain an initial igneous rock depth domain velocity model; and adding a well-to-seismic error and a construction error into the target function of the initial igneousrock depth domain speed model to obtain a final igneous rock depth domain speed model. According to the invention, the velocity model depicts igneous rock more finely, and the overall velocity changeis more reasonable. After the model is used for depth migration, the influence of igneous rock on an underlying stratum can be well eliminated, false structures and false fractures are eliminated, andthe real structural form and characteristics are recovered. And meanwhile, the tiny cracks are depicted more clearly and truly, the fracture-cavity energy is more focused, the imaging effect is better, and a good effect is achieved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Hydrochloric-acid tropisetron freeze-dried powder injection for injection and preparation method thereof
ActiveCN105125505APrevent oxidationImprove stabilityPowder deliveryDigestive systemFreeze-dryingBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a hydrochloric-acid tropisetron freeze-dried powder injection for injection and a preparation method thereof. The hydrochloric-acid tropisetron freeze-dried powder injection comprises a medicinal active ingredient, excipients and pH (potential of hydrogen) stabilizers, wherein the medicinal active ingredient is hydrochloric-acid tropisetron, the excipients include mannitol and sodium chloride, and the pH stabilizers include citric acid and sodium citrate. According to the hydrochloric-acid tropisetron freeze-dried powder injection, the mannitol and the sodium chloride serve as the excipients, the pH value of a midbody solution is controlled to be stabilized within 4.6-5.2 by citric acid and sodium citrate buffer solutions, oxidation of a main drug is inhibited, and stability of the main drug is improved; in six months of an acceleration test, the injection is stable in content, changes of related substances are small, and the storage period of the injection is prolonged. The preparation method is simple in process and suitable for industrial production.
Owner:REYOUNG PHARMA
Point cloud denoising method combining feature detection method and vertex updating method
ActiveCN111640072ARecovery propertiesRestore sharp featuresImage enhancementInternal combustion piston enginesTensor votingPoint cloud
The invention discloses a point cloud denoising method combining a feature detection method and a vertex updating method, and the method comprises the steps: firstly defining a new discrete operator,called an anisotropic second-order operator, on a point cloud, and enabling the new discrete operator to serve as a regular term in optimization to recover a normal vector field of the point cloud; secondly, based on the normal vector field optimized in the previous step, providing a feature point detection method based on bilateral tensor voting, and performing feature point detection and classification; then, utilizing the classification feature points detected in the previous step, and calculating for each feature point based on a RanSAC algorithm to obtain multiple normal vectors; and finally, performing vertex updating by using the multiple normal vector information so as to obtain denoised point cloud data. Compared with the prior art, the point cloud denoising method has the advantages that a nonlinear smooth area can be better recovered while sharp geometrical characteristics are kept, and an ideal denoising effect is achieved.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Omeprazole sodium freeze-dried powder injection and preparation method thereof
PendingCN112807282AHigh content of the main drugRecovery propertiesOrganic active ingredientsPowder deliveryOmeprazole SodiumXylylene
The invention provides an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The preparation method comprises the following steps of: (1) adding sodium xylene sulfonate and sodium hydrogen sulfite into sterile injection water, stirring and dissolving to obtain first mixed solution; (2) adding omeprazole sodium into the sterile injection water, stirring and dissolving at the normal temperature to obtain second mixed solution; (3) adding the first mixed solution obtained in the step (1) into the second mixed solution obtained in the step (2) to obtain total mixed solution, adding the sterile injection water, then adding sodium citrate to adjust the pH value, and carrying out stirring and dissolving, wherein the mass ratio of the total mixed solution to the sterile injection water is 1: (8-12); and (4) filtering the mixed solution prepared in the step (3), pouring the filtered mixed solution into a glass bottle, and freeze-drying to obtain the omeprazole sodium freeze-dried powder injection. The omeprazole sodium freeze-dried powder injection prepared by the invention is clear in solution, good in stability and high in main drug content.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Organic preparation for sterilizing vegetable continuous cropping obstacles sick soil and application
InactiveCN107033914ARecovery propertiesEfficient killingAgriculture tools and machinesOrganic fertilisersContinuous croppingOxidation-Reduction Agent
The invention relates to an organic preparation for sterilizing vegetable continuous cropping obstacles sick soil and application. The organicpreparation is a composition of molasses and one or more dry powder of the following plant materials: reed, potato rattan, straw, wheat straw, pea straw, bagasse, broad bean straw and corn straw. The weight percent of the molasses is 0-100%. Before use, the dry plant powder and the molasses are respectively packed and are used sequentially. A use method comprises the following steps: uniformly spreading the dry plant powder or the molasses on a vegetable ground with continuous cropping obstacles according to a proportion set in advance, carrying out tillage by using a rotary cultivator, and mixing and buring the plant powder or the molasses in a soil plough layer; uniformly watering in 30-80 tons in per mu, and keeping soil moist; and covering the land by using plastic films to isolate the soil from air; controlling the temperature of the soil to be 25-50 DEG C, and controlling the redox potential to be minus 60 mv or below to form an anaerobic intense oxidation reduction state; and removing the films after keeping the anaerobic intense oxidation reduction state for 3-4 weeks. Fusariumoxysporum, root-knot nematode and the like in the soil can be killed effectively, and continuous cropping obstacles are eliminated.
