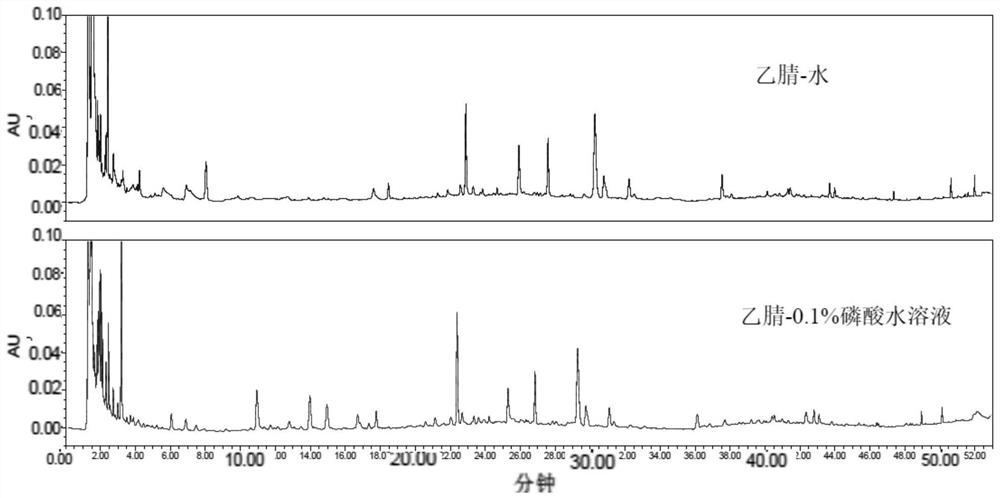
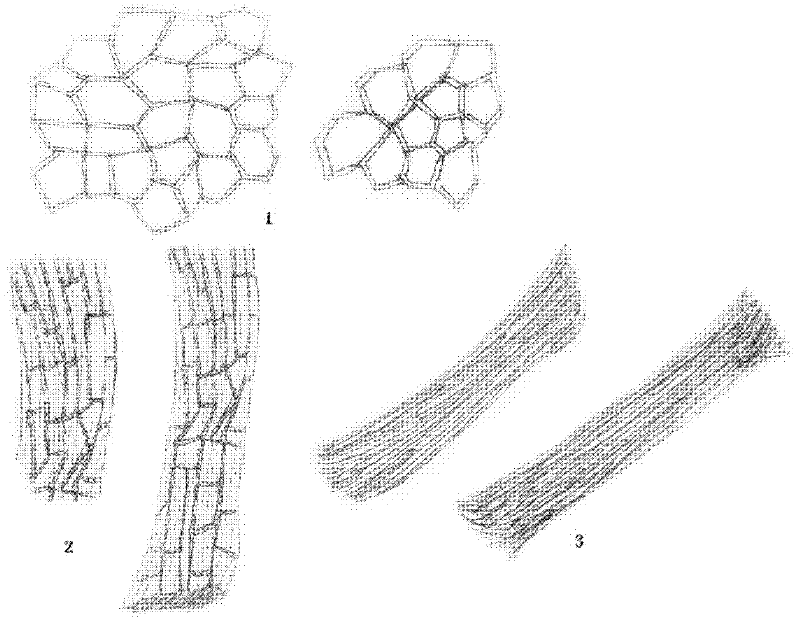
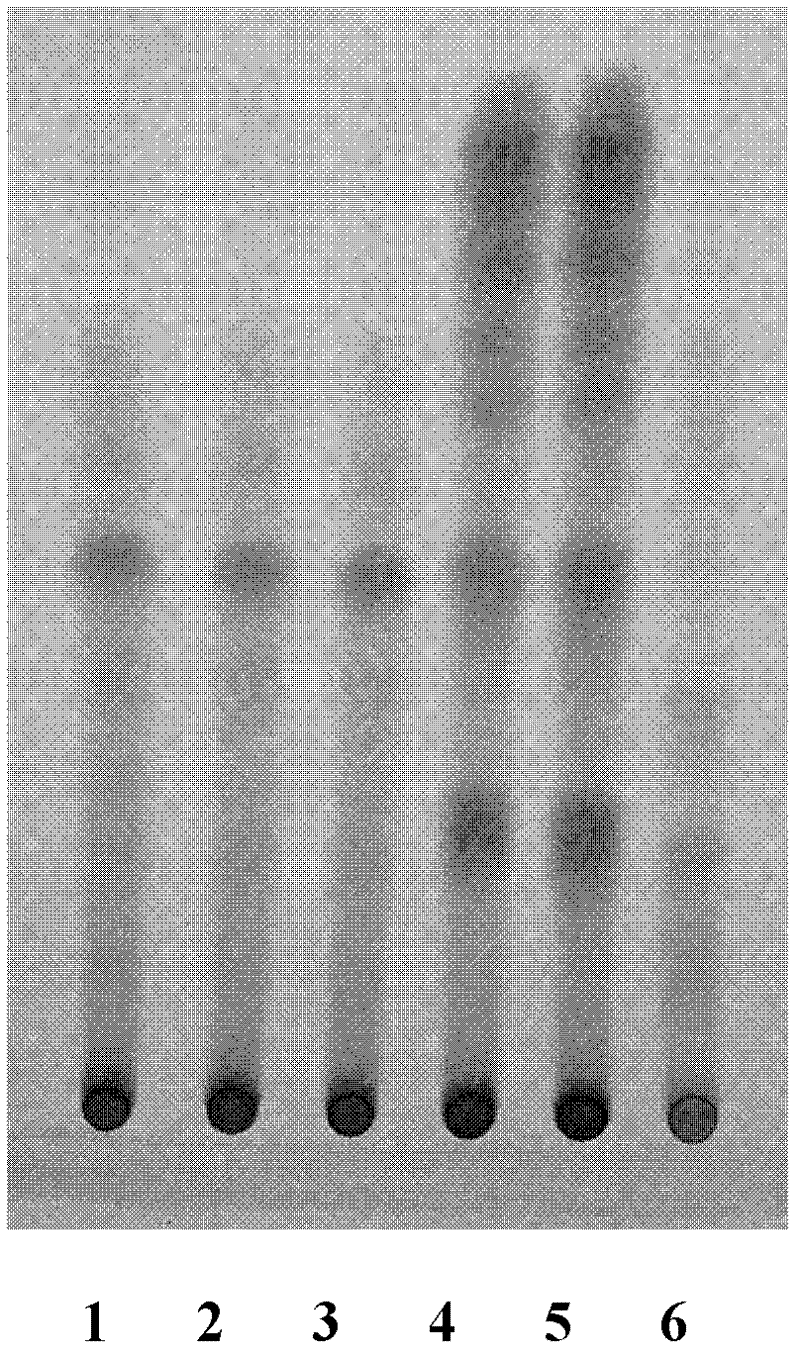
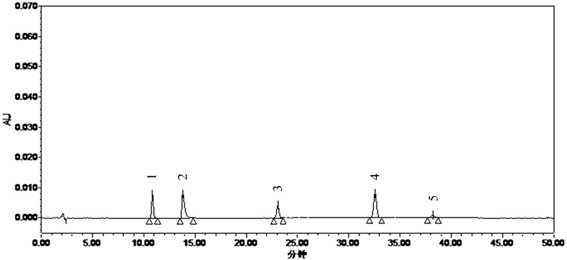
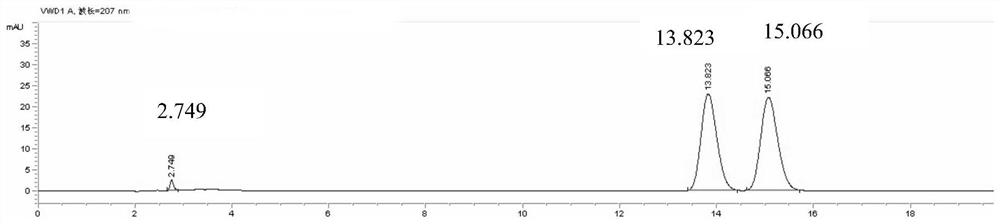
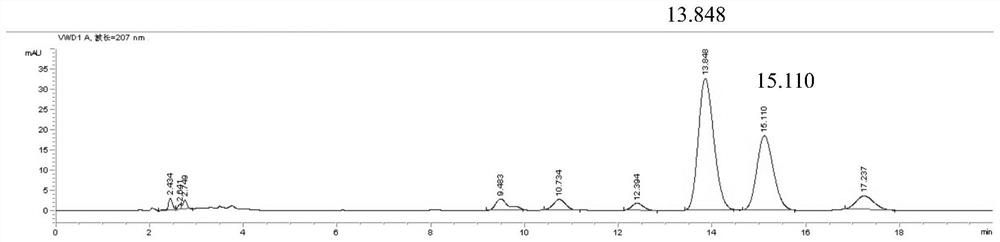
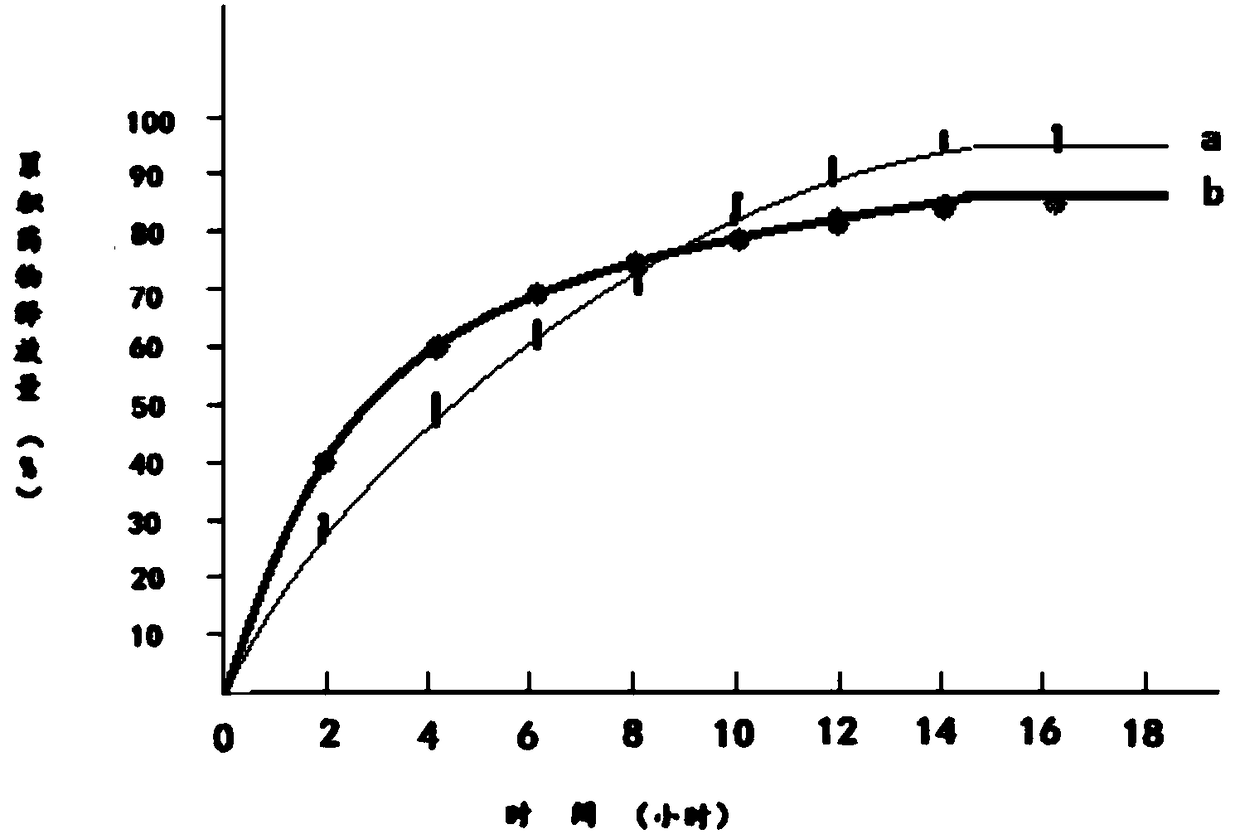
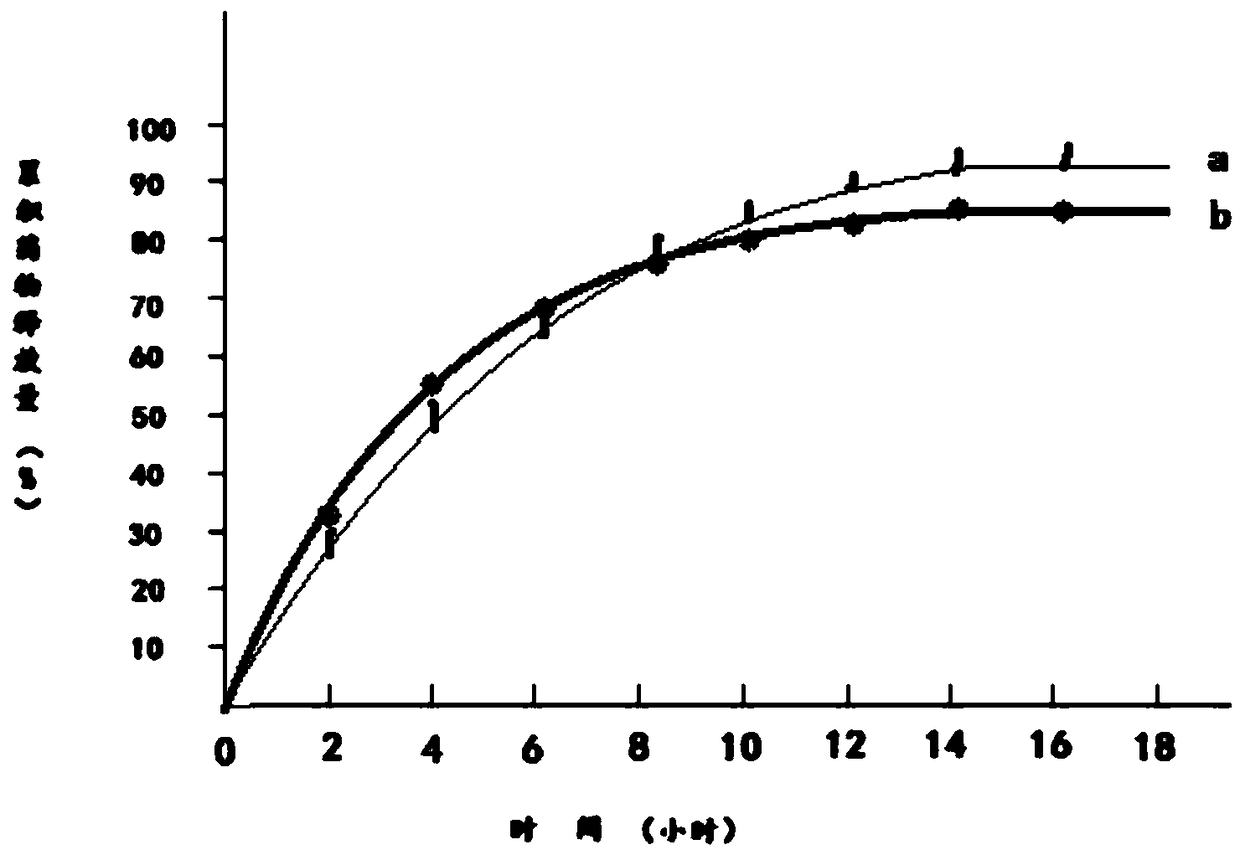
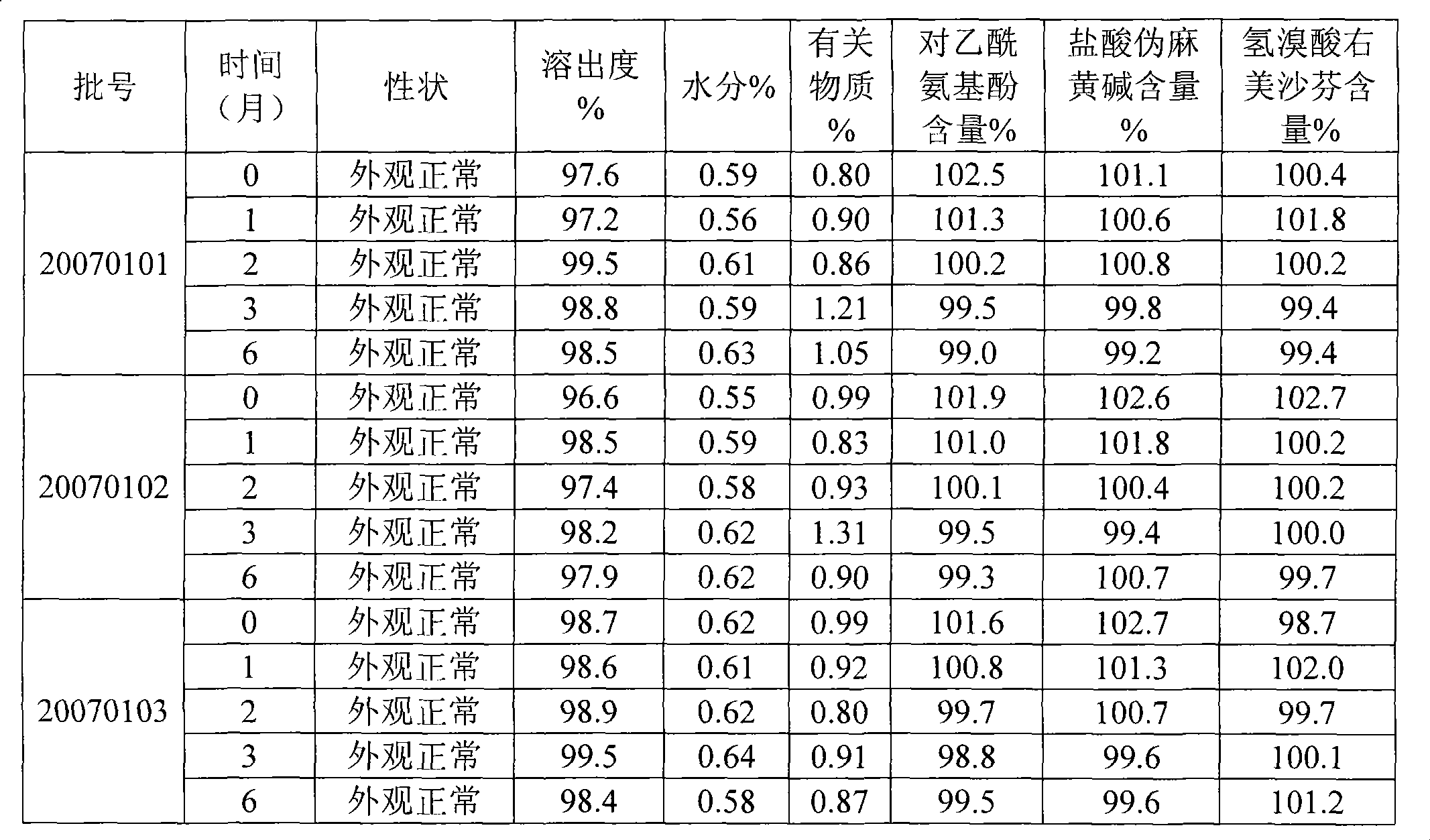
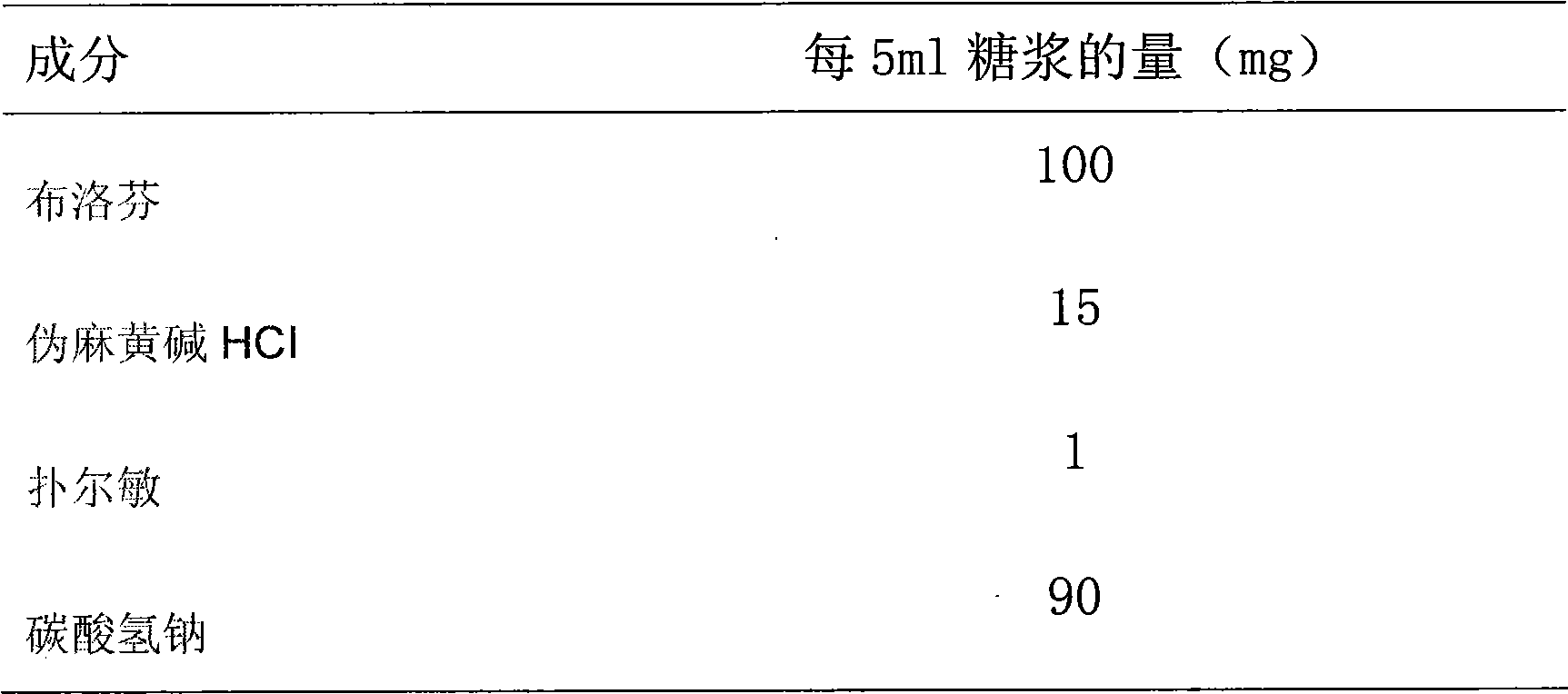
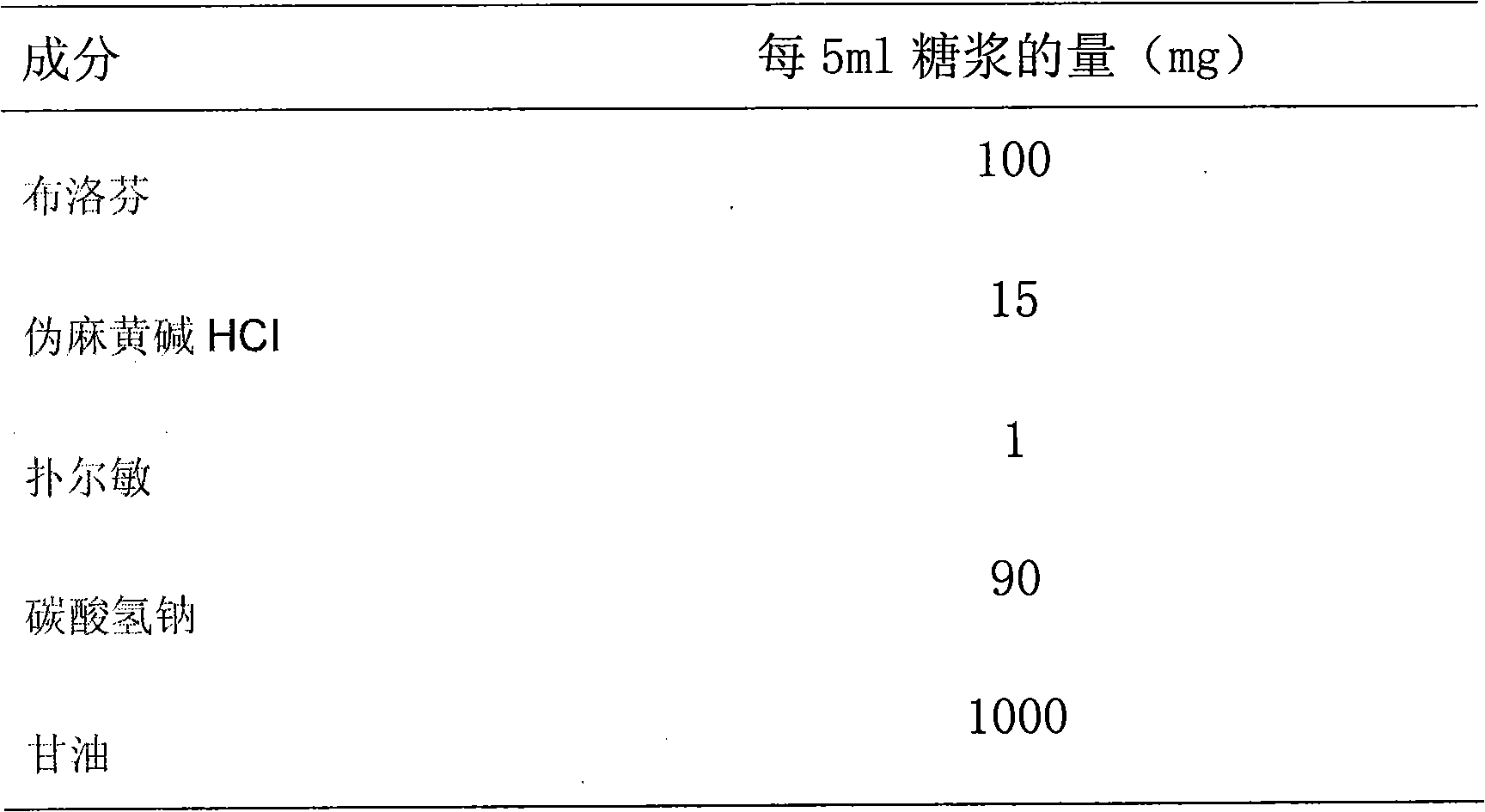
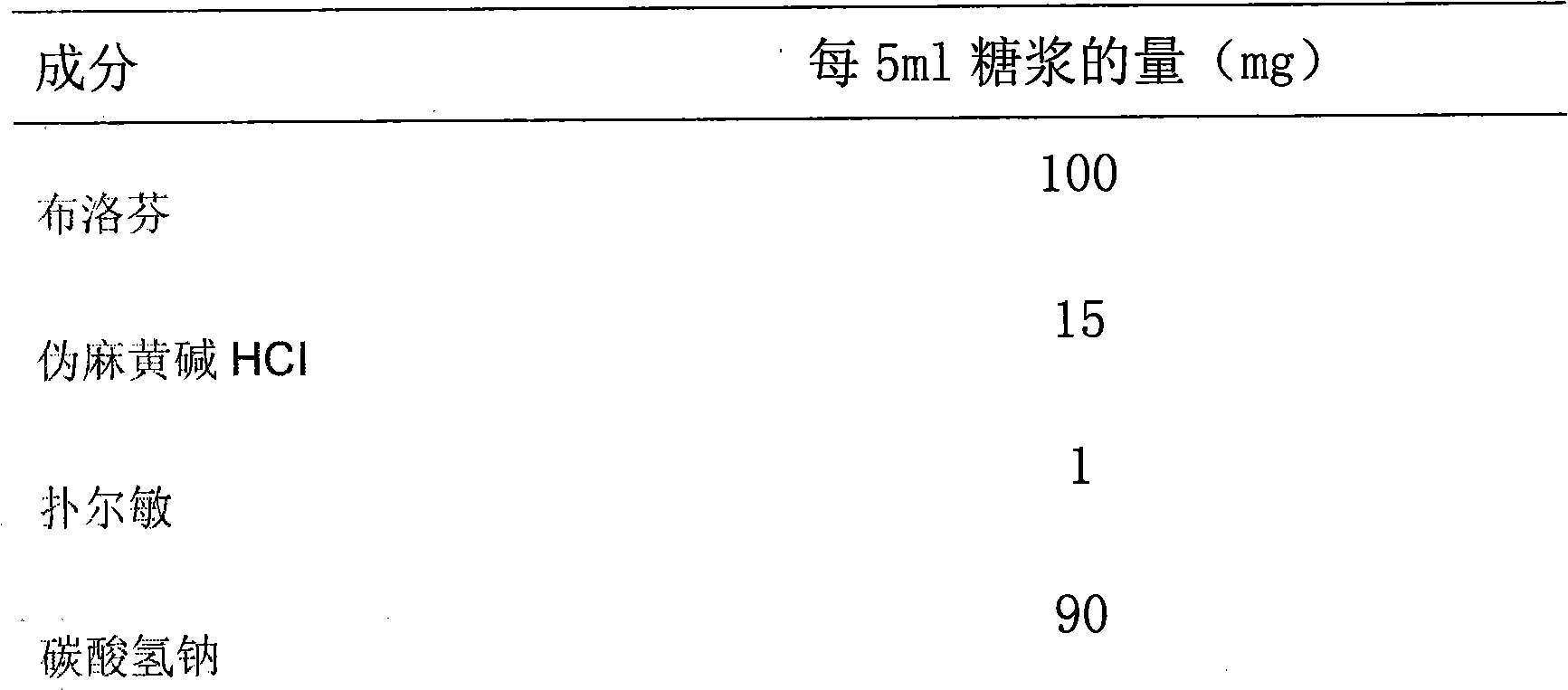
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Pseudoephedrine HCl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fingerprint spectrum construction method and detection method of Guizhishaoyaozhimu decoction composition
