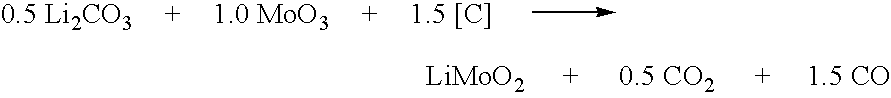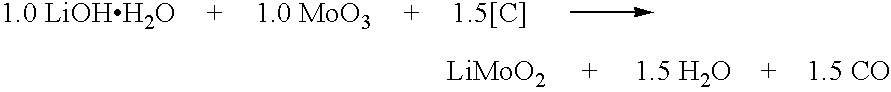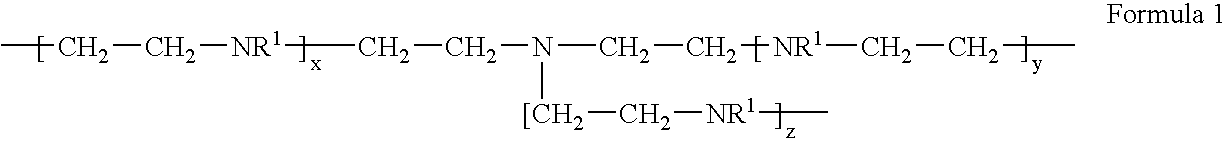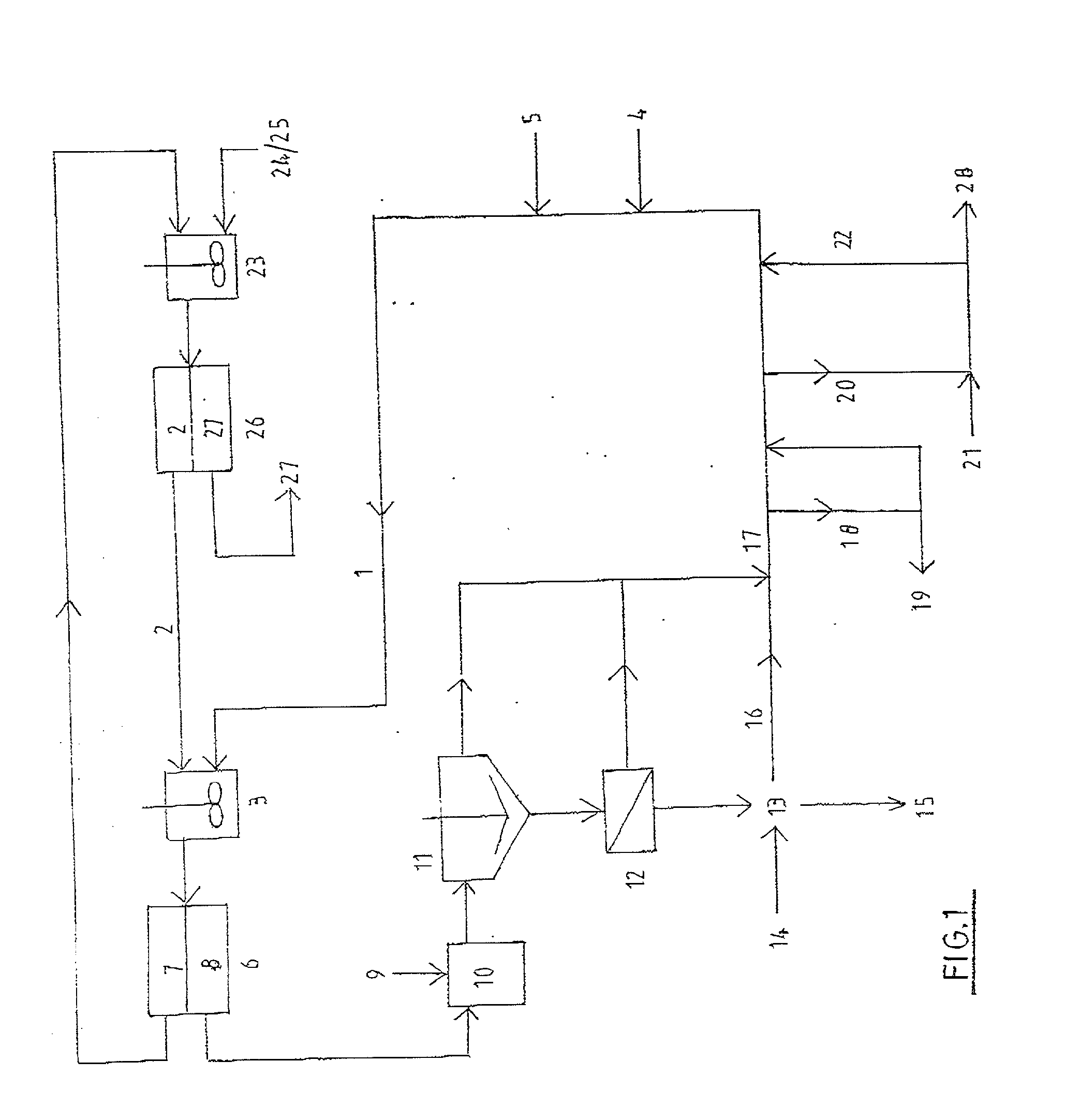Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
230results about "Magnesium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
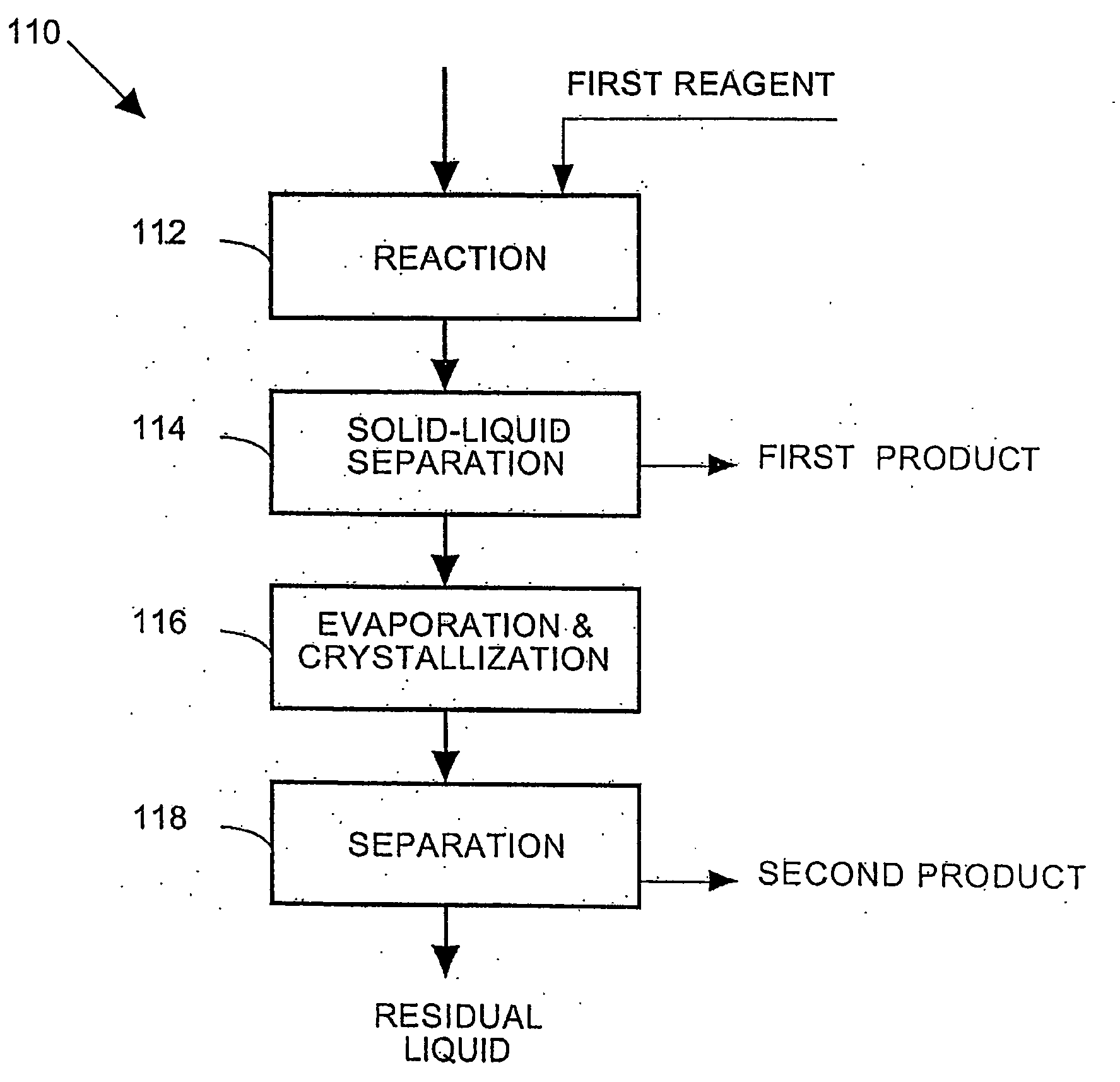
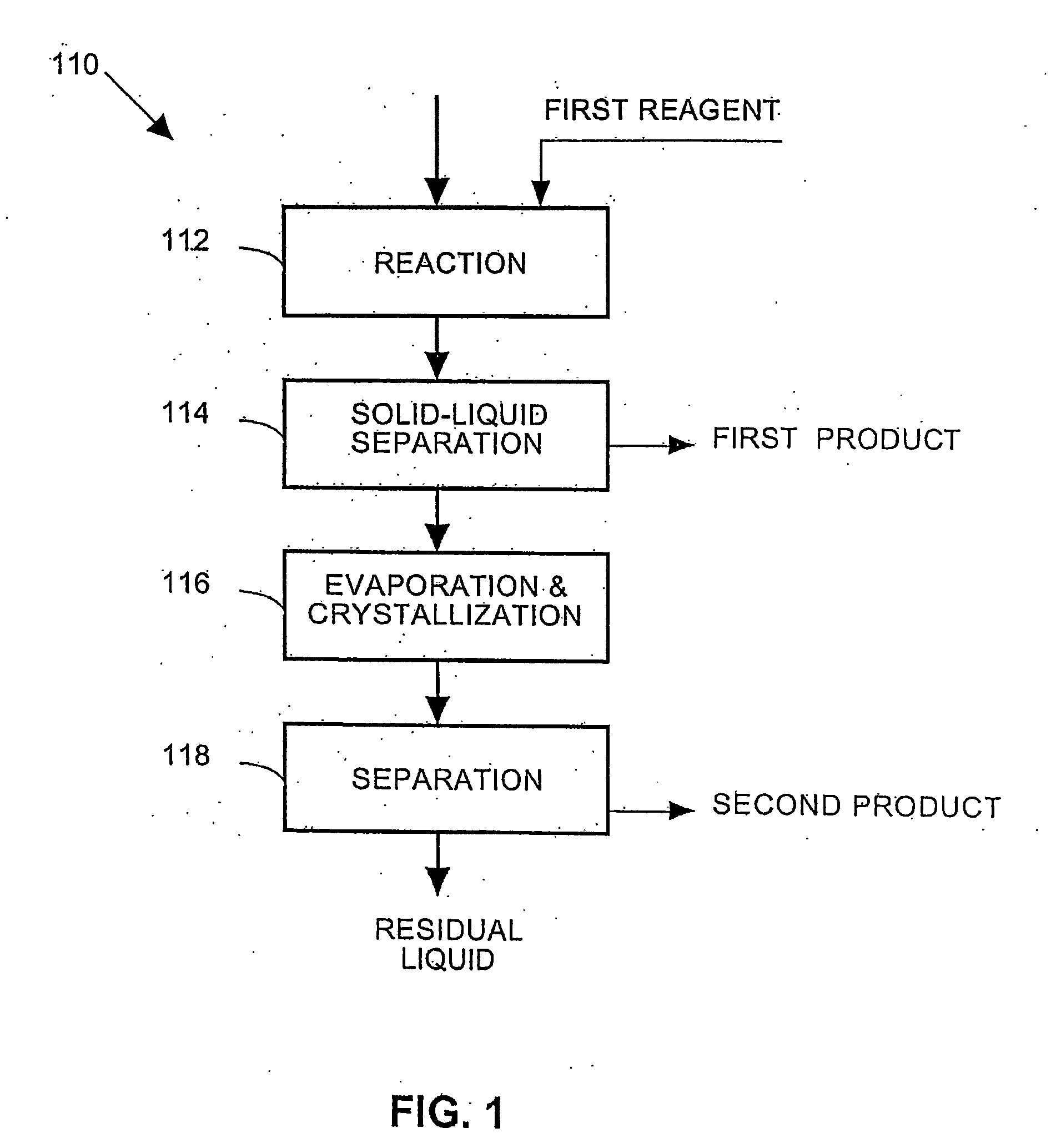
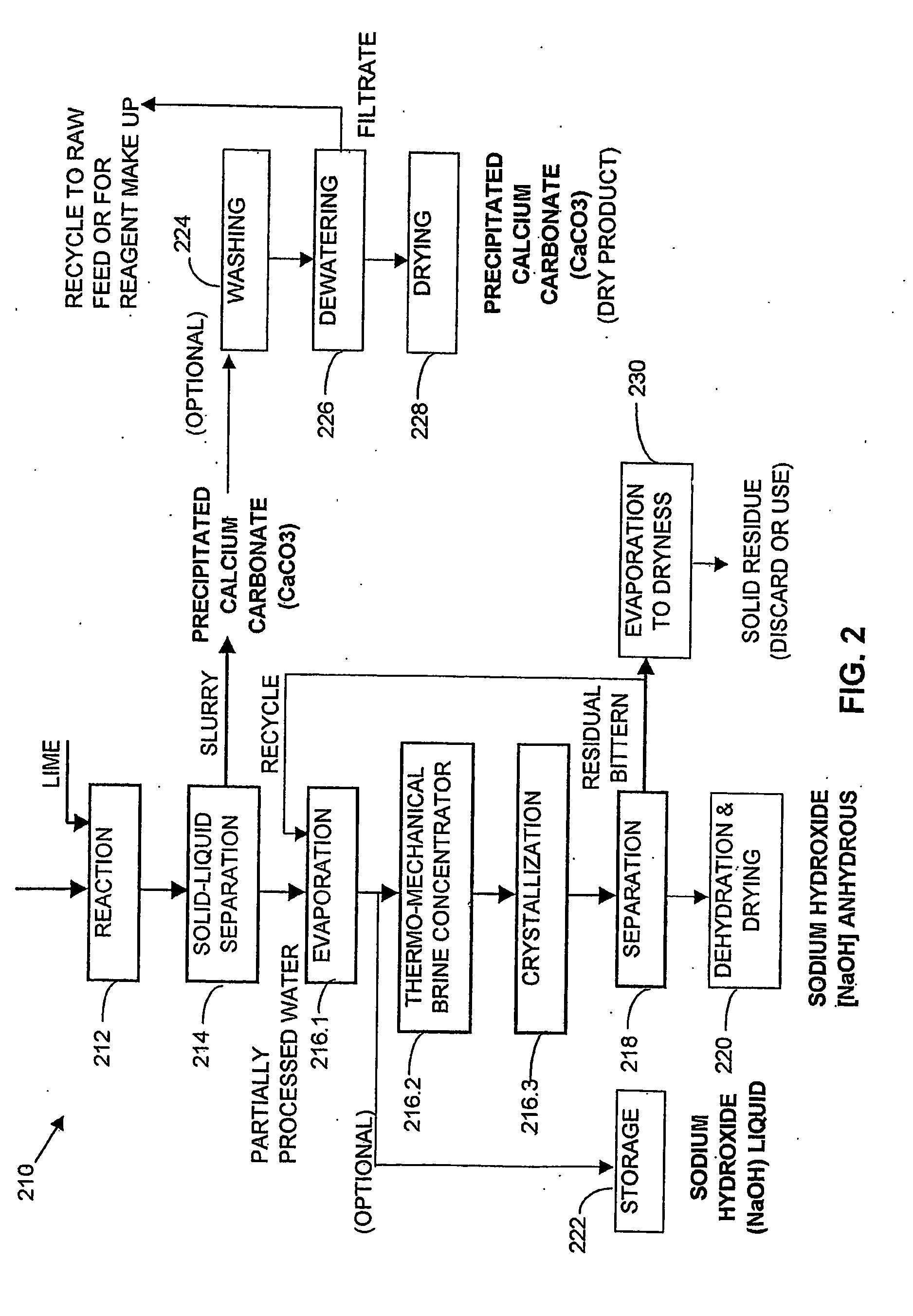
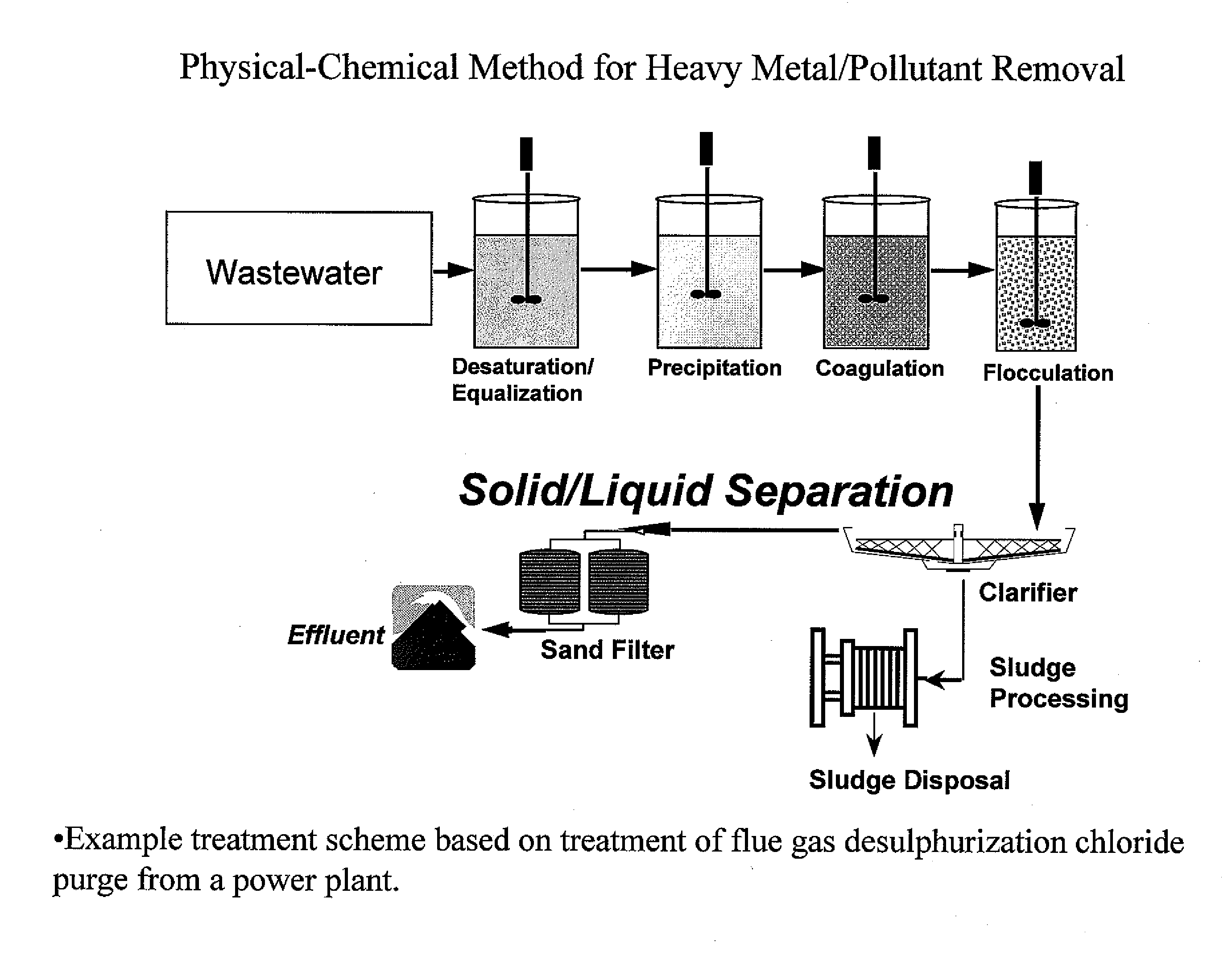
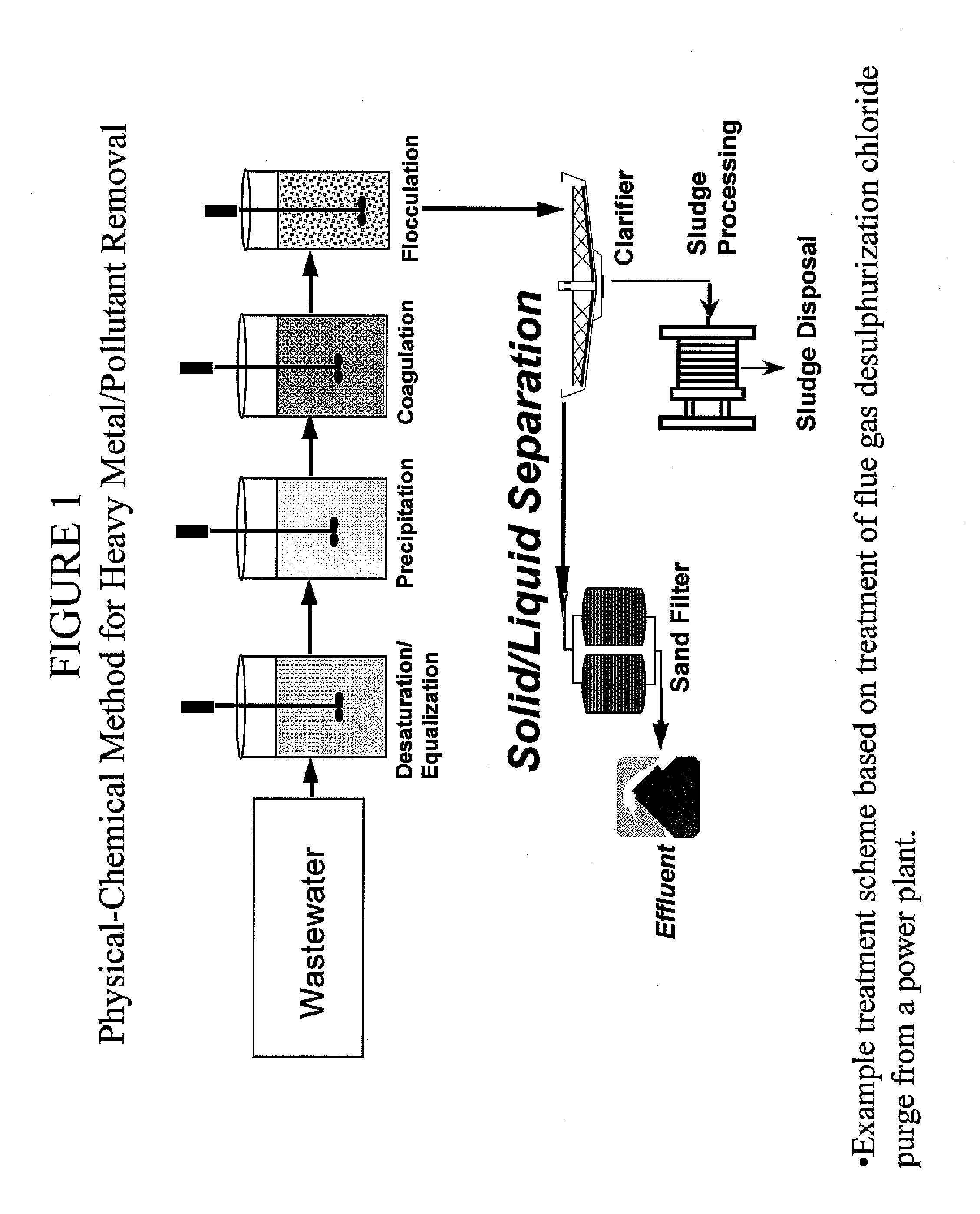
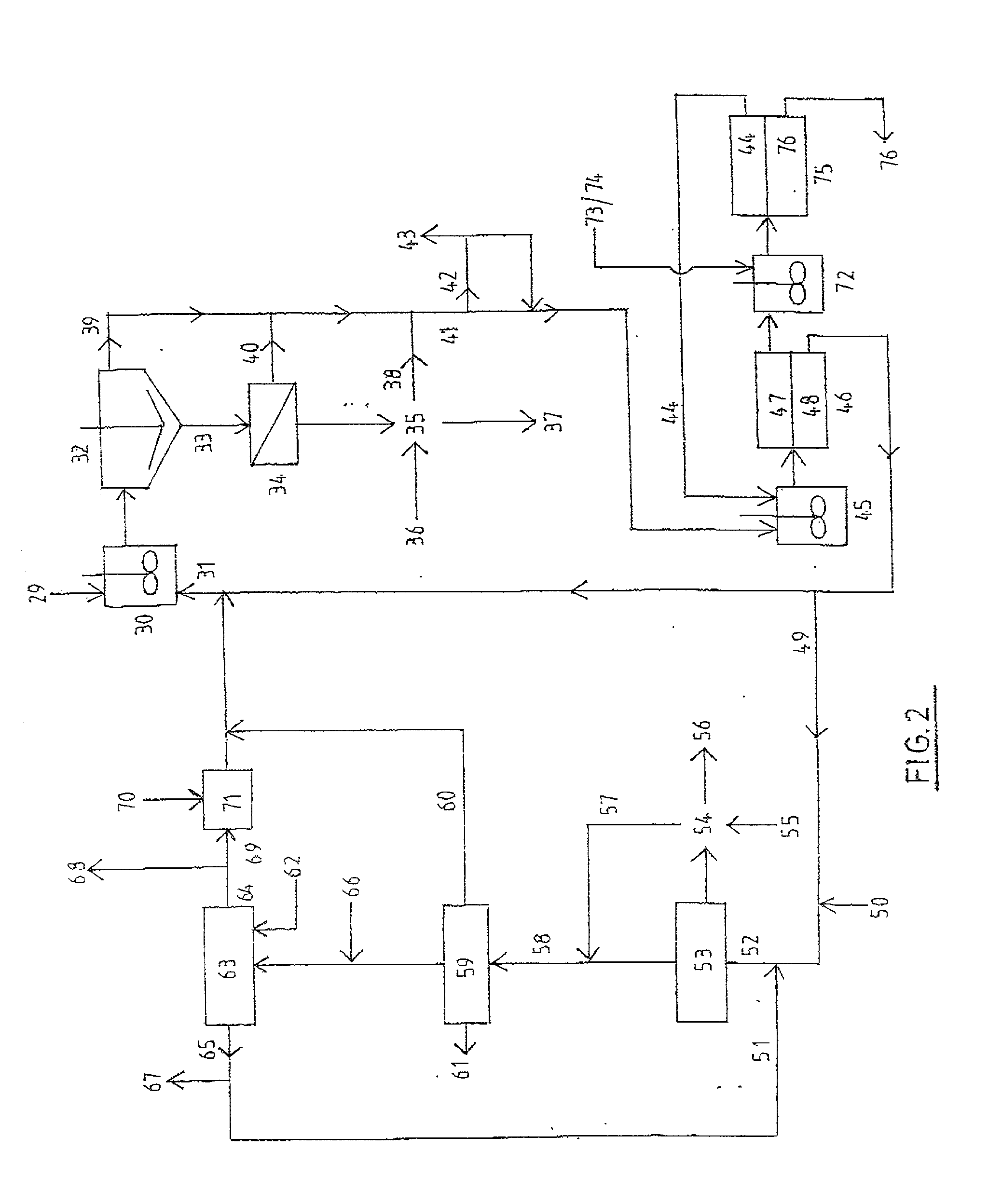
Process and apparatus for the treatment of saline water
InactiveUS20060196836A1Reduce ZLD costReduce loadWaste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
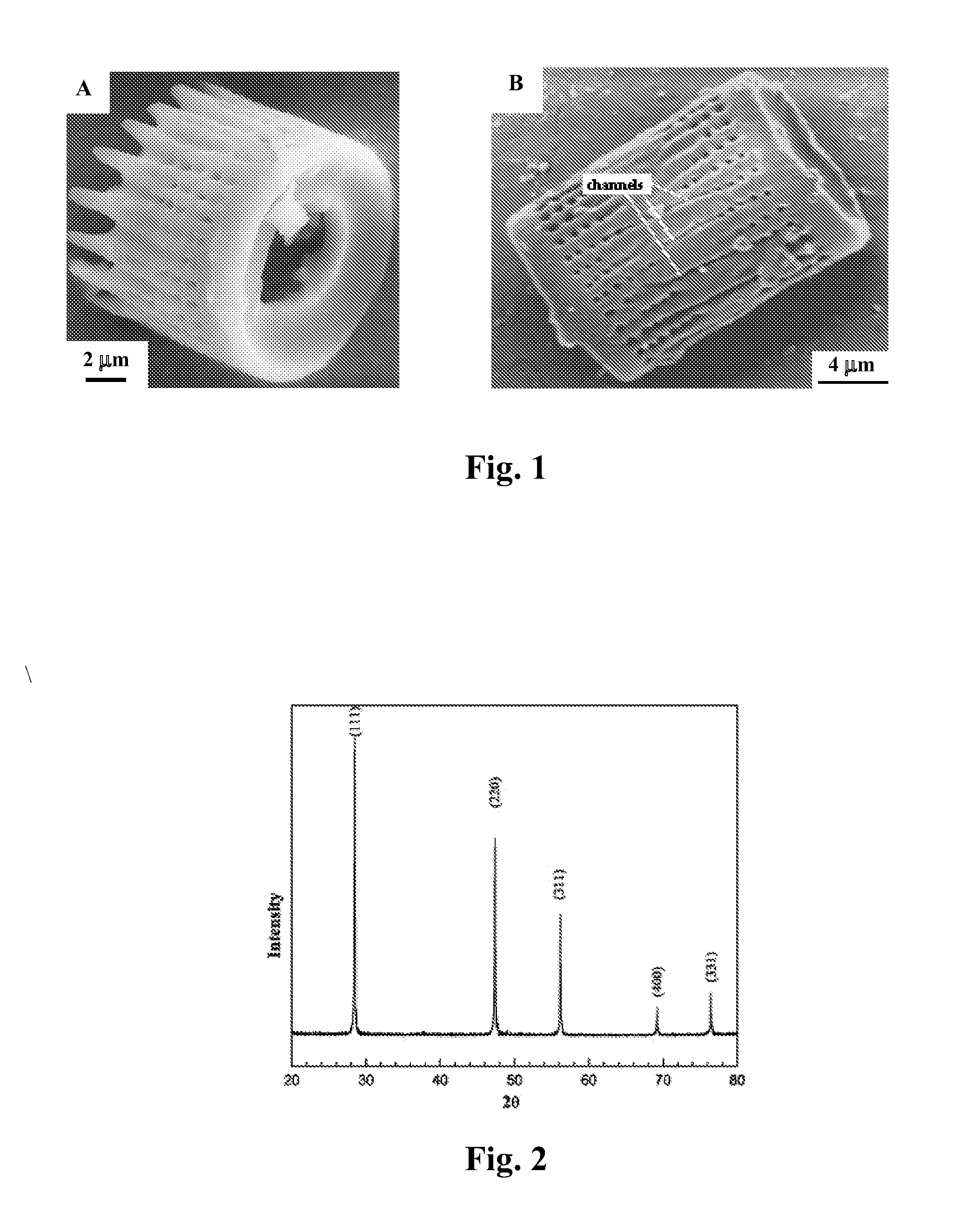
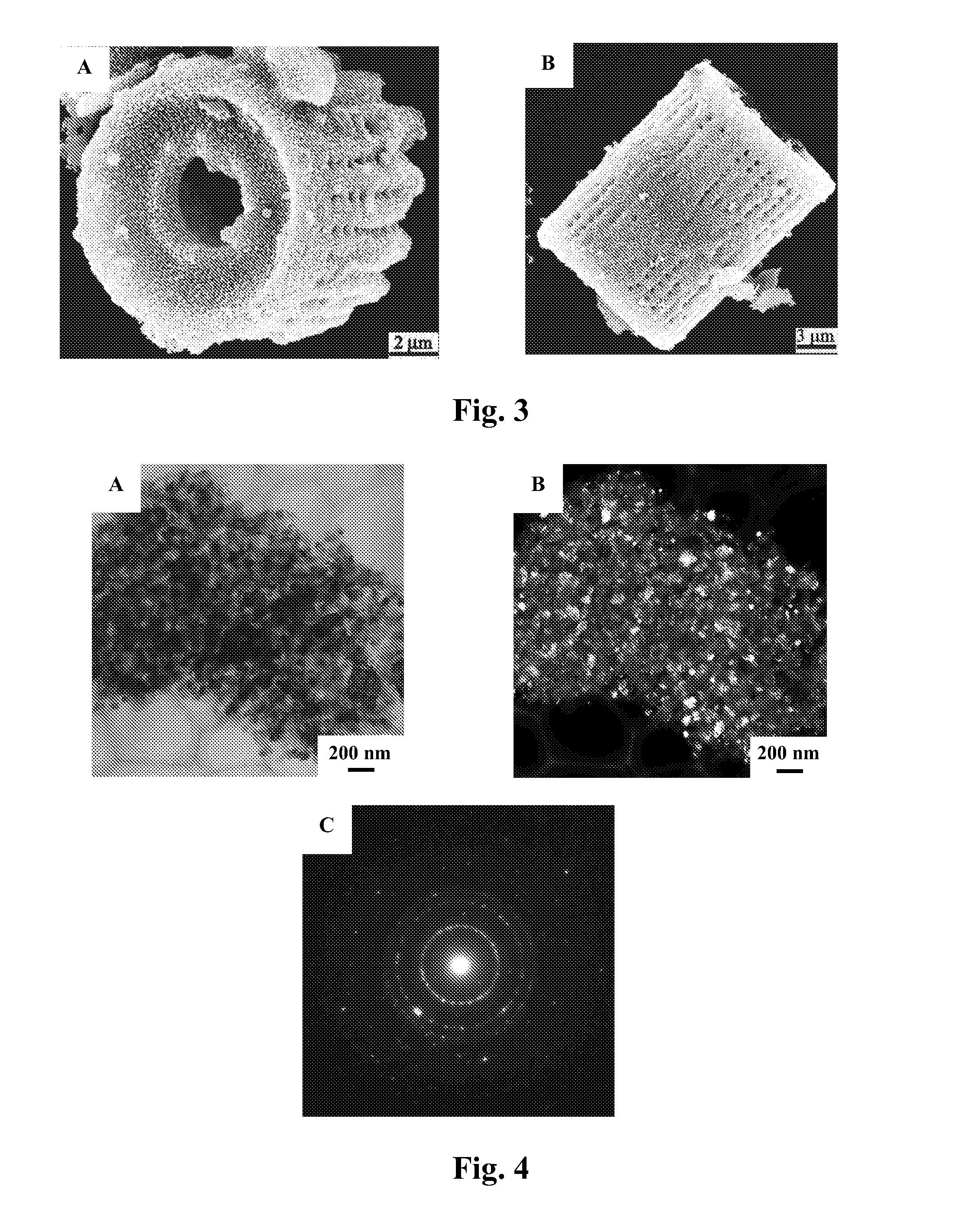
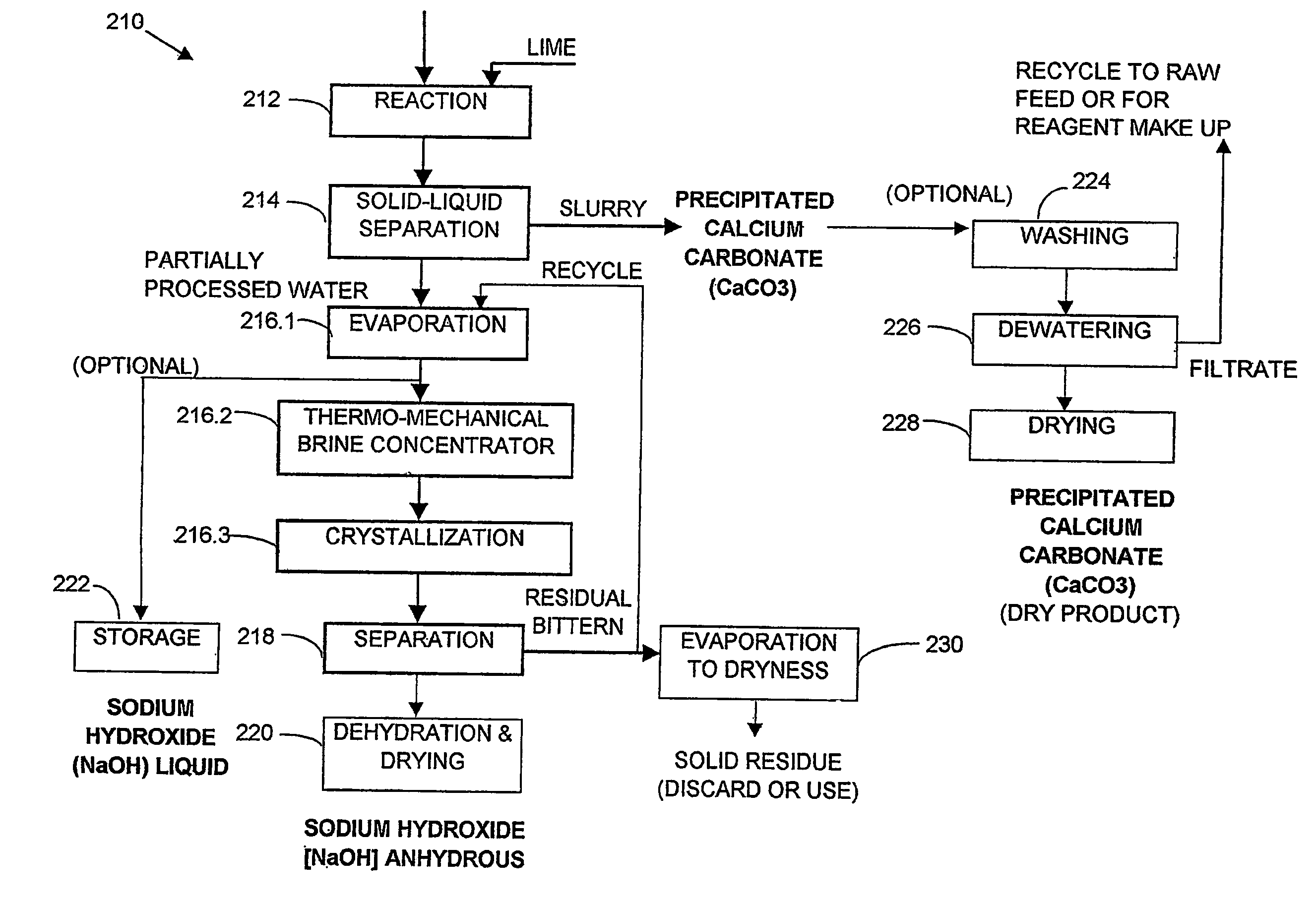
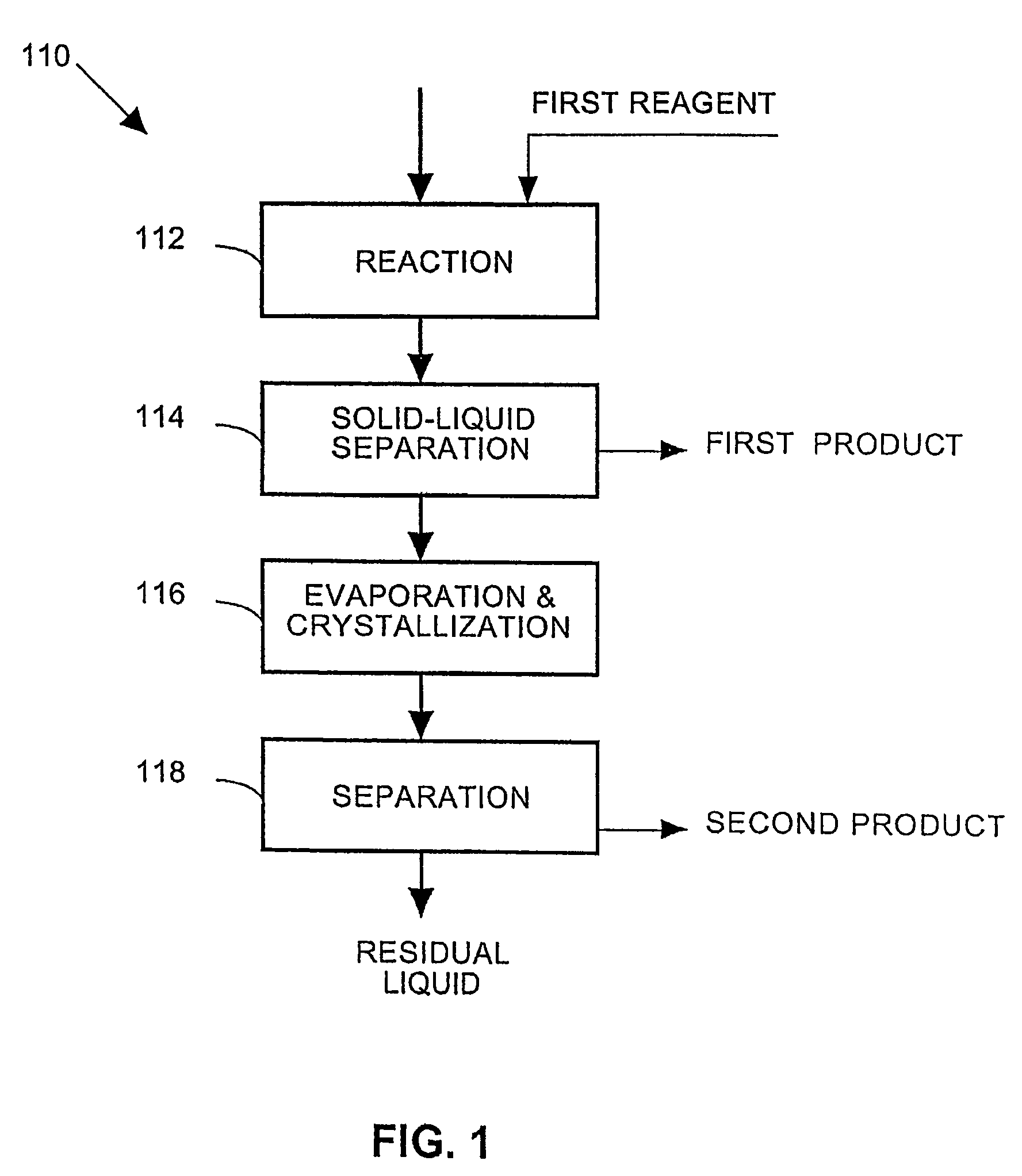
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
Methods of fabricating nanoscale-to-microscale structures
InactiveUS20080038170A1Attractive and optical propertyAttractive mechanical propertySilicaMixing methodsChemical compositionChemical reaction
Methods for the production of shaped nanoscale-to-microscale structures, wherein a nanoscale-to-microscale template is provided having an original chemical composition and an original shape, and the nanoscale-to-microscale template subjected to a chemical reaction, so as to partially or completely convert the nanoscale-to-microscale template into the shaped nanoscale-to-microscale structure having a chemical composition different than the original chemical composition and having substantially the same shape as the original shape, being a scaled version of the original shape. The shaped nanoscale-to-microscale structure formed comprises an element (such as silicon), a metallic alloy (such as a silicon alloy), or a non-oxide compound (such as silicon carbide or silicon nitride).