Owner:云南丽然农业科技发展有限公司
Freeze dried monophosphate adenine arabinoside powder injection for injection and preparation method thereof
ActiveCN102389403BProduct quality is stable and controllableSimple operation processOrganic active ingredientsPowder deliveryDrugs solutionMANNITOL/SORBITOL
The invention relates to freeze dried monophosphate adenine arabinoside powder injection for injection and a preparation method thereof. The freeze dried powder injection is prepared by freeze-drying a monophosphate adenine arabinoside liquid, wherein, the monophosphate adenine arabinoside liquid comprises 100 g of monophosphate adenine arabinoside, 25 g of mannitol, 3 g of disodium hydrogen phosphate, 0.3 g of sodium dihydrogen phosphate, 1 g of ethylenediaminetetraacetic acid, and 2000 mL water for injection. The preparation method comprises the following three steps: prefreezing, sublimating, and heating drying. Compared with common water injection in the prior art, the freeze dried powder injection and its preparation method disclosed herein have remarkable advantages in storage and transportation.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
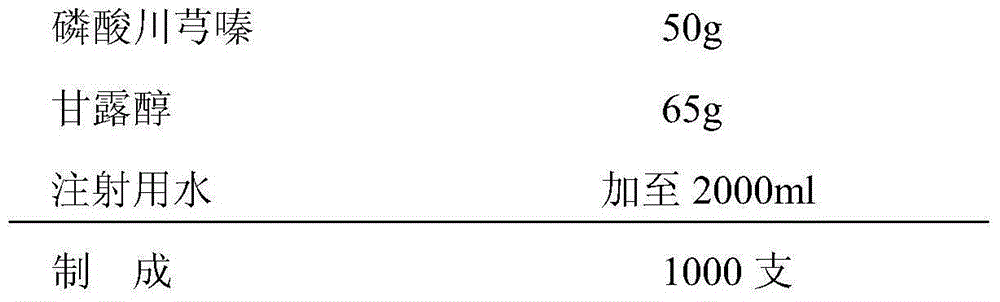
Freeze-dried powder injection of ligustrazine phosphate for injection and preparation method thereof
InactiveCN105213328AImprove stabilityLittle changePowder deliveryOrganic active ingredientsFreeze-dryingMass ratio
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to a freeze-dried powder injection of ligustrazine phosphate for injection and a preparation method thereof. The freeze-dried powder injection is prepared from ligustrazine phosphate and mannitol according to the mass ratio of 1 to 0.90-1.5. The stability of the freeze-dried powder injection is remarkably improved compared with that of liquid injections; moreover, since glycine and other substances used as stabilizers are omitted, the safety of medication is improved. The invention further provides the preparation method of the freeze-dried powder injection. According to the preparation method, mannitol is used as the excipient, phosphoric acid solution is used for regulating pH to be between 2.0 and 3.0, and therefore the stability of basic remedy is improved; acceleration tests show that within six months, the product is stable in content, related substances change a little, and therefore the storage period of the preparations is prolonged; the preparation method is simple in process and suitable for industrial production.
Owner:REYOUNG PHARMA
Hydrochloric acid diltiazem freeze-dried powder injection for injections and preparation method thereof
ActiveCN100563633CImprove stabilityReduced stabilityOrganic active ingredientsPowder deliveryMannitolPharmacology
The invention provides a diltiazem hydrochloride freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection comprises diltiazem hydrochloride and mannite, the proportion by weight of diltiazem hydrochloride and mannite is 1: 1 to 8. The freeze-dried powder injection has favorable clarity and stability and high content of main drug, thereby being freeze-dried powder injection with good quality.
Owner:HAINAN JINRUI PHARMA CO LTD
Pharmaceutical composition containing calcium folinate and fluorouracil
InactiveCN103393688AUniform colorSmall side effectsOrganic active ingredientsPharmaceutical non-active ingredientsDrugFluorouracil Injection
The invention relates to a pharmaceutical composition containing calcium folinate and fluorouracil, and particularly relates to a combined application package comprising a calcium folinate injection and an injection containing fluorouracil. When the combined application package is in use, the calcium folinate injection is intravenously injected and then the fluorouracil injection is intravenously injected.