ActiveCN113049724AImprove responseMultiple peaksComponent separationGallic acid esterHplc mass spectrometry
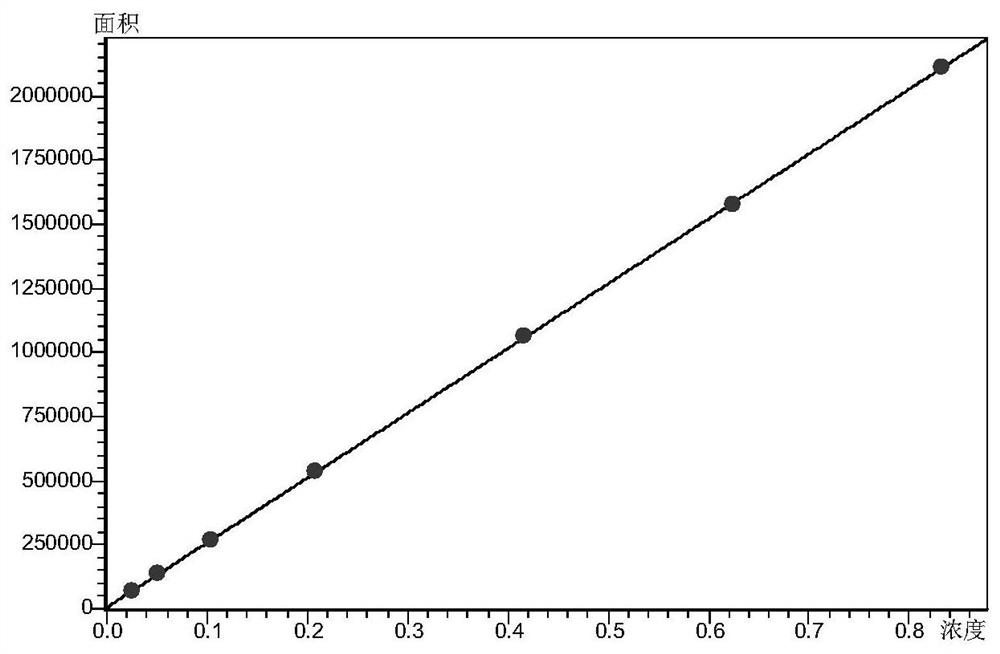
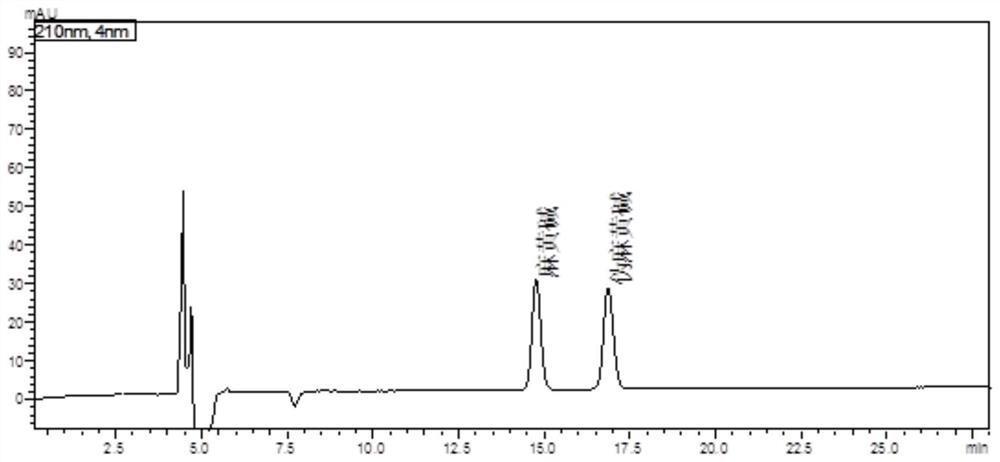
The invention discloses a fingerprint spectrum construction method and a detection method of a Guizhishaoyaozhimu decoction composition, and the fingerprint spectrum construction method comprises the following steps: (1) precisely weighing the Guizhishaoyaozhimu decoction composition, adding water, and carrying out ultrasonic treatment to obtain a test solution; (2) preparing 22 reference substances such as gallic acid, catechin, albiflorin, ephedrine hydrochloride and pseudoephedrine hydrochloride into a reference substance solution; and (3) precisely sucking