Owner:GEORGIA TECH RES CORP
Process for the treatment of saline water
InactiveUS7595001B2Waste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
Methods of fabricating nanoscale-to-microscale structures
InactiveUS7615206B2Attractive electronic and optical propertyAttractive mechanical propertySilicaMixing methodsChemical reactionChemical composition
Methods for the production of shaped nanoscale-to-microscale structures, wherein a nanoscale-to-microscale template is provided having an original chemical composition and an original shape, and the nanoscale-to-microscale template subjected to a chemical reaction, so as to partially or completely convert the nanoscale-to-microscale template into the shaped nanoscale-to-microscale structure having a chemical composition different than the original chemical composition and having substantially the same shape as the original shape, being a scaled version of the original shape. The shaped nanoscale-to-microscale structure formed comprises an element (such as silicon), a metallic alloy (such as a silicon alloy), or a non-oxide compound (such as silicon carbide or silicon nitride).
Owner:GEORGIA TECH RES CORP
Compositions and methods for generating hydrogen from water
InactiveUS20050232837A1Easy to separatePromote recoveryPhysical/chemical process catalystsCalcium/strontium/barium compoundsInorganic saltsHydrogen
The present invention relates to methods, compositions and systems for producing hydrogen from water involving reacting metal particles with water in the presence of an effective amount of catalyst. In particular the invention pertains to methods, compositions and systems for producing hydrogen upon reaction of metal particles selected from the group consisting of aluminum (Al), magnesium (Mg), silicon (Si) and zinc (Zn) with water, in the presence of an effective amount of a catalyst, wherein the catalyst is a water-soluble inorganic salt.
Owner:THE UNIV OF BRITISH COLUMBIA
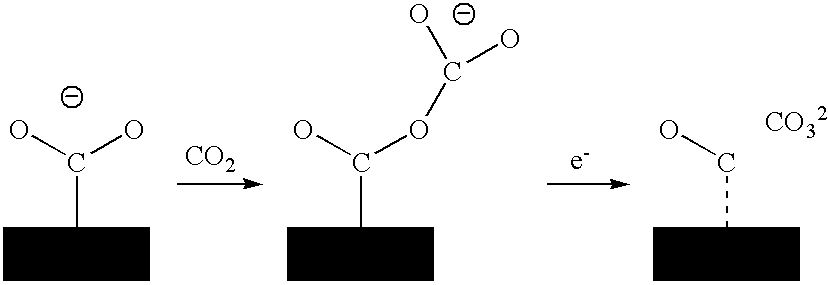
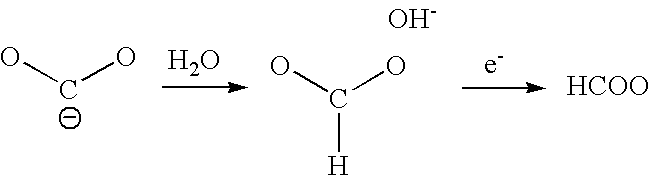
Process For Sequestrating Carbon In The Form Of A Mineral In Which The Carbon Has Oxidation Number +3
A process for sequestrating carbon emitted into the atmosphere in the form of CO2 comprises:a) a step for concentrating CO2 in the liquid phase;b) a step for electro-reduction in an aprotic medium to a compound in which the carbon changes to oxidation number +3 in the form of oxalic acid or formic acid;c) if appropriate, a step for re-extracting oxalic or formic acid in the aqueous phase; andd) a step for mineralization by reaction with a compound of an element M, resulting in a stable compound in which the atomic ratio C / M is about 2 / 1.
Owner:INST FR DU PETROLE
Synthesis of Metal Compounds Under Carbothermal Conditions
InactiveUS20050255026A1Economical and convenient processLower capability requirementsPhosphatesLithium compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4-11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV +1
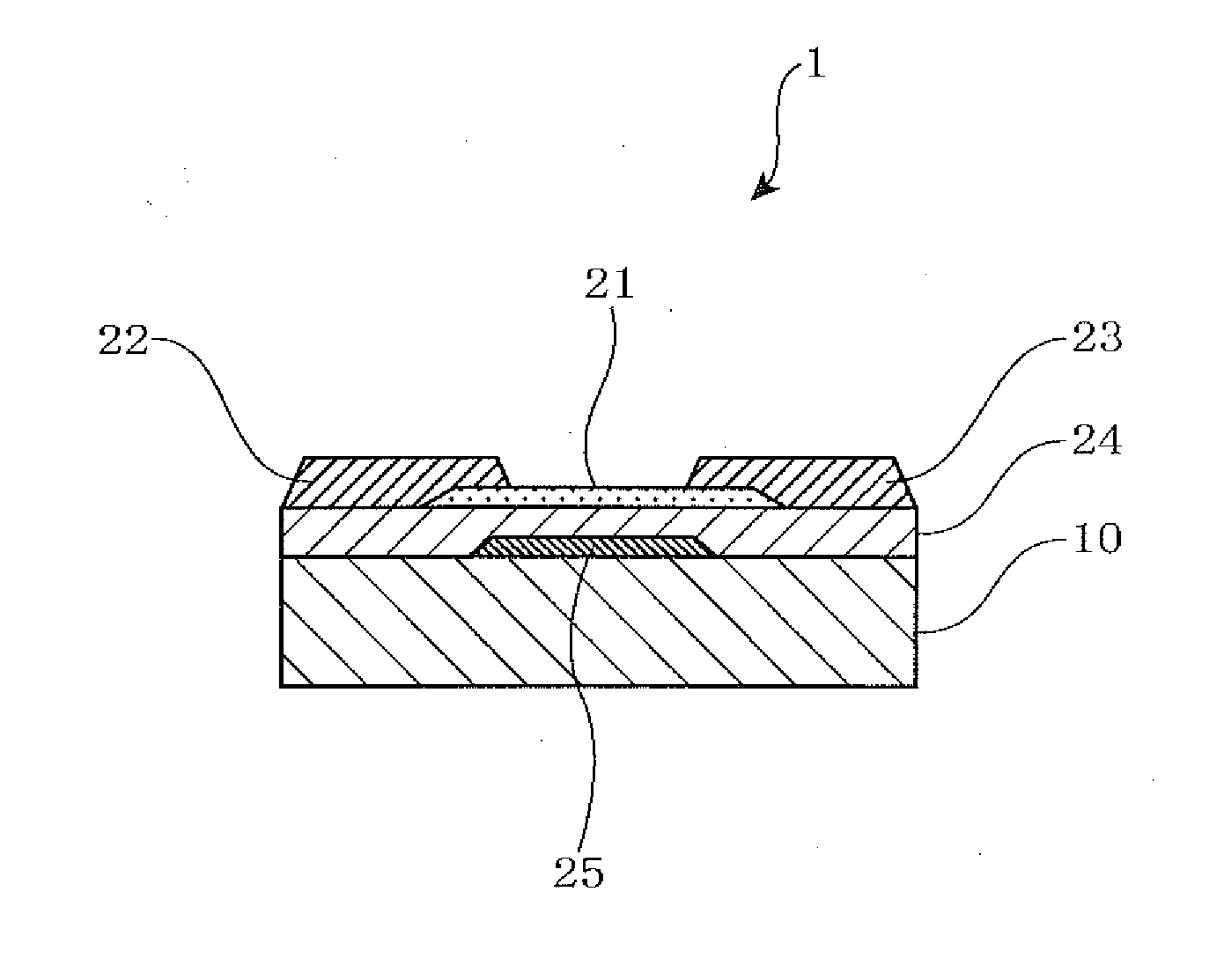
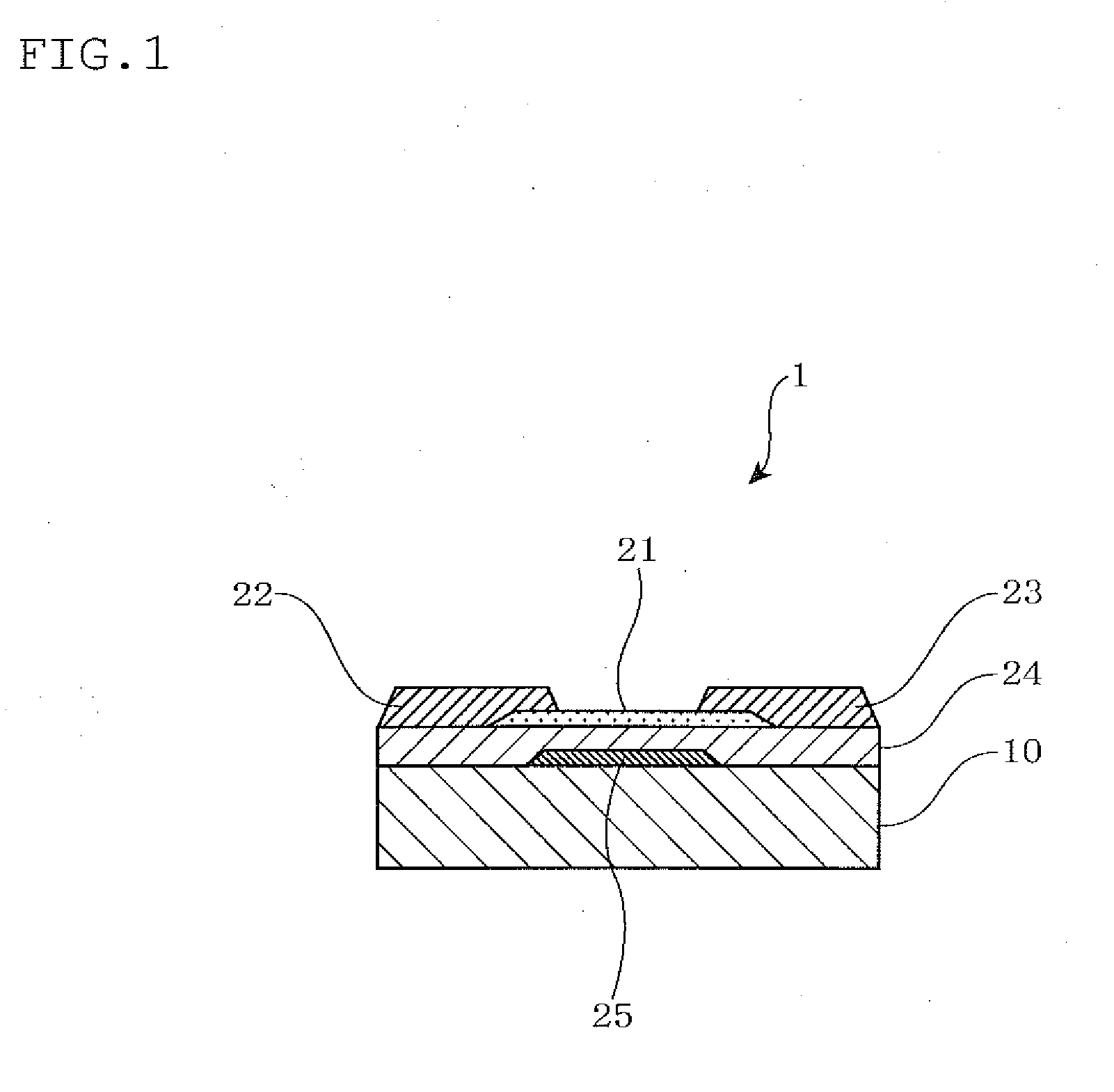
Sputtering target, oxide semiconductor film and semiconductor device
A sputtering target including an oxide sintered body, the oxide sintered body containing indium (In) and at least one element selected from gadolinium (Gd), dysprosium (Dy), holmium (Ho), erbium (Er) and ytterbium (Yb), and the oxide sintered body substantially being of a bixbyite structure.
Owner:IDEMITSU KOSAN CO LTD
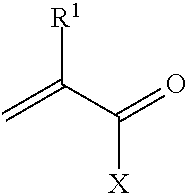
Metal scavenging polymers and uses thereof
ActiveUS20110243819A1Dispersed particle filtrationSelenium/tellurium compundsWastewater systemsMonomer
Uses for a composition comprising a polymer derived from at least two monomers: acrylic-x and an alkylamine, wherein said polymer is modified to contain a functional group capable of scavenging one or more compositions containing one or more metals are disclosed. These polymers have many uses in various mediums, including wastewater systems.
