Composition of ambroxol hydrochloride and arginine and preparation method thereof
A technology of ambroxol hydrochloride and arginine, applied in the field of medicine, can solve the problems of water solubility and stability of side effects, influence of main drug stability, and high dosage of auxiliary materials, and achieves excellent appearance, low packaging material requirements, and bioavailability. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Ambroxol Hydrochloride 15g
[0036] Arginine 0.15g
[0037] Lactose 70g
[0038] Add water for injection to 2000mL
[0039]
[0040] A total of 1000 bottles were made
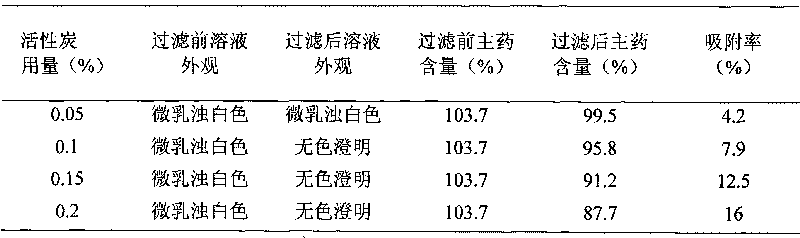
[0041] Weigh 15g of ambroxol hydrochloride and 0.15g of arginine, put them in a suitable container, add about 1500mL of water for injection, stir to dissolve completely, add 70g of lactose, stir to dissolve, add water for injection to 2000mL, add 0.1% (g / mL) activated carbon for injection and stirred for 30 minutes, filtered through a Buchner funnel to decarbonize, filtered through a No. 4 vertical fusion filter ball, filtered through a 0.22 μm filter membrane until clear, and filled in antibiotic glass at a volume of 2 mL per bottle Bottles are half-plugged with butyl rubber stoppers, plated, and sent to pre-freezing boxes for pre-freezing treatment. When the temperature of the material drops to 10-20°C below the eutectic point, quickly take out the sample to the freeze-dr...
Embodiment 2
[0043] Ambroxol Hydrochloride 15g
[0044] Arginine 0.15g
[0045] Mannitol 70g
[0046] Add water for injection to 2000mL
[0047]
[0048] A total of 1000 bottles were made
[0049] Weigh 15g of ambroxol hydrochloride and 0.15g of arginine, put them in a suitable container, add about 1500mL of water for injection, stir to dissolve completely, add 70g of mannitol, stir to dissolve, add water for injection to 2000mL, and the rest are the same as Example 1.
Embodiment 3
[0051] Ambroxol Hydrochloride 15g
[0052] Arginine 0.75g
[0053] Lactose 70g
[0054] Add water for injection to 2000mL
[0055]
[0056] A total of 1000 bottles were made
[0057] Weigh 15g of ambroxol hydrochloride and 0.75g of arginine, put them in a suitable container, add about 1500mL of water for injection, stir to dissolve completely, add 70g of lactose, stir to dissolve, add water for injection to 2000mL, and implement the rest in the same way example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com