the test solution and the reference solutions, injecting the test solution and the reference solutions into an ultra-high performance liquid chromatograph for chromatographic analysis at the detection wavelength of 210 nm to obtain a test fingerprint spectrum and a reference chromatographic spectrum, and formulating a standard fingerprint spectrum of the Guizhishaoyaozhimu decoction composition. The quality of a product to be detected can be comprehensively reflected through the standard fingerprint spectrum, and the product quality of the Guizhishaoyaozhimu decoction preparation can be effectively controlled.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD +1
Detection method of Wuweiganlu preparation
ActiveCN102645493AQuality improvementStable quality detection methodComponent separationPreparing sample for investigationPseudoephedrine HydrochlorideJuniperus formosana
The invention provides a detection method of a Wuweiganlu preparation. The method comprises the following steps of: performing microscopic identification of the microscopic characteristics of juniperus formosana and myricaria in the Wuweiganlu preparation; performing thin-layer chromatography identification of rhododendron anthopogonoide, juniperus formosana and artemisia sieversiana in the Wuweiganlu preparation; and measuring the content of ephedrine hydrochloride and pseudoephedrine hydrochloride in ephedra and the content of artemisetin in artemisia sieversiana in the preparation by a high performance liquid chromatography. The detection method provided by the invention has the advantages of good reproducibility and stability, high precision, strong specificity, clear spot color, high separation degree, accurate content and the like, and is simple to operate; and by creating a reliable quality detection method with strong specificity, the quality of the Wuweiganlu preparation can be effectively controlled so that the quality of the Wuweiganlu preparation is stable, safe and controllable.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Quality control method of dampness-resolving toxin-vanquishing composition
ActiveCN111983106AGuaranteed stabilityQuality improvementComponent separationRadix Astragali seu HedysariPinellia
The invention discloses a quality control method of a dampness-resolving toxin-vanquishing composition. The dampness-resolving toxin-vanquishing composition is mainly prepared from the following components: herba ephedrae, fried semen armeniacae amarae, gypsum, liquorice root, herba pogostemonis, cortex magnoliae officinalis, rhizoma atractylodis fried with bran, fried semen tsaoko, rhizoma pinellinae praeparata, poria cocos, radix et rhizoma rhei, radix astragali, semen lepidii and radix paeoniae rubra. The quality control method of the dampness-resolving toxin-vanquishing composition comprises the following steps: (1) determining the content of total anthraquinone, the content of free anthraquinone, the content of ephedrine hydrochloride, the content of pseudoephedrine hydrochloride andthe content of paeoniflorin in the dampness-resolving toxin-vanquishing composition by adopting high performance liquid chromatography, and calculating the content of combined anthraquinone, wherein the combined anthraquinone content is equal to the sum of the total anthraquinone content and the free anthraquinone content; and (2) identifying ephedra, liquorice and mangnolia officinalis by adopting thin-layer chromatography. By implementing the method, each link in the production process of the dampness-resolving toxin-vanquishing composition can be well controlled, and the quality stability and controllability of the product are effectively ensured.