Owner:ECOLAB USA INC
Synthesis of metal compounds under carbothermal conditions
InactiveUS7060206B2Economical and convenient processLower capability requirementsCell electrodesSulfur compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4–11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV
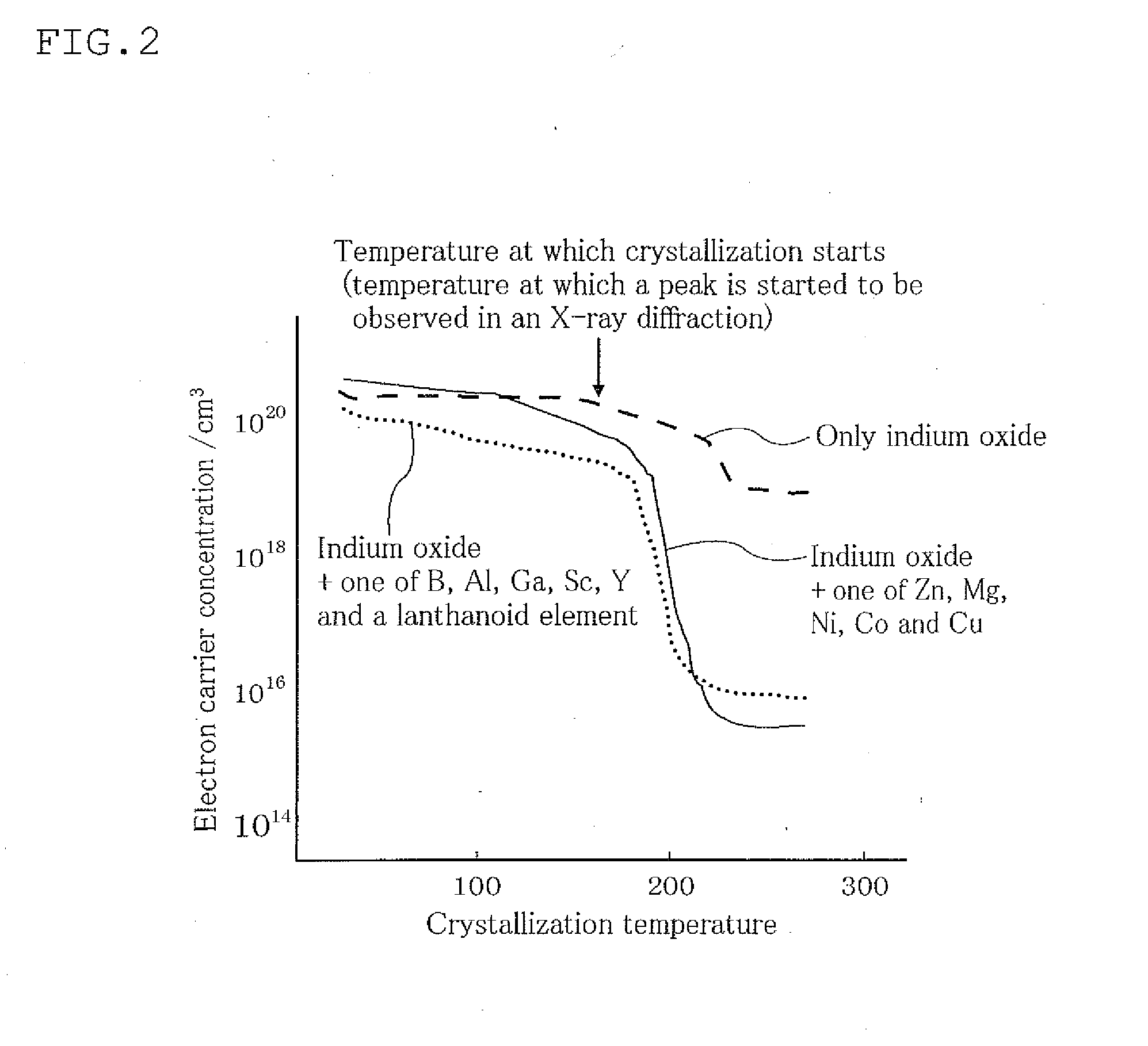
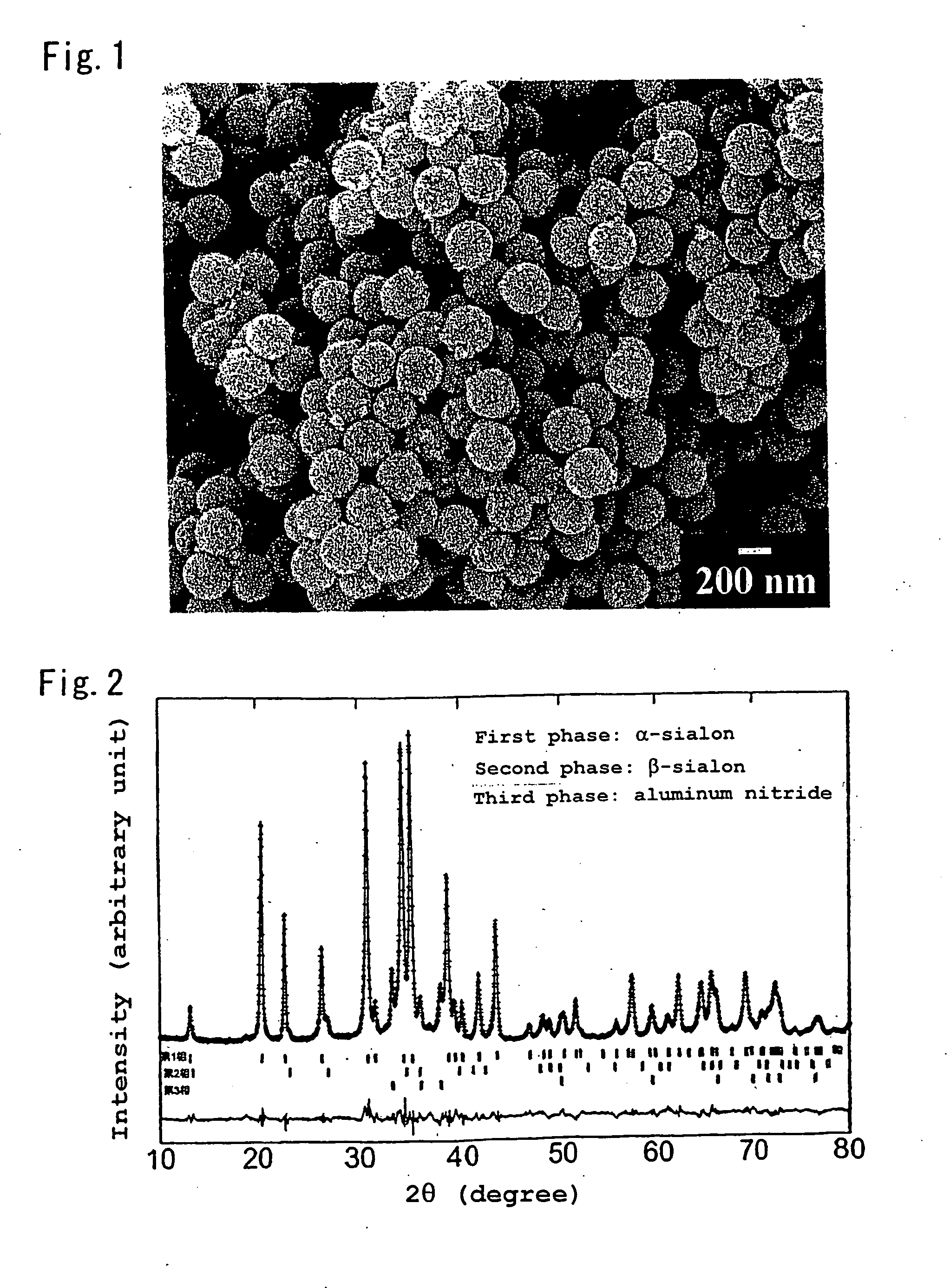
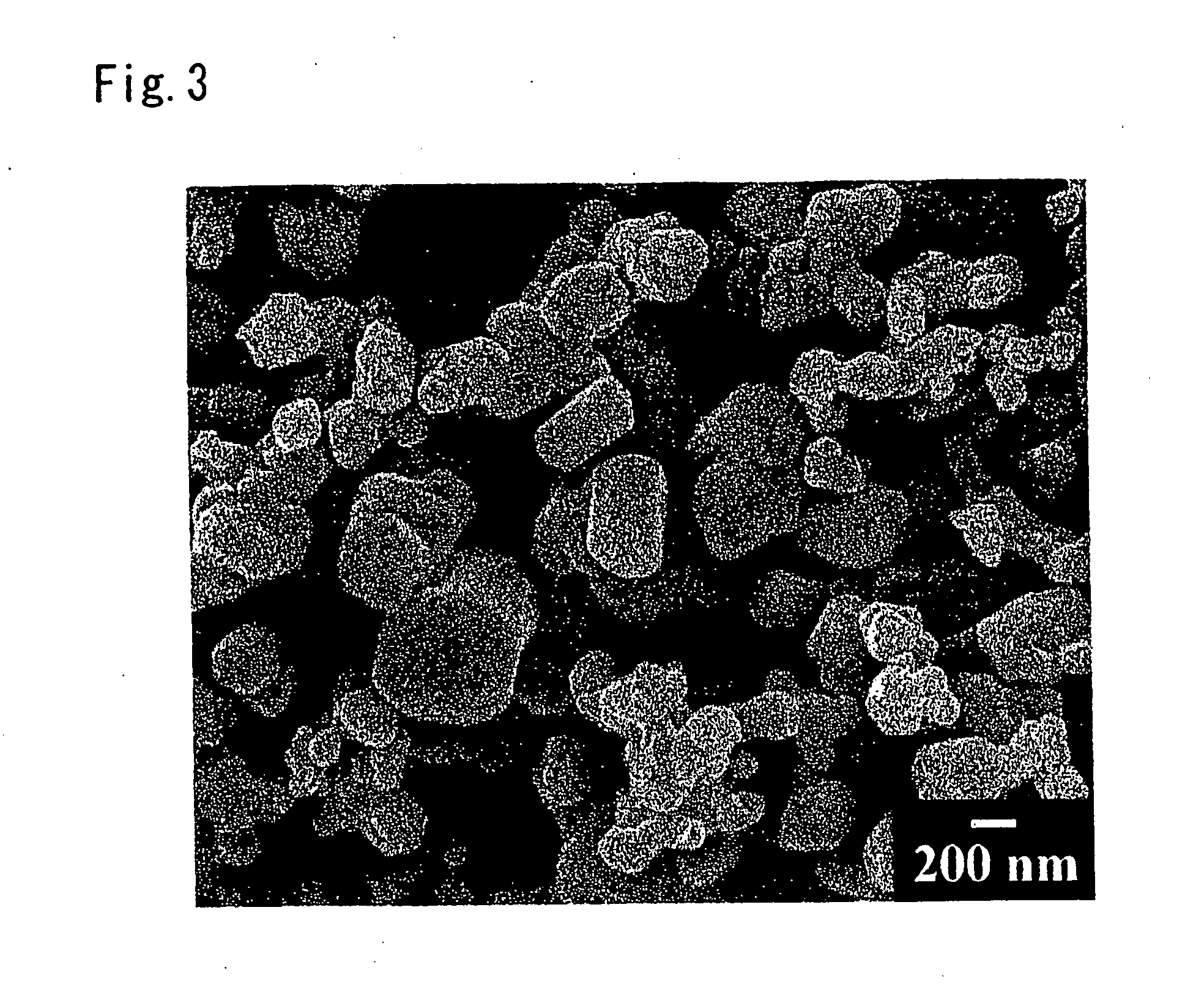
Oxynitride powder and production method thereof
ActiveUS20070166218A1Low oxygenHigh nitrogen contentAluminium compoundsAluminium silicatesPhosphorNitrogen
It is aimed at providing an oxynitride powder, which is suitable for usage as a phosphor, is free from coloration due to contamination of impurities, and mainly includes a fine α-sialon powder. An oxynitride powder is produced by applying a heat treatment in a reducing and nitriding atmosphere, to a precursor compound including at least constituent elements M, Si, Al, and O (where M is one element or mixed two or more elements selected from Li, Mg, Ca, Sr, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), thereby decreasing an oxygen content and increasing a nitrogen content of the precursor.
Owner:NAT INST FOR MATERIALS SCI
Method for effecting solvent extraction of metal ions using hydrocarbon soluble aminomethylene phosphonic acid compounds
Solvent extraction of one or more metal ions from an aqueous solution in the presence of hydrocarbon-soluble aminomethylenephosphonic acid derivatives.
Owner:BASF AG +1
Lithium-manganese composite oxide granular secondary particle, method for production thereof and use thereof
ActiveUS20050123832A1Improve output characteristicsMaintain good propertiesAluminium compoundsLithium compoundsMicrometerCompound (substance)
The invention provides granular secondary particles of a lithium-manganese composite oxide which are granular secondary particles made up of aggregated crystalline primary particles of a lithium-manganese composite oxide and have many micrometer-size open voids therein, the open voids having an average diameter in the range of from 0.5 to 3 μm and the total volume of the open voids being in the range of from 3 to 20 vol. % on average based on the total volume of the granules. These particles are suitable for use as a constituent material for non-aqueous electrolyte secondary batteries showing high-output characteristics. The invention further provides a process for producing the granular secondary particles of a lithium-manganese composite oxide which includes spray-drying a slurry prepared by dispersing a fine powder of a manganese oxide, a lithium source, an optional compound containing at least one element selected from Al, Co, Ni, Cr, Fe, and Mg, and an agent for open-void formation to thereby granulate the slurry and then calcining the granules at a temperature of from 700 to 900° C.
Owner:TOSOH CORP
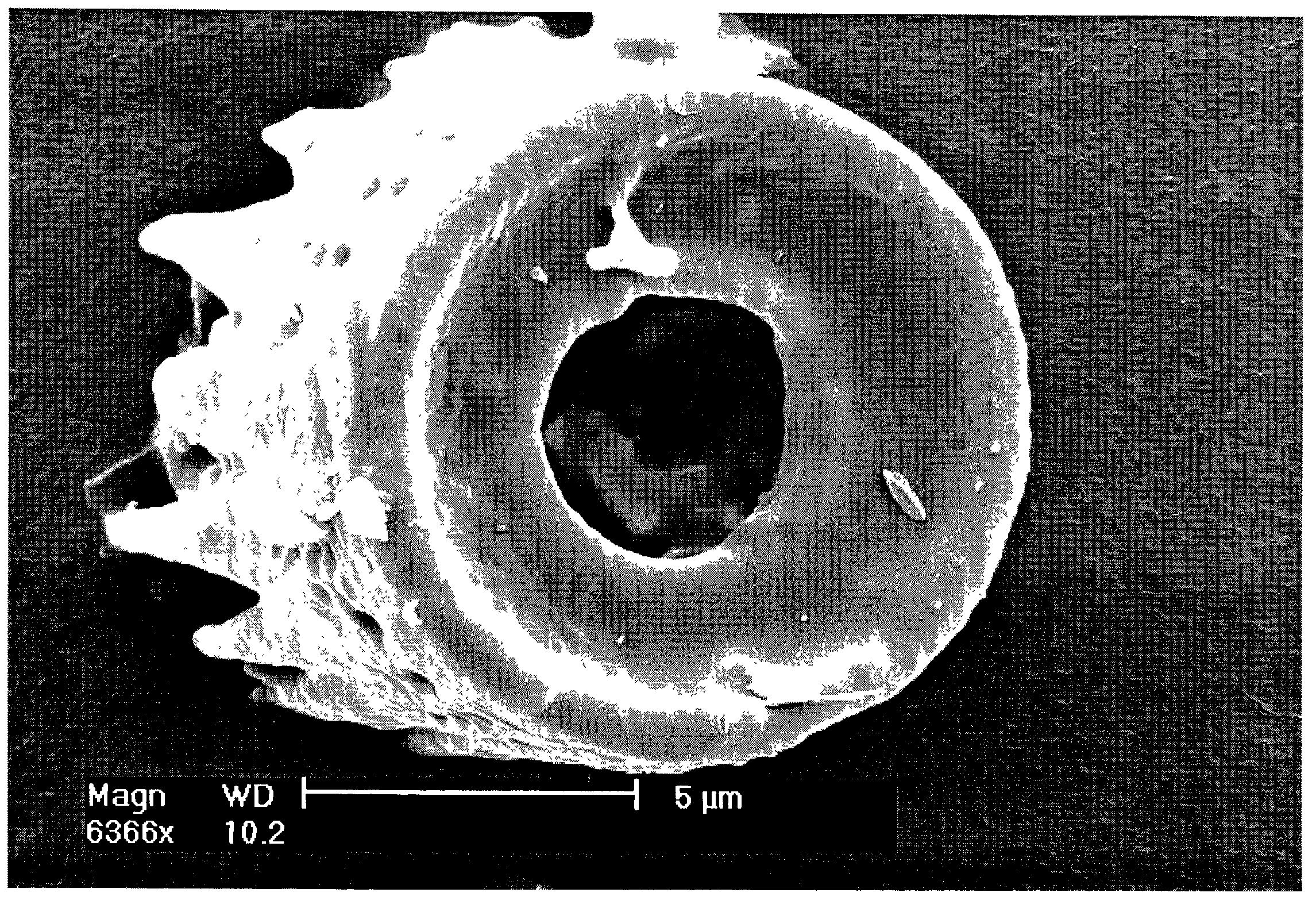
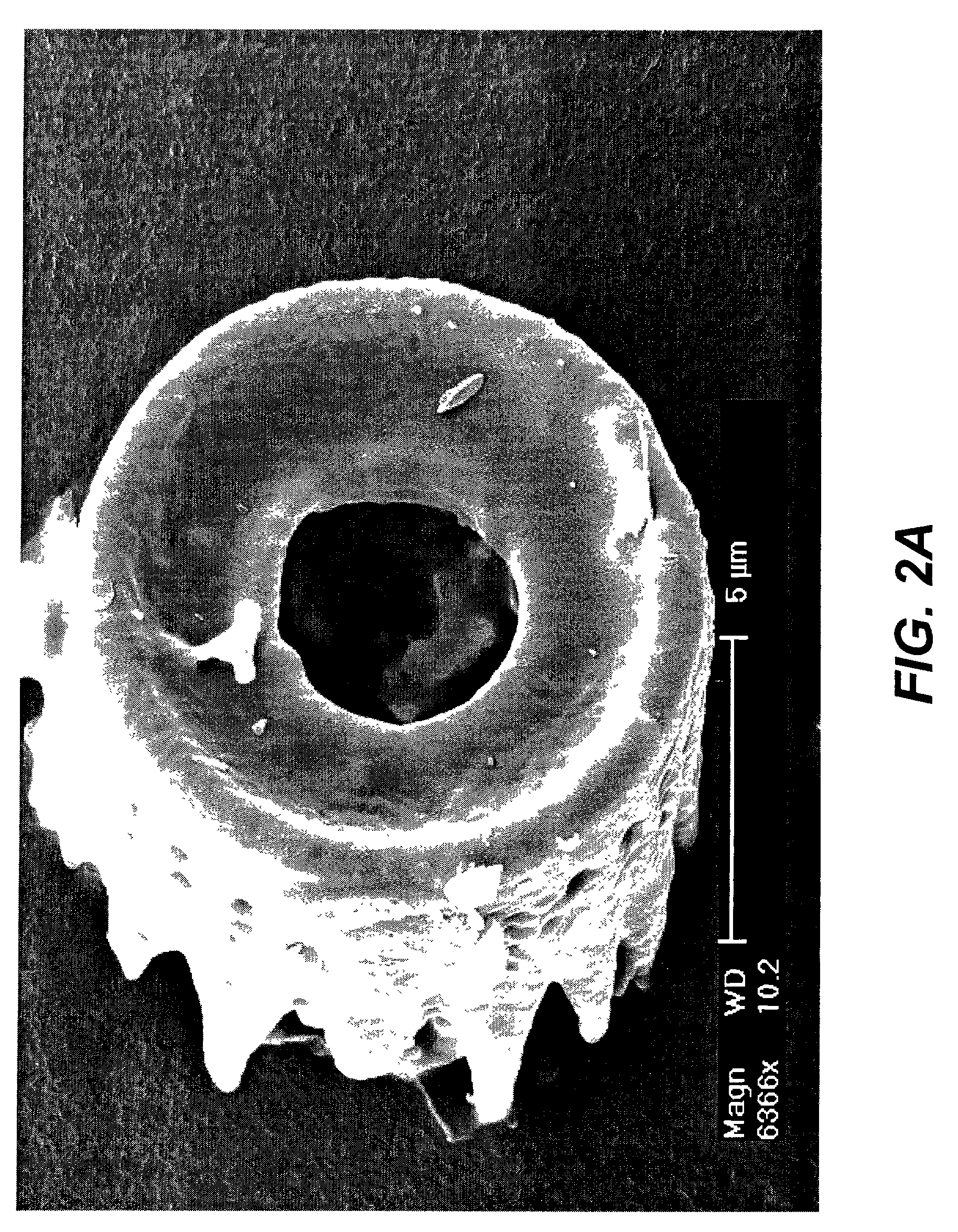
Shaped microcomponent via reactive conversion of biologically-derived microtemplates
InactiveUS7067104B2Well mixedLow-cost (highly-scaleablePowder deliveryZirconium compoundsGenetic engineeringReproduction
The present invention is focused on a revolutionary, low-cost (highly-scaleable) approach for the mass production of three-dimensional microcomponents: the biological reproduction of naturally-derived, biocatalytically-derived, and / or genetically-tailored three-dimensional microtemplates (e.g., frustules of diatoms, microskeletons of radiolarians, shells of mollusks) with desired dimensional features, followed by reactive conversion of such microtemplates into microcomponents with desired compositions that differ from the starting microtemplate and with dimensional features that are similar to those of the starting microtemplate. Because the shapes of such microcomponents may be tailored through genetic engineering of the shapes of the microtemplates, such microcomposites are considered to be Genetically-Engineered Materials (GEMs).
Owner:THE OHIO STATES UNIV
Polymeric chelant and coagulant to treat metal-containing wastewater
The present invention is directed to the use of a combination of a polymeric chelant and coagulant to treat metal containing wastewater. More particularly, the invention is directed at removing copper from CMP wastewater. The composition includes a combination of (a) a polymeric chelant derived from a polyamine selected from the group consisting of diethylenetriamine (DETA), triethylenetetraamine (TETA), tertraethylenepentaamine (TEPA), poly[vinylamine], and branched or linear poly[ethylenimine] (PEI); and (b) a water soluble or dispersible copolymer of a tannin and a cationic monomer selected from the group consisting of methyl chloride or dimethyl sulfate quaternary salt of dimethyl aminoethyl acrylate, diethylaminoethyl acrylate, dimethylaminoethyl methacrylate, diethylaminoethyl methacrylate, dimethylaminopropyl methacrylamide, dimethylaminopropyl acrylamide, and diallyl dimethyl ammonium chloride.
