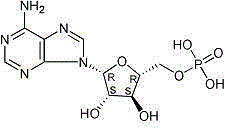
Officinal composition of vidarabine monophosphate for injection
A technology of adenosine vidarabine monophosphate and composition, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Inconvenient, affecting the quality of medicines and other problems, to achieve the effect of good appearance, prevent spray bottles, improve appearance shape and porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0062] Vidarabine Monophosphate 2g
[0063] Lysine 8g
[0064] Mannitol 50g
[0065] Disodium hydrogen phosphate 2g
[0066] Sodium dihydrogen phosphate 0.2g
[0067] A medicinal composition of adenosine monophosphate vidarabine for injection. Take adenosine monophosphate and add appropriate amount of absolute ethanol until it is completely dissolved, add water for injection, then slowly add lysine under stirring, add mannitol, and Dissolve, take a pH regulator (disodium hydrogen phosphate-sodium dihydrogen phosphate) to adjust the pH of the solution within the range of 7.0, stir at room temperature, centrifuge, take the supernatant, filter it with a 0.22 μm microporous membrane, and the filtrate Packed into vials, and then freeze-dried.
[0068] 1000 bottles of the present invention consume 1005ml of water for injection.
[0069] Wherein, the freeze-drying process comprises successively:
[0070] For pre-freezing, put the product into a freeze-drying cabinet that is...
specific Embodiment 2
[0075] Composition of pharmaceutical composition (based on 1000 bottles)
[0076] Vidarabine Monophosphate 2g
[0077] Water-soluble chitosan derivative 10g
[0078] Lysine 2g
[0079] Mannitol 20g
[0080] Disodium hydrogen phosphate 2 g
[0081] Sodium dihydrogen phosphate 0.2g
[0082] A medicinal composition of adenosine monophosphate vidarabine for injection. Take adenosine monophosphate and add an appropriate amount of absolute ethanol until it is completely dissolved, take a water-soluble chitosan derivative and dissolve it in water for injection, and then slowly add lycine while stirring. Amino acid, add mannitol, dissolve all, take pH regulator (disodium hydrogen phosphate-sodium dihydrogen phosphate) to adjust the pH of the solution within the range of 7.0, stir at room temperature, centrifuge, take the supernatant, and use a 0.22μm micropore After aseptic filtration with a filter membrane, the filtrate was divided into vials and then freeze-dried.
[00...
specific Embodiment 3
[0090] Composition of pharmaceutical composition (based on 1000 bottles)
[0091] Vidarabine Monophosphate 2g
[0092] Water-soluble chitosan derivative 10g
[0093] Lysine 2g
[0094] Mannitol 20g
[0095] Disodium hydrogen phosphate 2g
[0096] Citric acid 0.2g
[0097] A medicinal composition of adenosine monophosphate vidarabine for injection. Take adenosine monophosphate and add an appropriate amount of absolute ethanol until it is completely dissolved, take a water-soluble chitosan derivative and dissolve it in water for injection, and then slowly add lycine while stirring. Amino acid, add mannitol, dissolve all, take pH regulator (disodium hydrogen phosphate-citric acid) to adjust the pH of the solution in the range of 7.0 to 7.5, stir at room temperature, centrifuge, take the supernatant, 0.22μm micropore After aseptic filtration with a filter membrane, the filtrate was divided into vials and then freeze-dried.
[0098] The drug solution of vidarabine monophosp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com