Freeze-dried powder injection of ligustrazine phosphate for injection and preparation method thereof
A technology of ligustrazine phosphate and freeze-dried powder injection, which is applied in freeze-dried delivery, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem that the safety of stabilizers is not fully guaranteed and affects The problems of product solubility, hygroscopicity, and poor product stability have achieved the effect of good resolubility, suitable for industrial production, and improved drug safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
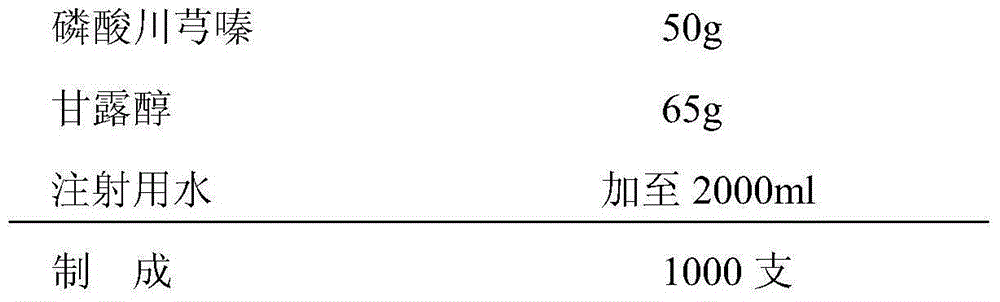
[0028] The formula of described ligustrazine phosphate freeze-dried powder for injection consists of:
[0029]
[0030] Its preparation method:
[0031] (1) Add the prescribed amount of water for injection into the batching tank.
[0032] (2) Weigh the prescribed amount of mannitol, add it into the batching tank, stir and dissolve, and cool down to below 25°C.
[0033] (3) Weigh the prescribed amount of Ligustrazine Phosphate into the batching tank and stir until fully dissolved, and control the pH at 2.0-3.0. If the pH>3.0, add phosphoric acid solution to adjust the pH to 2.0-3.0.
[0034] (4) Filling after sterilization and filtration
[0035] (5) Drugs are loaded into a freeze dryer and pre-frozen at -42°C for 1.5 hours, raised to -5°C for 10 hours, raised to 0°C for 2 hours, raised to 15°C for 3 hours.
[0036] (6) Capping, packaging, full inspection, and storage.
[0037]
[0038] Its preparation method:
[0039] (1) Add the prescribed amount of water for injec...
Embodiment 3
[0047]
[0048] Its preparation method:
[0049] (1) Add the prescribed amount of water for injection into the batching tank.
[0050] (2) Weigh the prescribed amount of mannitol, add it into the batching tank, stir and dissolve, and cool down to below 25°C.
[0051] (3) Weigh the prescribed amount of Ligustrazine Phosphate into the batching tank and stir until fully dissolved, and control the pH at 2.0-3.0. If the pH>3.0, add phosphoric acid solution to adjust the pH to 2.0-3.0.
[0052] (4) Filling after sterilizing and filtering.
[0053] (5) Put the drug into the freeze dryer for pre-freezing at -42°C, maintain for 1.8h, raise the medium to -5°C, maintain for 11h, raise the medium to 0°C, maintain for 2.5h, raise the medium to 15°C, and maintain for 3.5h.
[0054] (6) Capping, packaging, full inspection, and storage.
Embodiment 4
[0056]
[0057] Its preparation method:
[0058] (1) Add the prescribed amount of water for injection into the batching tank.
[0059] (2) Weigh the prescribed amount of mannitol, add it to the batching tank, stir and dissolve, and cool down to below 25°C.
[0060] (3) Weigh the prescribed amount of Ligustrazine Phosphate into the batching tank and stir until fully dissolved, and control the pH at 2.0-3.0. If the pH>3.0, add phosphoric acid solution to adjust the pH to 2.0-3.0.
[0061] (4) Filling after sterilizing and filtering.
[0062] (5) Put the drug into the lyophilizer for pre-freezing at -42°C, maintain for 1.5h, raise the medium to -5°C, maintain for 10h, raise the medium to 0°C, maintain for 2h, raise the medium to 15°C, and maintain for 3h.
[0063] (6) Capping, packaging, full inspection, and storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com