Owner:GENERAL ELECTRIC CO
Method for the treatment of salt brine
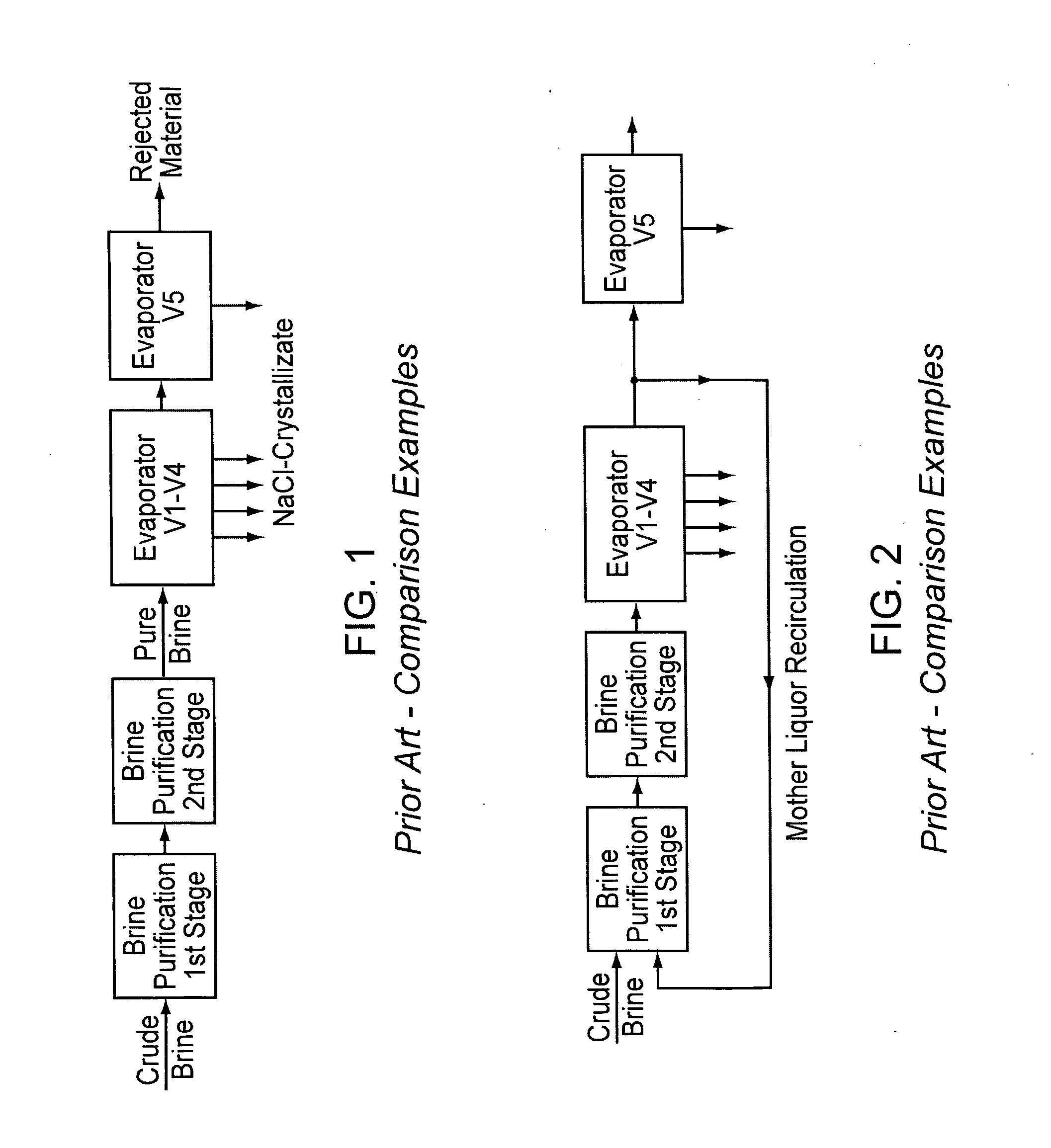
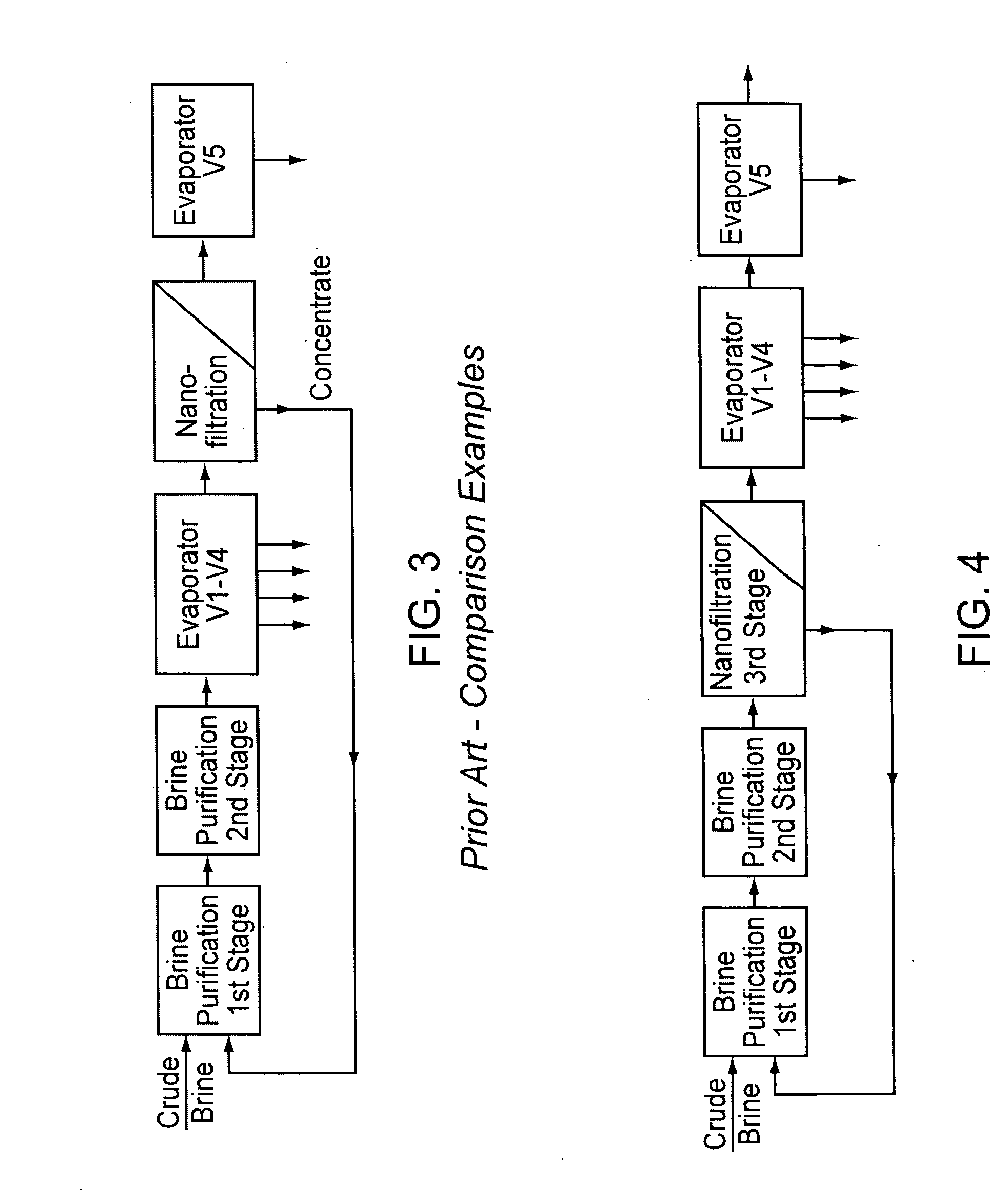
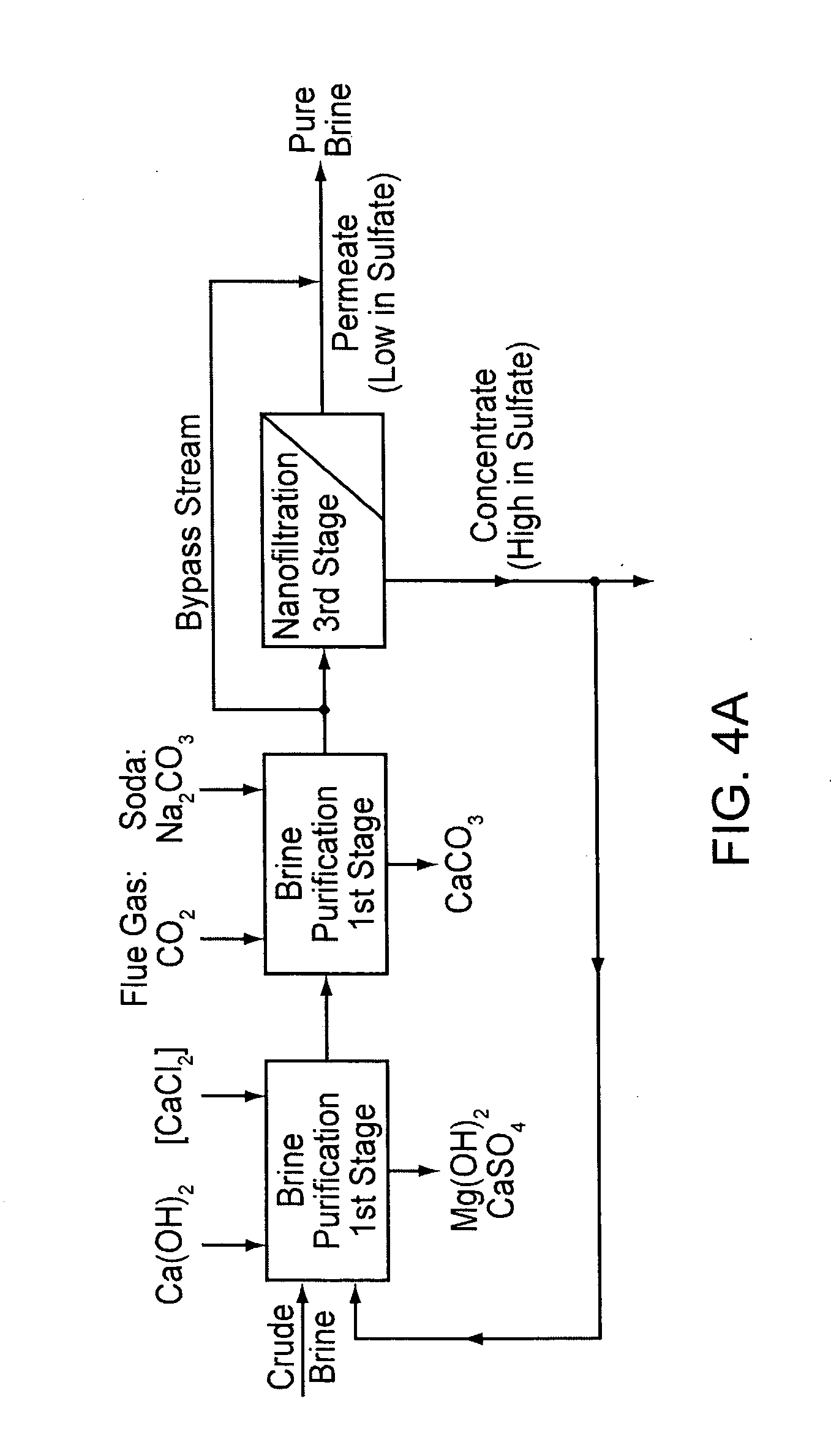
InactiveUS20070189945A1Low costReduce contentCalcium/strontium/barium compoundsMagnesium compoundsNanofiltrationCrystallization
A method for purifying salt brine to obtain a highly pure sodium chloride from the purified brine by means of crystallization. Nanofiltration directly follows a two-stage brine purification according to the Schweizerhalle method, as a third purification stage, and the permeate of the nanofiltration is a highly pure brine. The concentrate from this step is recirculated into the first stage of the brine purification.
Owner:ESCO - EURON SALT
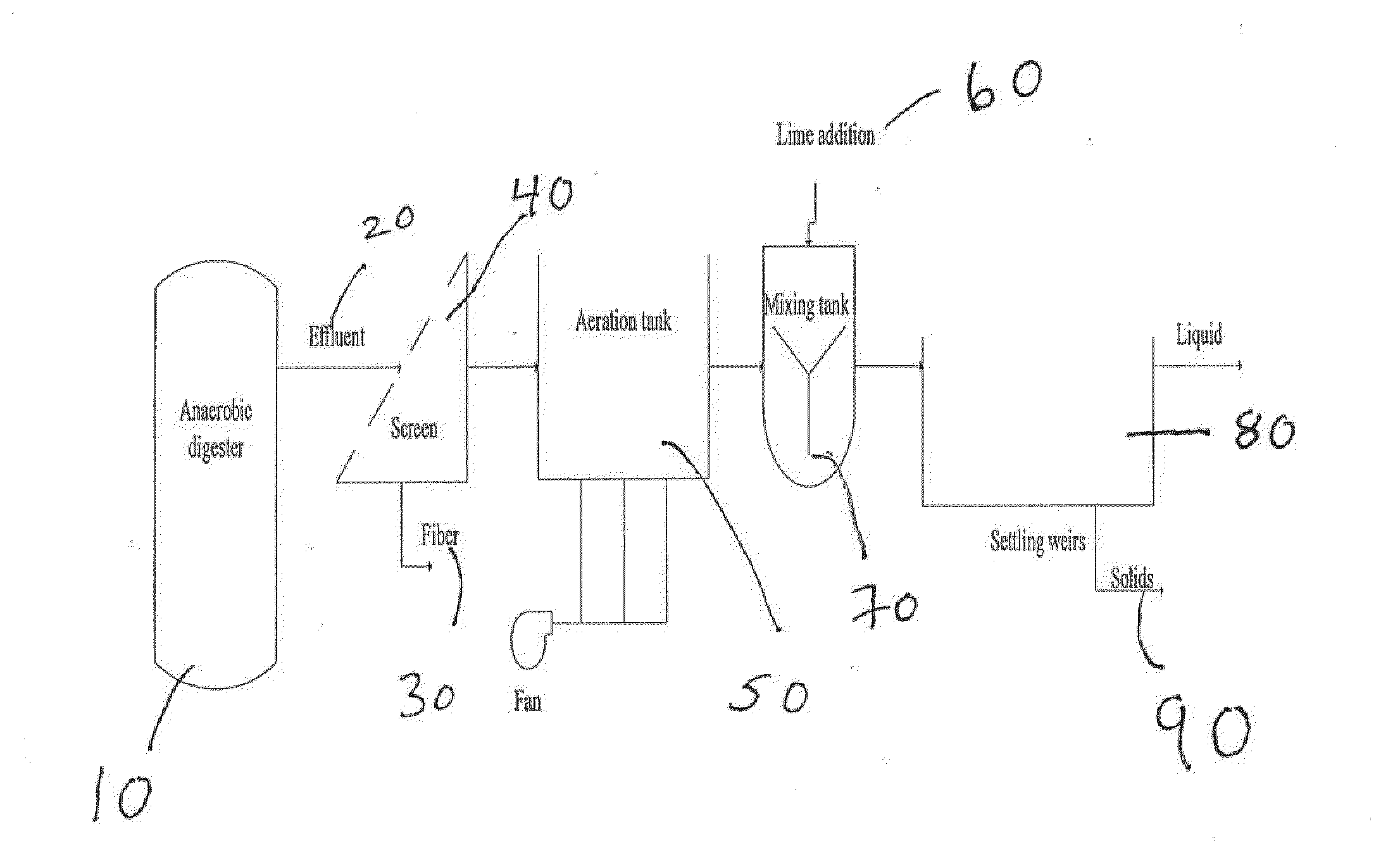
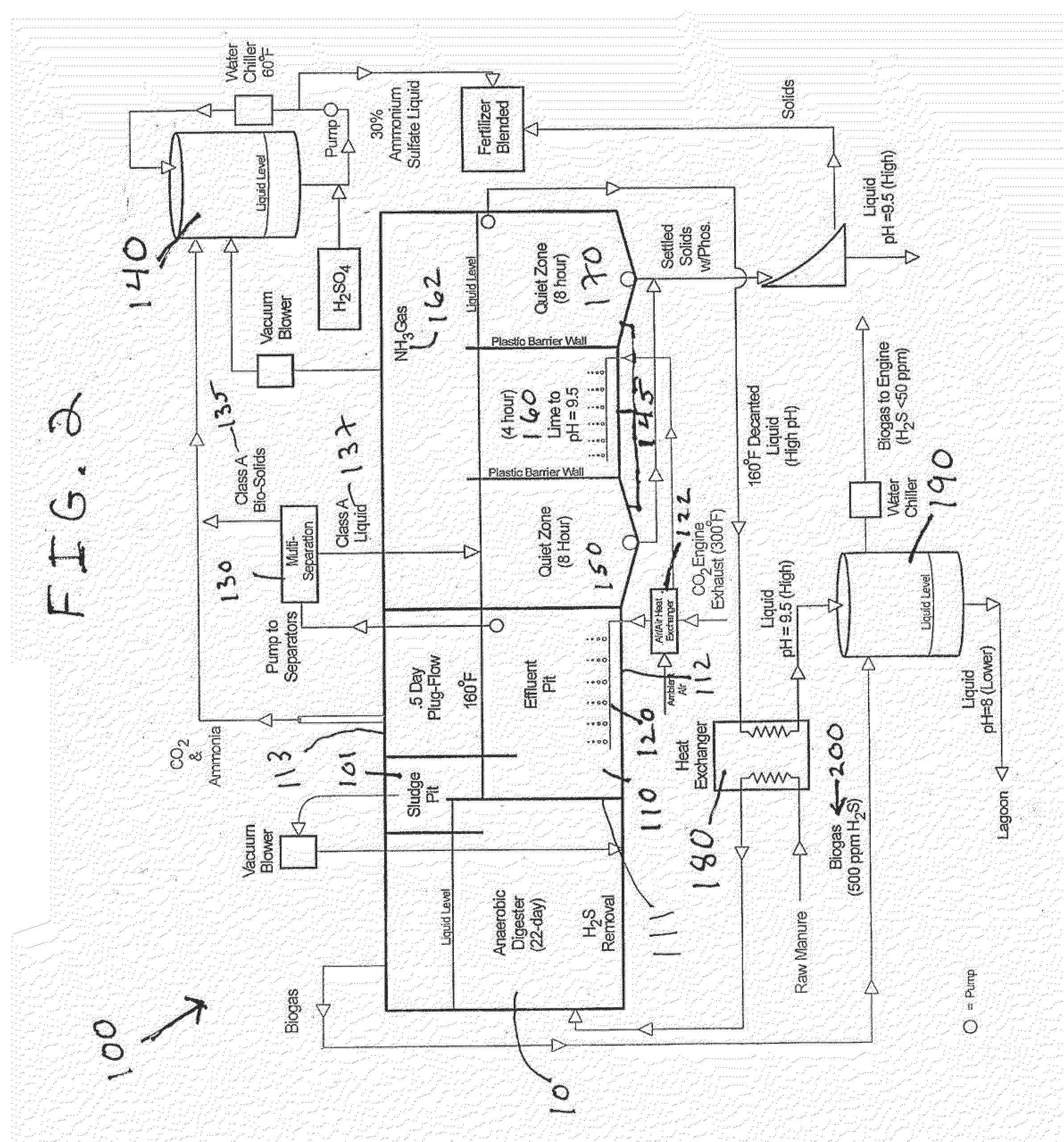
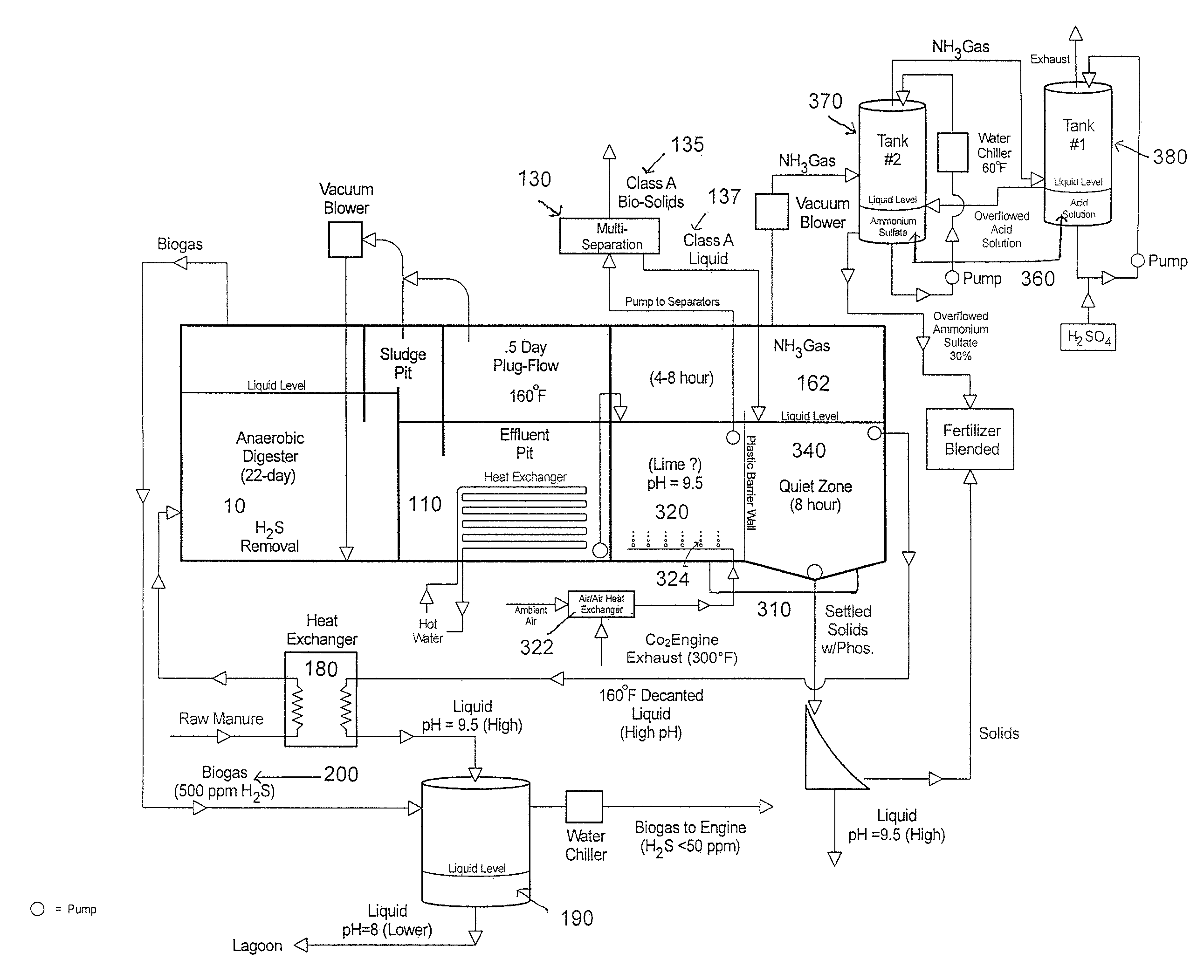
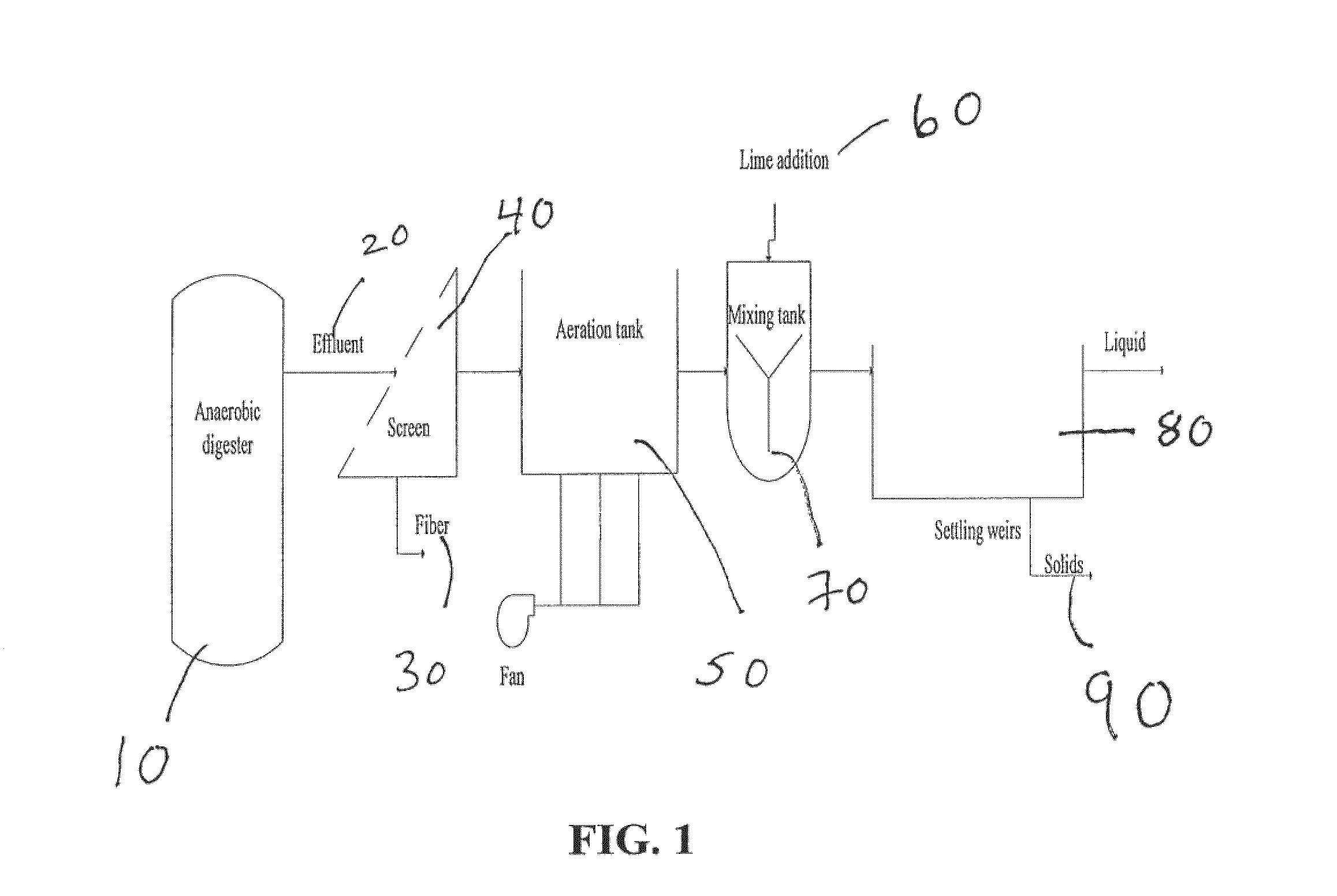
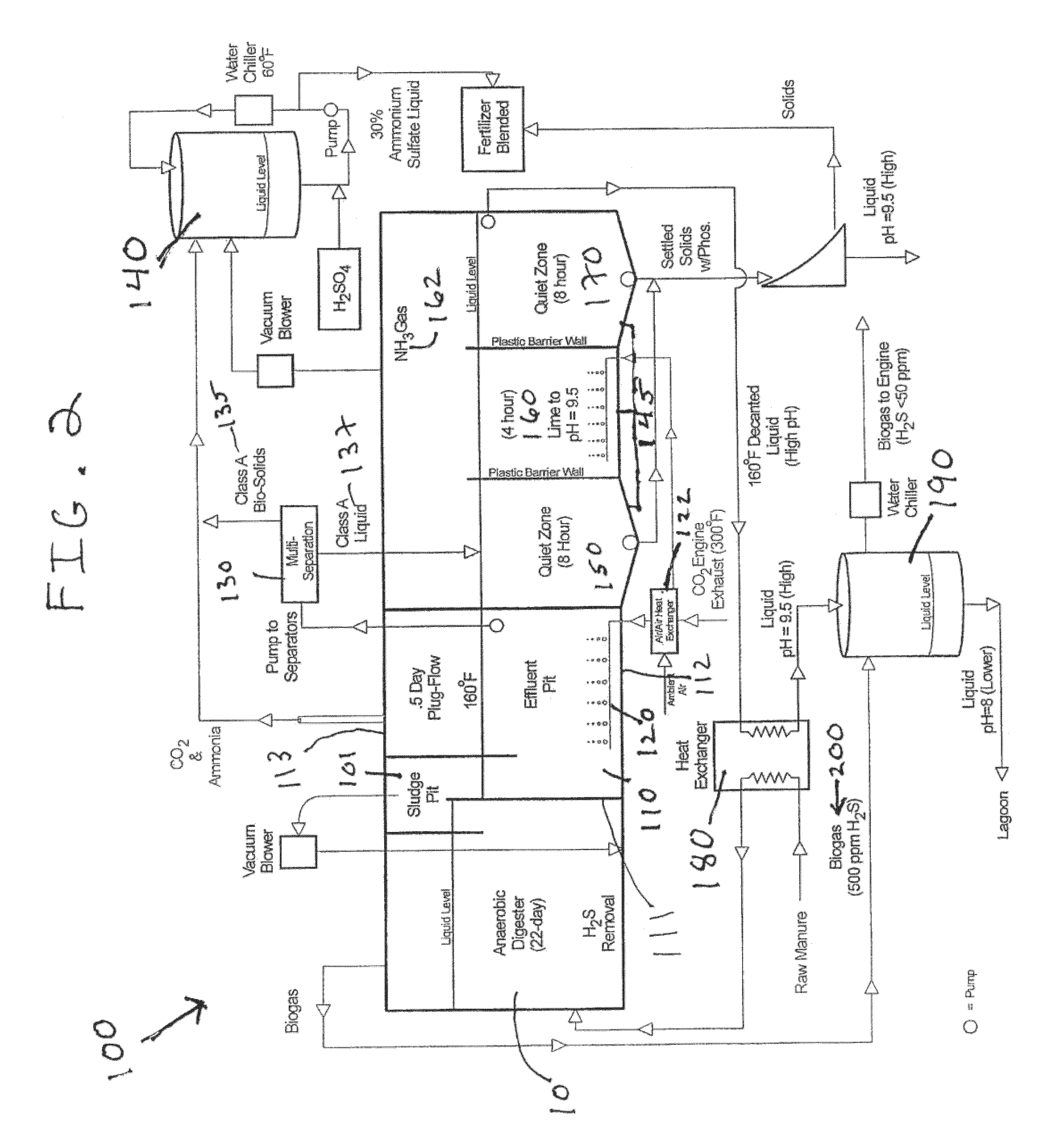
Nutrient recovery systems and methods
ActiveUS20120118035A1Large levelHigh recovery rateBio-organic fraction processingBioloigcal waste fertilisersAeration rateBiology
Methods, systems, and apparatuses for anaerobic digestion of waste fibrous material and the recovery of nutrients are provided. Methods, systems, and apparatuses disclosed herein provide mechanisms to release dissolved gases from anaerobic digester effluent. Methods, systems and apparatuses disclosed herein can recover one or more nutrients from anaerobic digested effluent using a range of temperatures, aeration rates, aeration times, pH ranges, and settling times.
Owner:DVO +2
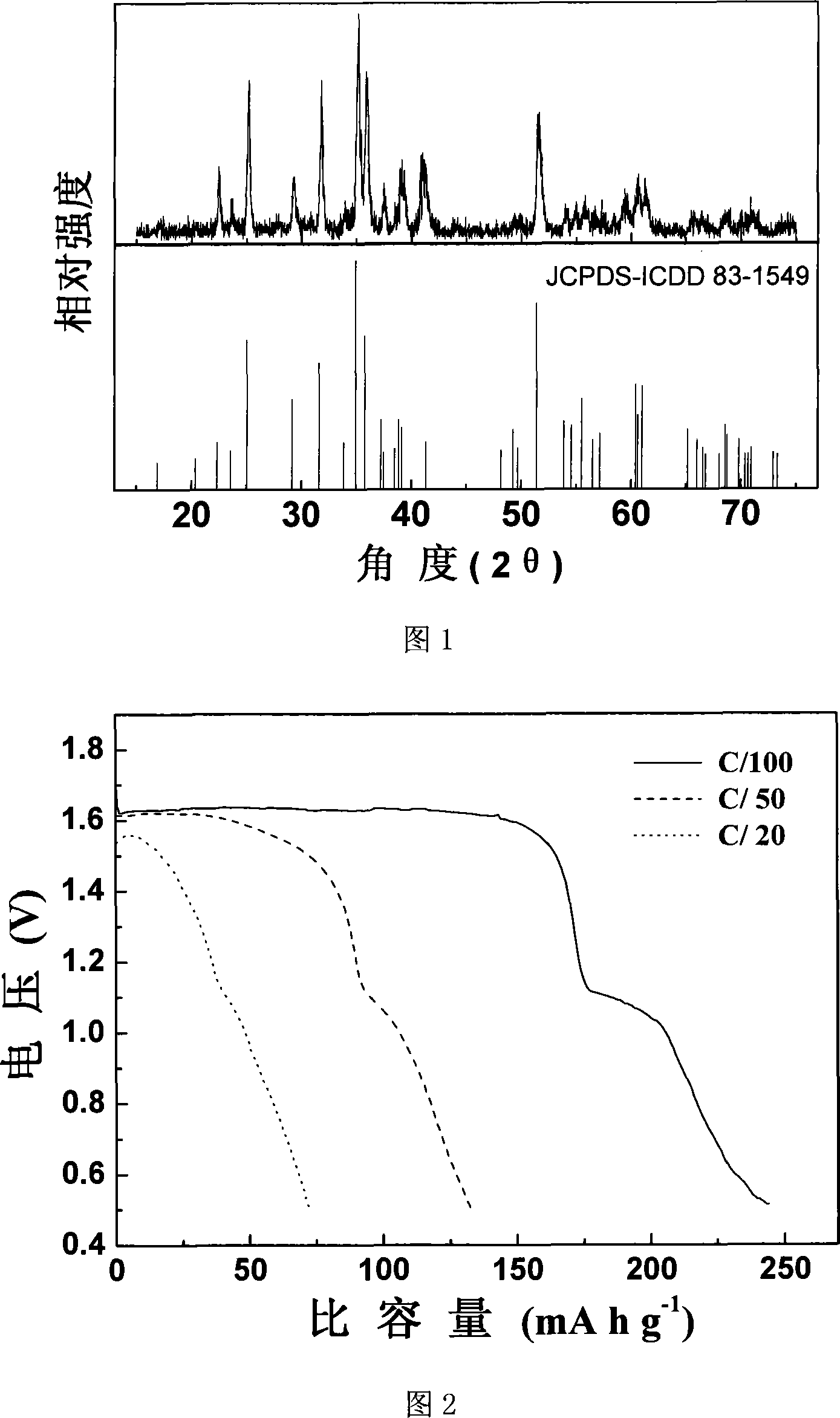
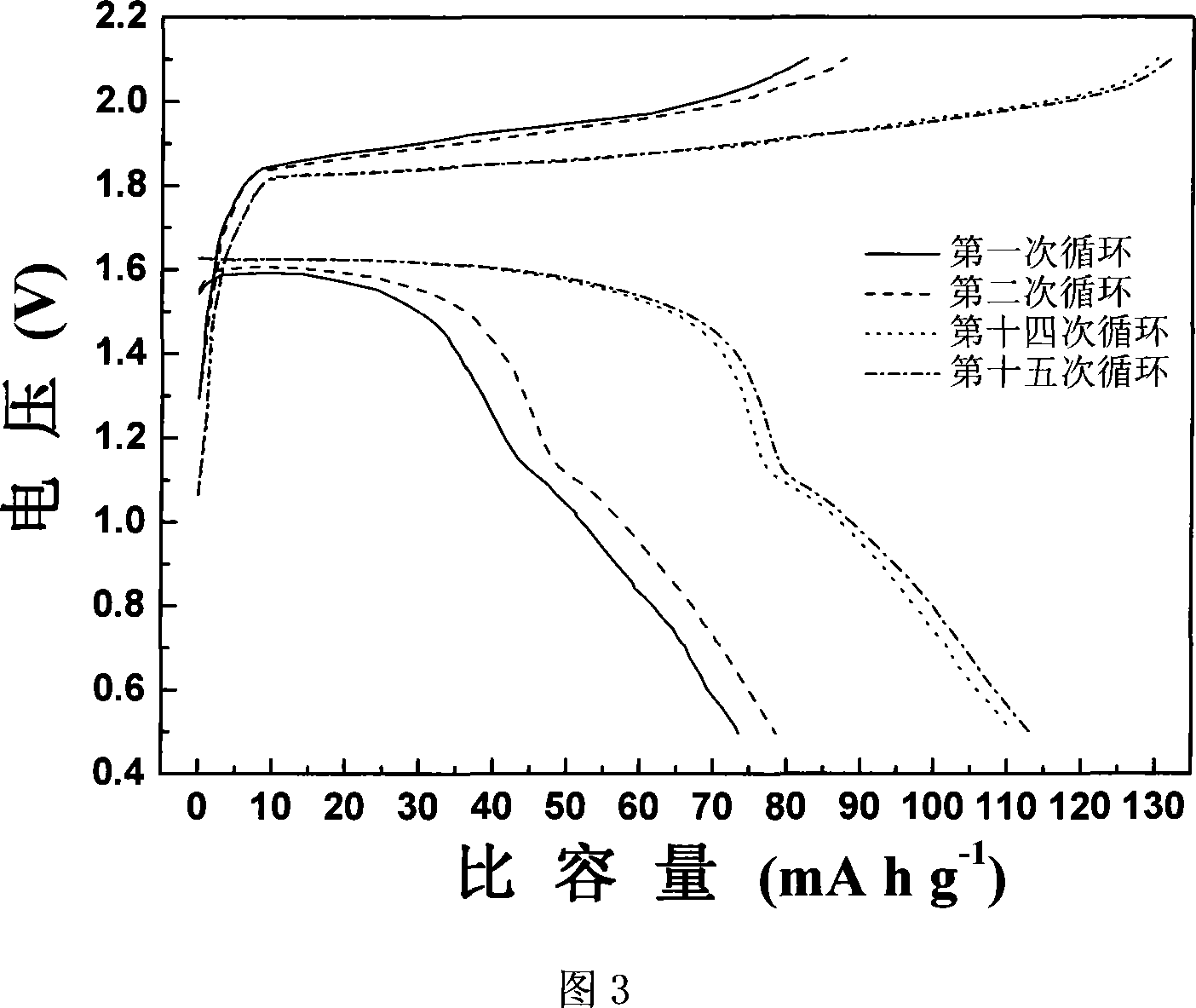
A magnesium secondary battery anode material and the corresponding preparation method
InactiveCN101217194AHigh voltage platformIncrease capacityElectrode manufacturing processesMagnesium compoundsManganeseCharge discharge