Owner:HAINAN LINGKANG PHARMA CO LTD
Grease lubrication bearing system
InactiveCN107701901ASolve the technical problem of lubrication without cooling functionAvoid pollutionRolling contact bearingsShaftsEngineeringLubrication
The invention discloses a grease lubrication bearing system. The grease lubrication bearing system comprises a bearing structure and a lubricating grease circular drive device; according to the bearing structure, a lubricating space used for containing lubricating oil is arranged between the outer ring and the inner ring of the bearing structure, an oil injection hole and an oil draining hole communicating with the lubricating space are formed in the bearing structure, the oil draining hole is connected with a grease absorbing opening of the lubricating grease circular drive device, a grease outlet of the lubricating grease circular drive device is connected with the oil injection hole of the bearing, and a lubricating grease filter device and / or a cooling device are / is arranged between the lubricating grease circular drive device and the oil injection hole in a series manner. The lubricating grease filter device and / or a cooling device are / is arranged, harmful substance can be cleanedthrough the lubricating grease circular device, the optimal lubricating state can be maintained, the time of the optimal lubricating state is prolonged, the good lubricating is obtained, the servicelife of the bearing is prolonged, through the cooling device, the lubricating grease temperature is reduced, the lubricating grease liquidity is improved, the lubricating grease is injected into the bearing again, and the lubricating grease characteristic effect can be restored.
Owner:AUTOL TECH
Red sage root ligustrazine freeze dried injecta and preparation method thereof
InactiveCN100361672CGood curative effectQuality improvementOrganic active ingredientsPowder deliveryFreeze-dryingCurative effect
The invention provides a red sage root ligustrazine freeze dried injecta and its preparation, wherein the injection is in powder form, and comprises red sage root extract, Chuangxiongzine Hydrochlorid and right amount of filling agent by the weight proportion of 400-1100:50-150:0-200, the preparing process comprises extracting red sage root liquid, configuring the soup, freeze drying and packaging.
Owner:CSPC OUYI PHARM CO LTD
Cyclic adenosine meglumine freeze-dried powder for injection and preparation method thereof
ActiveCN105213329BImprove stabilityLittle changeOrganic active ingredientsPowder deliveryFreeze-dryingAdenosine
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to a lyophilized adenosine monophosphate meglumine powder for injection and a preparation method thereof. It is made of cyclic adenosine monophosphate and meglumine, and the mass ratio of cyclic adenosine monophosphate and meglumine is 1:0.55‑0.65. The invention does not need to add glycine, mannitol, sodium chloride, sodium citrate, citric acid, disodium hydrogen phosphate, etc., and improves the safety and stability of medication. The present invention also provides a preparation method thereof, first using cyclic adenosine monophosphate and meglumine in a molar ratio of 1:1 to form a salt, and then using meglumine to adjust the pH to 5.8-7.0, which improves the stability of the main drug. According to the test, the content of the product is stable within 6 months, the change of related substances is small, and the storage period of the preparation is prolonged. The preparation method has simple process and is suitable for industrial production.
Owner:REYOUNG PHARMA
Soil continuous cropping protector and preparation method and application thereof
InactiveCN102766470BRecovery propertiesCorrect acid-base balanceOrganic fertilisersSoil conditioning compositionsContinuous croppingLicorice roots
Owner:张鹰
Calcium folinate and phenyl alanine composition and preparation method thereof
ActiveCN101780083BSmall toxicityImprove anti-tumor activityPowder deliveryOrganic active ingredientsLeucovorin CalciumPhenylalanine
Owner:BEIJING SIHUAN PHARMA +1
Vidarabine monophosphate freeze-dried powder injection for injection and preparation method thereof
ActiveCN101642440BProduct quality is stable and controllableSimple operation processOrganic active ingredientsPowder deliveryDrugs solutionMANNITOL/SORBITOL
The invention relates to a vidarabine monophosphate freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is obtained by freeze-drying a vidarabine monophosphate drug solution. The formula is: 100 g of vidarabine monophosphate, 25 g of mannitol, 3 g of disodium hydrogen phosphate, 0.3 g of sodium dihydrogen phosphate, 1 g of disodium edetate, and add water for injection to 2000 mL. The freeze-drying process in the preparation method of the freeze-dried powder injection includes three processes of pre-freezing, sublimation and temperature-rising drying. The freeze-dried powder injection and the preparation method thereof of the present invention have significant advantages in storage and transportation compared with the commonly used water injection in the prior art.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Ambroxol hydrochloride freeze-dried powder injection and preparing method thereof
ActiveCN100506217CAvoid interactionImprove stabilityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
The invention relates to a freeze-dried powder injection of ambroxol hydrochloride and a preparation method thereof. The freeze-dried powder injection is composed of ambroxol hydrochloride and mannitol, and the ambroxol hydrochloride freeze-dried powder injection is obtained by a freeze-drying process. Good stability, fast dissolution, and other indicators are in compliance with the regulations.
Owner:SHANDONG YUXIN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com