The invention discloses a magnesium rechargeable battery anode material and the preparation method. The anode material is a carbon-coated magnesium manganese silicate, the chemical structural formula is MgxMnySiO4 / C, wherein, x is not less than 1 and not more than 1.03, y is not less than 0.97 and not more than 1, the content of the magnesium manganese silicate is 83.2 to 100 percent and the content of the carbon is 0 to 16.8 percent according to weight percentage, and the invention is a grey to black powder. The invention takes nano-silicon dioxide as a silicon source and adopts a modified sol-gel method for obtaining a magnesium manganese silicate precursor, and the magnesium rechargeable battery anode material is obtained by carbon coating processing and subsequent heat processing under the condition of protection atmosphere. The invention has good electrochemical charge-discharging behaviors, a discharging plateau is stabilized to achieve 1.6V (vs.Mg / Mg<2 PLUS>), the discharging capacity under the small current charge-discharging condition (C / 100) can achieve 243.9mAh.g<MINUS 1> (78 percent of theoretical capacity); compared with the ideal anode material Mo3S4 of a current magnesium rechargeable battery, the carbon-coated magnesium manganese silicate has the advantages of simple preparation, large capacity and high voltage plateau ,etc.
Owner:SHANGHAI JIAO TONG UNIV
Solid ammonia storage and delivery material
InactiveUS20090280047A1Cobalt ammonia complexesNitrogen compoundsAlkaline earth metalAmmonia storage
A solid ammonia storage and delivery material A solid ammonia storage material comprising: an ammonia absorbing salt, wherein the ammonia absorbing salt is an ionic salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkaline earth metals, and / or one or more transition metals, such as Mn, Fe, Co, Ni, Cu, and / or Zn, X is one or more anions, a is the number of cations per salt molecule, z is the number of anions per salt molecule, and ri is the coordination number of 2 to 12, wherein M is Mg provides a safe, light-weight and cheap compact storage for ammonia to be used in the automotive industry.
Owner:AMMINEX EMISSIONS TECH
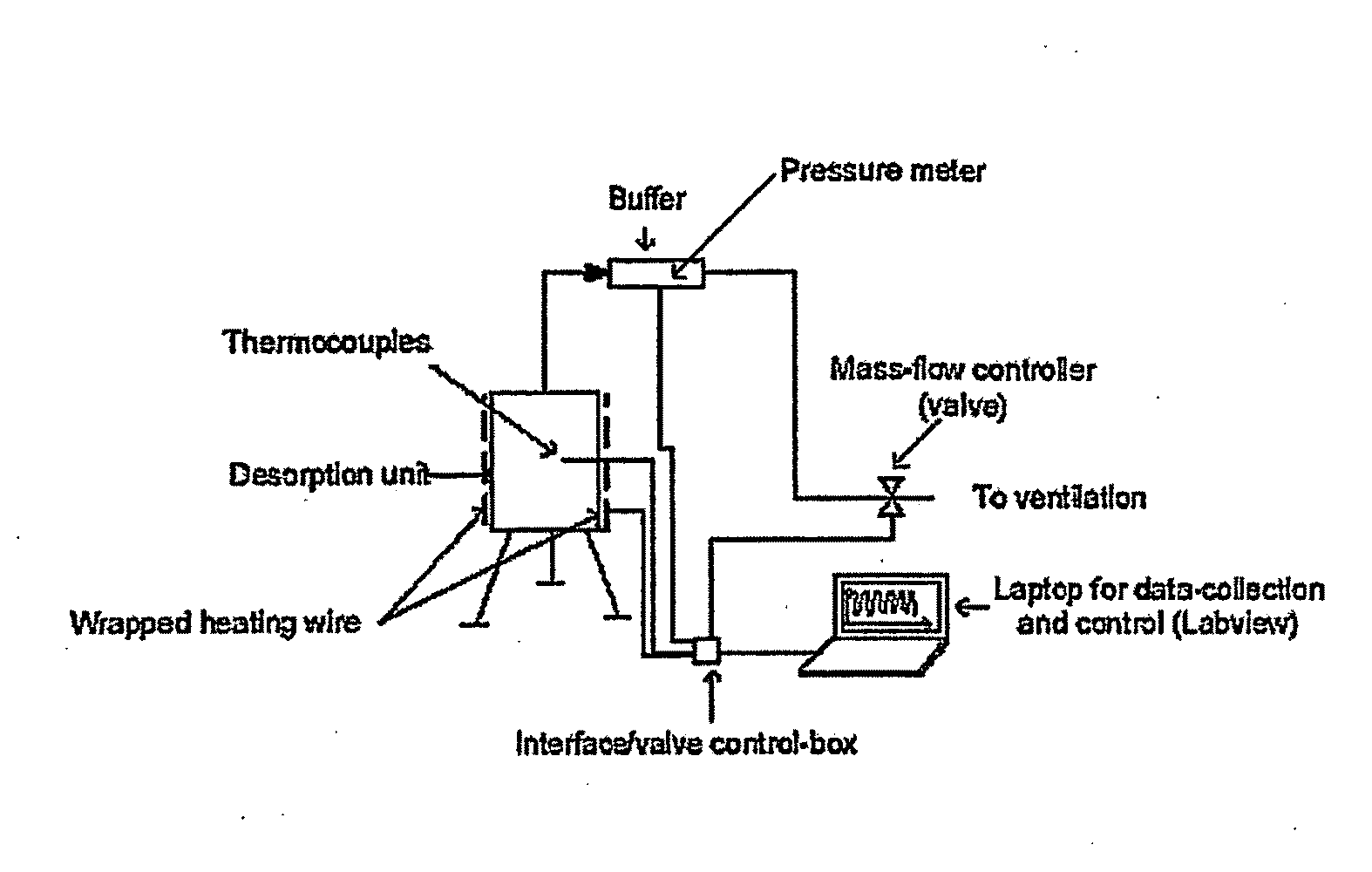
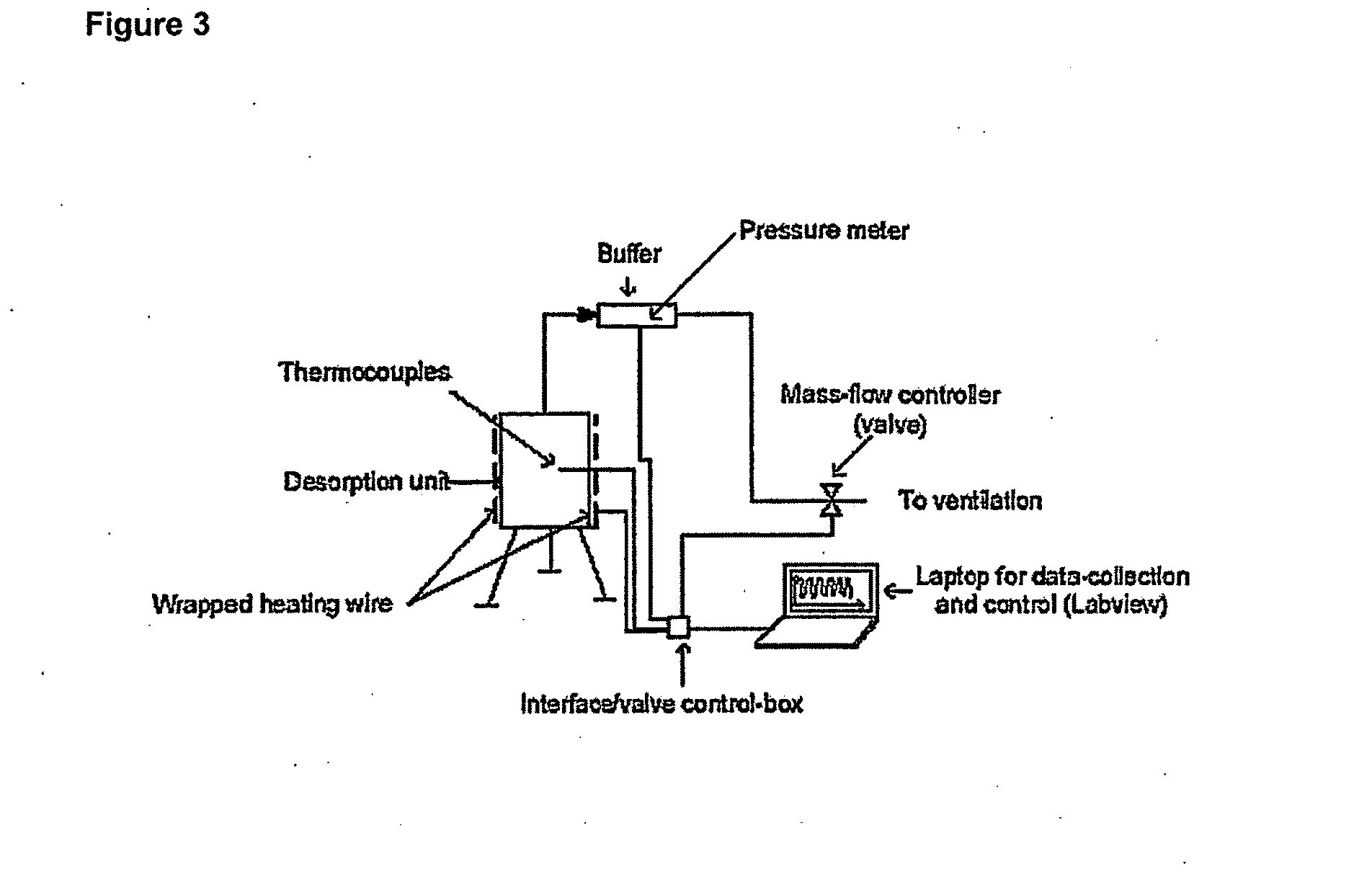
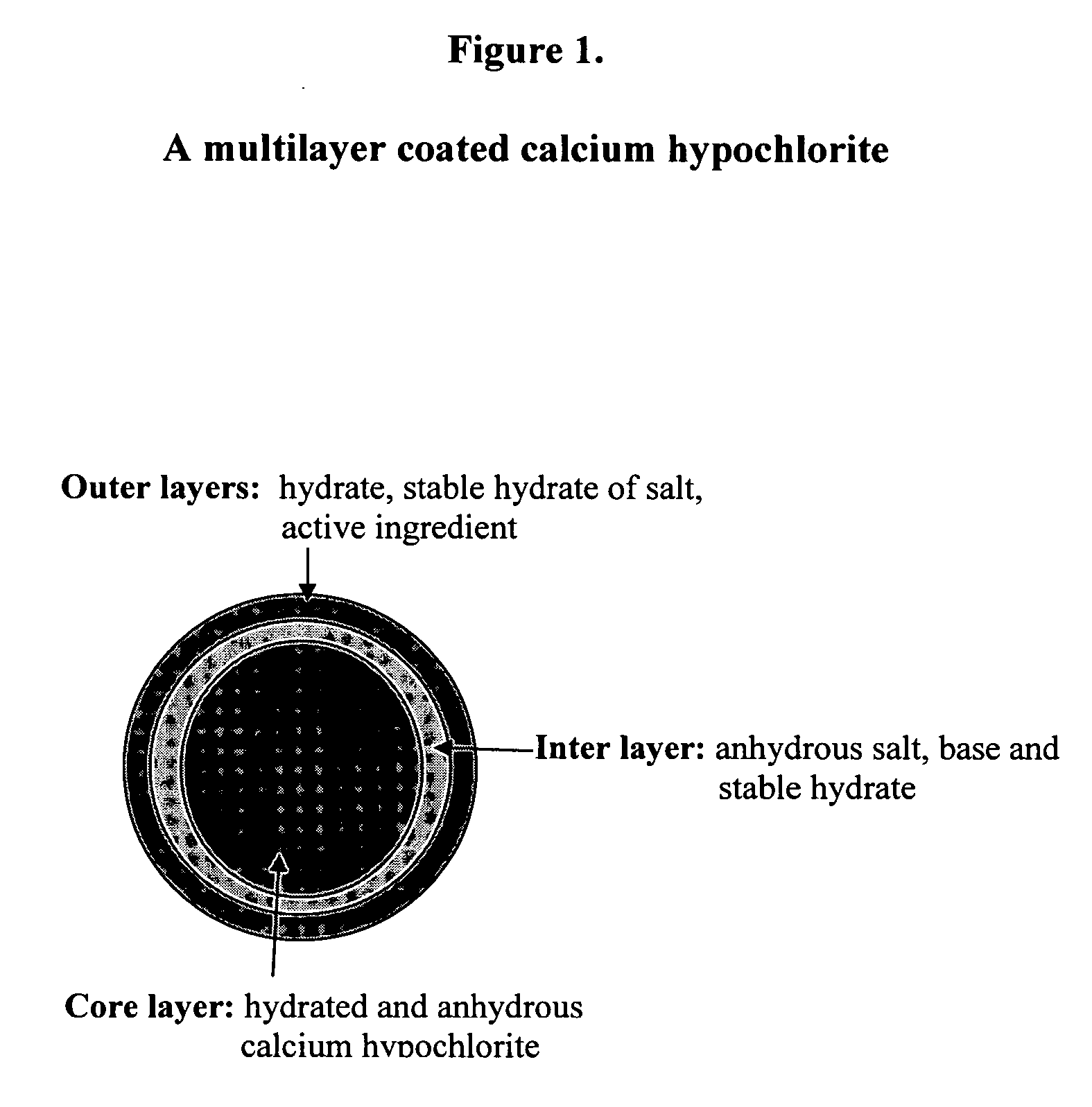
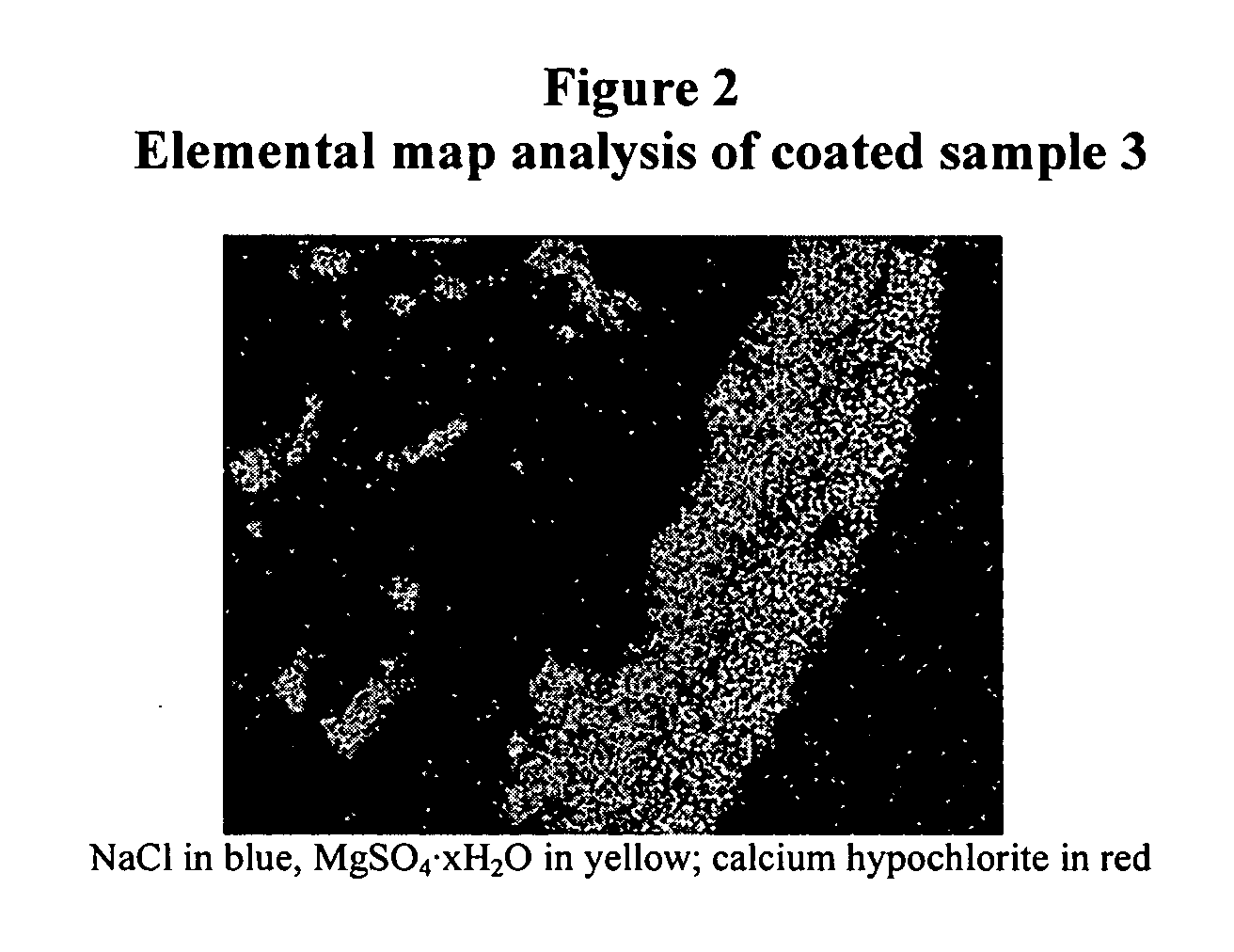
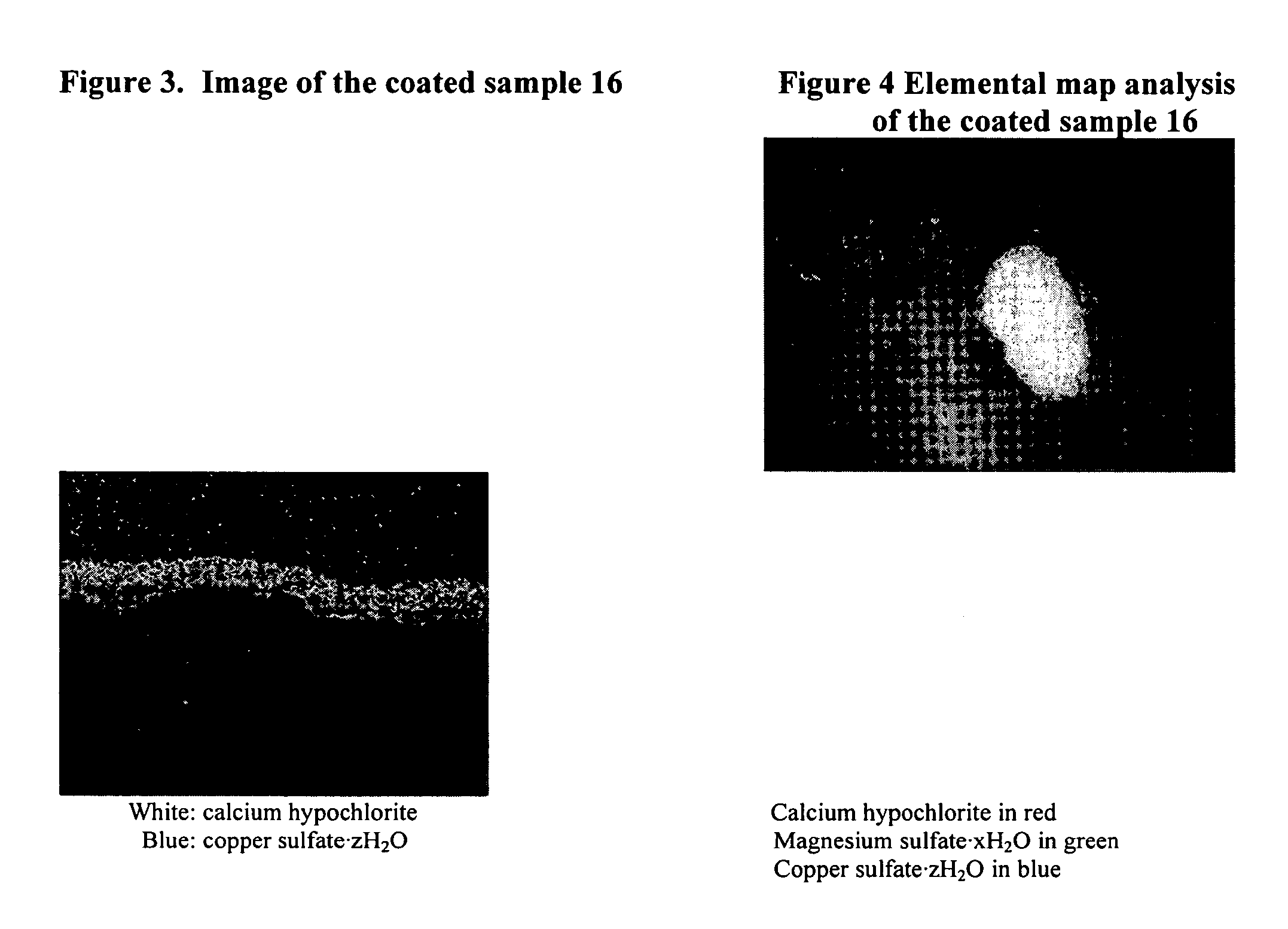
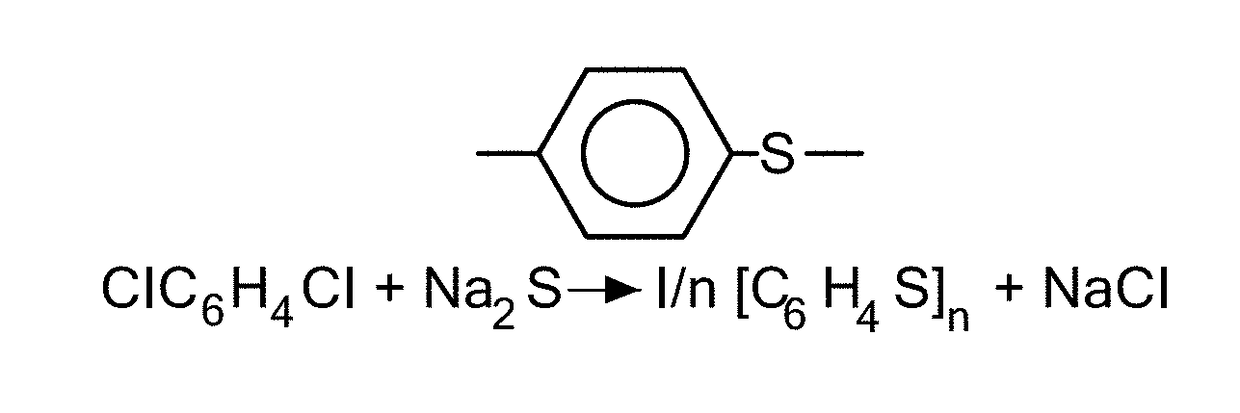
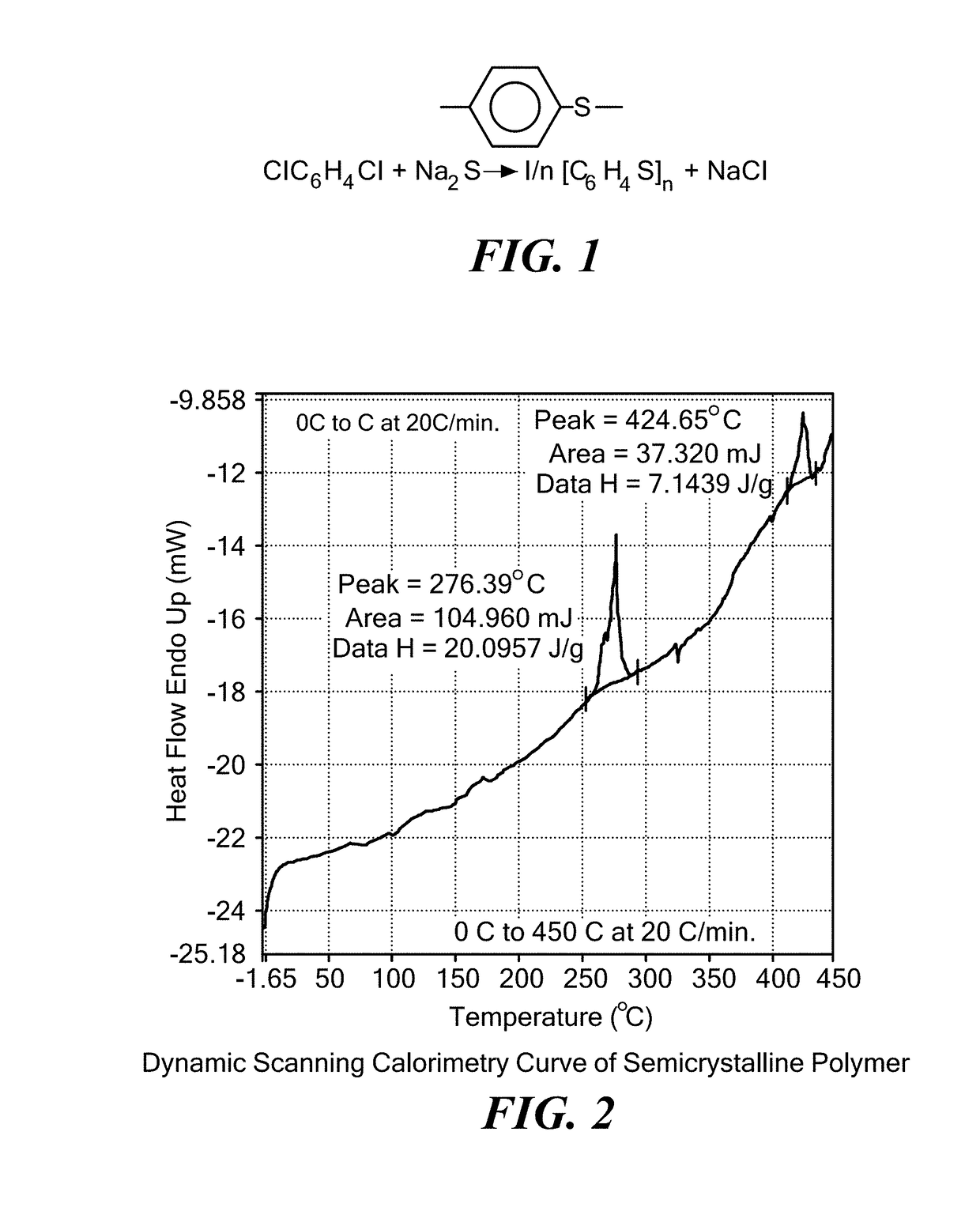
Coated calcium hypochlorite composition
InactiveUS20070125979A1Water softeningOrganic/inorganic per-compounds compounding agentsInter layerCalcium hypochlorite
The present invention is directed to a water treatment composition, comprising: calcium hypochlorite coated with a coating comprising at least one hydrated or anhydrous salt. The present invention is also directed to a water treatment composition, comprising: (a) an inner core layer comprising calcium hypochlorite; (b) one or more interlayers of selected salts positioned on top of said inner core layer, and (c) one or more outer layers of selected salts positioned on top of said interlayer(s).
Owner:INNOVATIVE WATER CARE LLC
Electrochemical cell having solid ionically conducting polymer material
ActiveUS20180006308A1Low effective aqueous porosityLarge specific surface areaSolid electrolytesAlkaline accumulatorsConductive polymerElectrochemical cell
Owner:IONIC MATERIALS INC
Lithium-manganese composite oxide granular secondary particle, method for production thereof and use thereof
InactiveUS7217406B2Improve output characteristicsMaintain good propertiesAluminium compoundsLithium compoundsMicrometerSlurry
Granular secondary particles of a lithium-manganese composite oxide suitable for use in non-aqueous electrolyte secondary batteries showing high-output characteristics which are granular secondary particles made up of aggregated crystalline primary particles of a lithium-manganese composite oxide and have many micrometer-size open voids therein with a defined average diameter and total volume of open voids. A process for producing the granular secondary particles which includes spray-drying a slurry of at least a manganese oxide, a lithium source, and an agent for open-void formation to thereby granulate the slurry and then calcining the granules.
Owner:TOSOH CORP
Layered Double Hydroxides That Delaminate In Water, Their Manufacturing Process And Use
ActiveUS20080021115A1High light transmittanceImprove corrosion resistanceBiocideCosmetic preparationsWater basedLotion
Disclosed is a layered double hydroxide capable of delamination in water comprising a plurality of basal layers of a double hydroxide of Formula: M(II)1-xM(III)x(OH)2 wherein M(II) is Mg, Zn or a combination thereof, M(III) is Al, and x is 0.2 to 0.33 and a plurality of intercalated layers between each adjacent basal layers of Mg acetate, Zn acetate or Ce acetate and water of intercalation. A process for producing the layered double hydroxide is also disclosed. The layered double hydroxide find use as an vehicle component or an anti-corrosive pigment of water-based protective coatings for metallic substrates, and as a humectant or a stabilizing agent for cosmetic preparations such as lotions, creams or foundations.
Owner:TAYCA CORP
Process for extraction of metals
InactiveUS20020053260A1Avoid less flexibilityHigh activitySolvent extractionOther chemical processesOrganic solventMagnesium salt
A process for the extraction of metals from an aqueous solution of metals in the form of their metal ions. The preparation and use of an efficient organic extractant and also the recovery from the organic extractant of the metal ions. A process for preparing an extractant solution where the extractant solution is a solution of a calcium ion or magnesium ion loaded extractant in a water-immiscible organic solvent, and where the extractant solution is suitable for extracting metal ions from an aqueous solution containing the metal ions, including mixing an aqueous solution of calcium ions or magnesium ions with a basic calcium salt or a basic magnesium salt and with a solution of an organic extractant in a water-immiscible organic solvent to form the extractant solution, and then separating the extractant solution from the aqueous solution.
Owner:CANOPEAN
Cobalt oxide particles and process for producing the same, cathode active material for non-aqueous electrolyte secondary cell and process for producing the same, and non-aqueous electrolyte secondary cell
ActiveUS6998071B2Stable crystal structureImprove thermal stabilityMaterial nanotechnologyConductive materialElectrical batteryCrystal structure
Owner:TODA IND
Sulfite control to reduce mercury re-emission
ActiveUS8828341B1Reduce mercury emissionReduce re-emissionAluminium compoundsGas treatmentFlue gasSlurry
A method for reducing mercury emission and / or re-emission in cleaned flue gas through control of sulfite concentration within a wet flue gas desulfurization (WFGD) system is disclosed. One method for reducing mercury emission and / or re-emission through control of sulfite concentration is to measure the sulfite concentration of an aqueous alkaline slurry used in a WFGD system and comparing the same to a predetermined sulfite concentration value. If the comparison reveals the measured sulfite concentration is above the predetermined values, the amount of oxidation air supplied to the system is increased. If the comparison reveals the measured sulfite concentration is below the predetermined values, the amount of oxidation air supplied to the system is decreased.
Owner:GENERAL ELECTRIC TECH GMBH
Metal scavenging polymers and uses thereof
Uses for a composition comprising a polymer derived from at least two monomers: acrylic-x and an alkylamine, wherein said polymer is modified to contain a functional group capable of scavenging one or more compositions containing one or more metals are disclosed. These polymers have many uses in various mediums, including wastewater systems.
Owner:ECOLAB USA INC
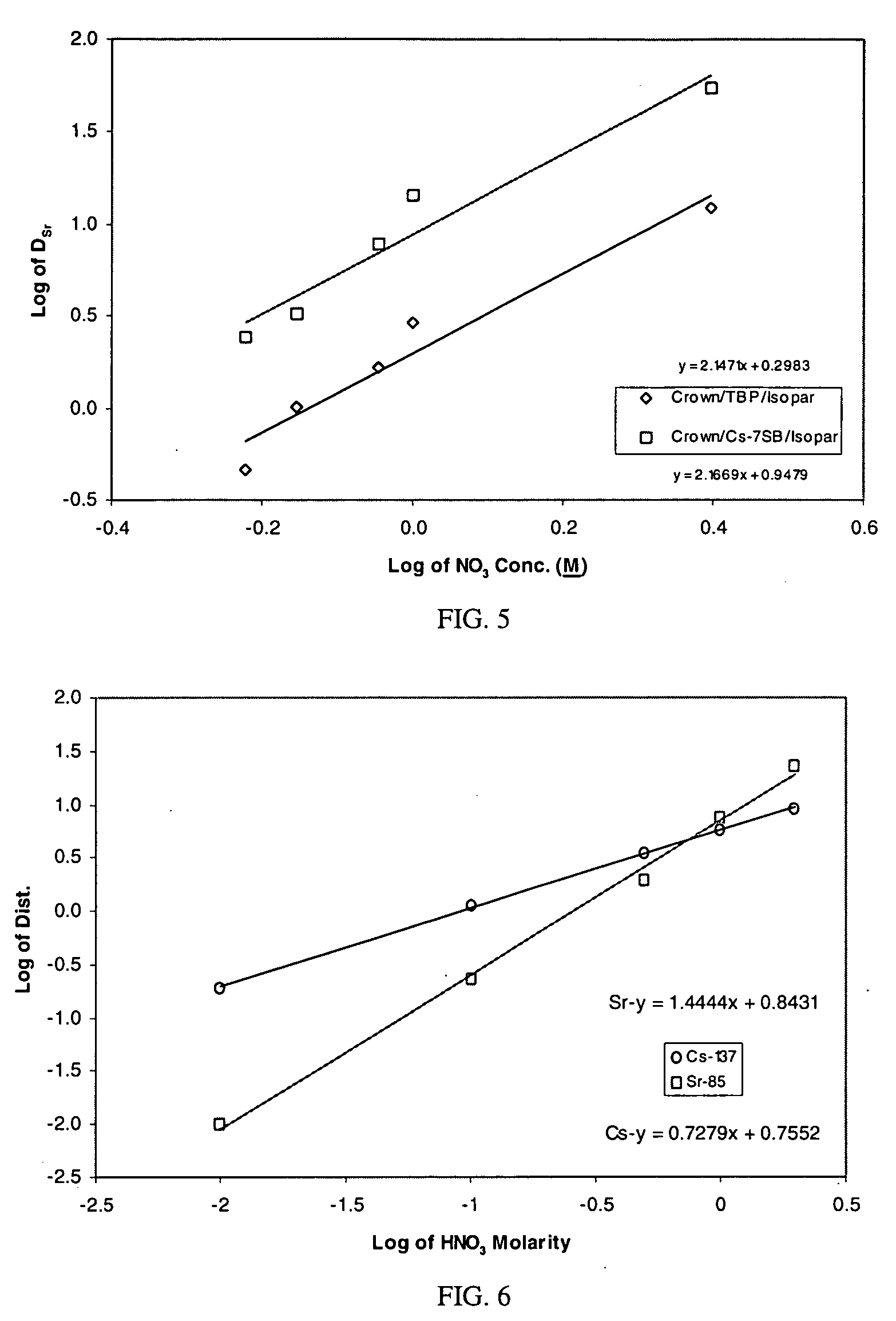
Cesium and strontium extraction using a mixed extractant solvent including crown ether and calixarene extractants
A mixed extractant solvent including calix[4]arene-bis-(tert-octylbenzo)-crown-6 (“BOBCalixC6”), 4′,4′,(5′)-di-(t-butyldicyclo-hexano)-18-crown-6 (“DtBu18C6”), and at least one modifier dissolved in a diluent. The mixed extractant solvent may be used to remove cesium and strontium from an acidic solution. The DtBu18C6 may be present from approximately 0.01 M to approximately 0.4M, such as from approximately 0.086 M to approximately 0.108 M. The modifier may be 1-(2,2,3,3-tetrafluoropropoxy)-3-(4-sec-butylphenoxy)-2-propanol (“Cs-7SB”) and may be present from approximately 0.01M to approximately 0.8M. In one embodiment, the mixed extractant solvent includes approximately 0.15M DtBu18C6, approximately 0.007M BOBCalixC6, and approximately 0.75M Cs-7SB modifier dissolved in an isoparaffinic hydrocarbon diluent. The mixed extractant solvent may form an organic phase in an extraction system that also includes an aqueous phase. Methods of extracting cesium and strontium as well as strontium alone are also disclosed.
Owner:BATTELLE ENERGY ALLIANCE LLC
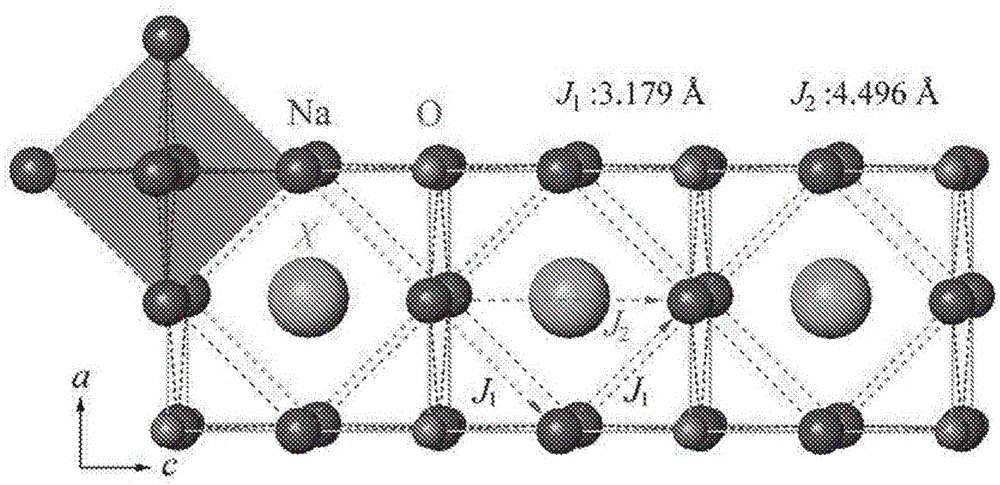
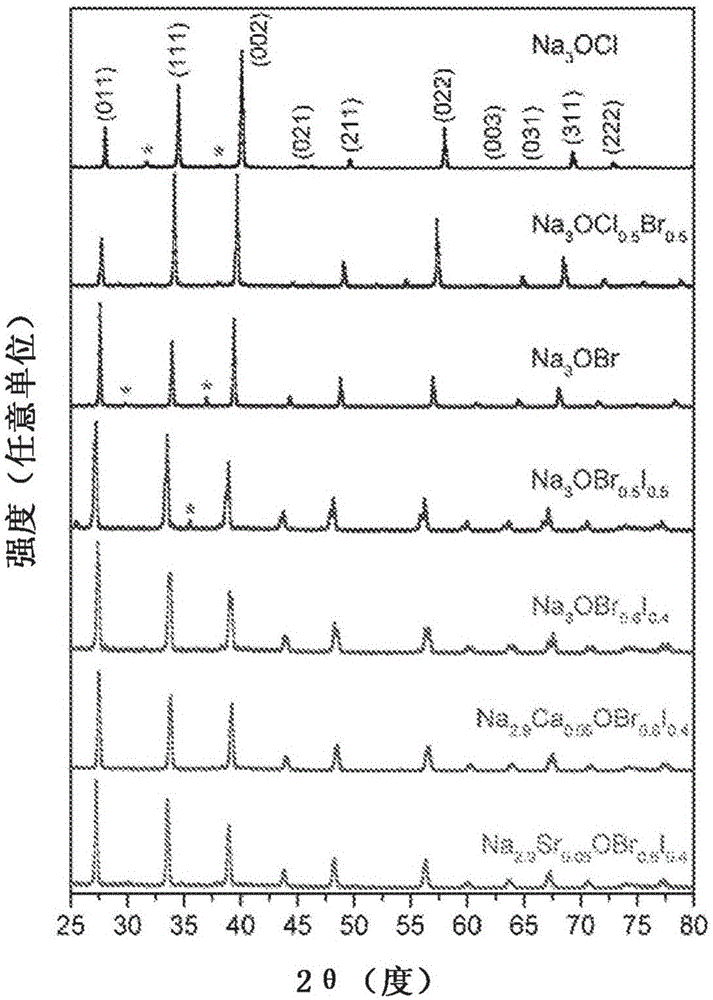
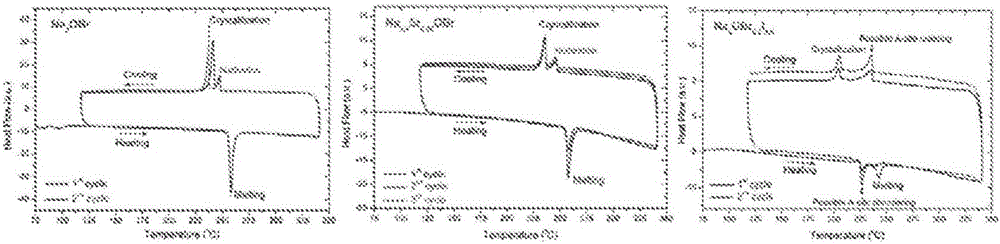
Sodium anti-perovskite solid electrolyte compositions
Na-rich electrolyte compositions provided herein can be used in a variety of devices, such as sodium ionic batteries, capacitors and other electrochemical devices. Na-rich electrolyte compositions provided herein can have a chemical formula of Na3OX, Na3SX, Na (3-delta) M delta / 2OX and Na (3-delta) M delta / 2SX wherein 0 ;lt; delta & lt; 0.8, wherein X is a monovalent anion selected from fluoride, chloride, bromide, iodide, H-, CN-, BF4-, BH4-, ClO4-, CH3-, NO2-, NH2- and mixtures thereof, and wherein M is a divalent metal selected from the group consisting of magnesium, calcium, barium, strontium and mixtures thereof. Na-rich electrolyte compositions provided herein can have a chemical formula of Na (3-delta) M delta / 3OX and / or Na (3-delta) M delta / 3SX; wherein 0 & lt;delta & lt; 0.5, wherein M is a trivalent cation M+3, and wherein X is selected from fluoride, chloride, bromide, iodide, H-, CN-, BF4-, BH4-, ClO4-, CH3-, NO2-, NH2- and mixtures thereof. Synthesis and processing methods of NaRAP compositions for battery, capacitor, and other electrochemical applications are also provided.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
Nutrient recovery systems and methods
ActiveUS8613894B2High recovery rateLow costBio-organic fraction processingBioloigcal waste fertilisersAeration rateBiology
Methods, systems, and apparatuses for anaerobic digestion of waste fibrous material and the recovery of nutrients are provided. Methods, systems, and apparatuses disclosed herein provide mechanisms to release dissolved gases from anaerobic digester effluent. Methods, systems and apparatuses disclosed herein can recover one or more nutrients from anaerobic digested effluent using a range of temperatures, aeration rates, aeration times, pH ranges, and settling times.
Owner:DVO +2
Popular searches
Sedimentation separation Reverse osmosis Energy based wastewater treatment Differential sedimentation Water/sewage treatment by neutralisation Alkali metal oxides/hydroxides Water/sewage treatment by flocculation/precipitation Alkali metal halide purification Membranes Water/sewage treatment by ion-exchange
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com