Owner:GUANGDONG YIFANG PHARMA
Medicinal composition containing ibuprofen
ActiveCN101028258AGood curative effectOrganic active ingredientsAntipyreticPseudoephedrine HydrochlorideCurative effect
A composite medicine containing ibuprofen, which may be the free acid of ibuprofen, salt of ibuprofen, their combination, or the combination of ibuprofen and pseudoephedrine hydrochloride, is disclosed.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Preparation containing Cetirizine Hydrochloride and hydrochloric pseudoephedrine and its prepn. method
InactiveCN1498617AOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideUse medication
A medicine in the form of tablet is composed of the core tablet prepared from pseudoephedrine hydrochloride and pharmacologically receptable carrier and the coated layer prepared from cetirizine hydrochloride and pharmacologically receptable carrier. Its advantages are quick release of cetirizine hydrochloride, slow release of pseudoephedrine hydrochloride.
Owner:SHANGHAI SINE PHARMA LAB
Quality detection method of dampness-resolving toxin-vanquishing composition
ActiveCN111735889AGuaranteed stabilityEnsure controllabilityComponent separationPaeoniae RadixOfficinalis
The invention discloses a quality detection method of a dampness-resolving toxin-vanquishing composition. The dampness-resolving toxin-vanquishing composition is mainly prepared from the following components: herba ephedrae, fried semen armeniacae amarae, gypsum, liquorice root, herba pogostemonis, cortex magnoliae officinalis, rhizoma atractylodis fried with bran, fried semen tsaoko, rhizoma pinellinae praeparata, poria cocos, rheum officinale, astragalus membranaceus, semen lepidii, and radix paeoniae rubra. The quality detection method of the dampness-resolving toxin-vanquishing compositioncomprises the following steps: measuring the content of total anthraquinone, the content of free anthraquinone, the content of ephedrine hydrochloride, the content of pseudoephedrine hydrochloride and the content of paeoniflorin in the dampness-resolving toxin-vanquishing composition by adopting high performance liquid chromatography, and calculating the content of combined anthraquinone; whereinthe combined anthraquinone content = the total anthraquinone content - the free anthraquinone content. The method is high in specificity and stability, high in durability and capable of effectively guaranteeing the stability and controllability of the product quality of the dampness-resolving toxin-vanquishing composition in the large-scale production process.
Owner:GUANGDONG YIFANG PHARMA
Cetirizine hydrochloride pseudo ephedrine sustained-release pellet and preparation method thereof
InactiveCN104490880AIncreased surface distribution areaReduce absorption rateOrganic active ingredientsRespiratory disorderCetirizine HydrochlorideTherapeutic effect
The invention relates to a cetirizine hydrochloride pseudo ephedrine sustained-release pellet. The cetirizine hydrochloride pseudo ephedrine sustained-release pellet comprises a medicated pellet body and a coating layer; the coating layer wraps the medicated pellet body; the medicated pellet body comprises 5 mg of cetirizine hydrochloride, 125 mg of pseudoephedrine hydrochloride, 80 mg of core pellets, 100-200 mg of fillers, 25-125 mg of lubricating agents and 5-50 mg of binding agents; the coating layer comprises 45-225 mg of Eudragit NE30D and 14-68 mg of talcum powder; the preparation method includes the steps that (1) material preparing; (2) mixing; (3) binding agent preparing; (4) pelleting; (5) coating material preparing, (6) coating; (7) filling; and (8) conducting aluminum plastic and obtaining end products. The cetirizine hydrochloride pseudo ephedrine sustained-release pellet is used for remitting nasal symptoms or non-nasal symptoms caused by perennial or seasonal anaphylactic rhinitis; a novel sustained release preparation and a novel pellet preparation are adopted, the treatment effect is stable, and the bioavailability is higher.
Owner:HARBIN SHENGJI PHARMA
Cetirizine and pseudoephedrine sustained-release capsule and preparation method thereof
InactiveCN101474166AWell mixedQuick effectOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideSustained Release Capsule
The invention discloses a cetirizine pseudoephedrine slow release capsule. Cetirizine hydrochloride is prepared to quick release pellets and pseudoephedrine hydrochloride is prepared to slow release pellets, in accordance with the weight proportion that each 1000 preparations contain 5g of cetirizine hydrochloride and 120g of pseudoephedrine hydrochloride, the two pellets are uniformly mixed and encapsulated; or the two pellets are encapsulated respectively in proportion. The quick release part of the cetirizine hydrochloride and the slow release part of the pseudoephedrine hydrochloride are synthesized together subsequent to being prepared to the pellets respectively, so as to prepare the capsule with two different medicine-releasing speeds, the medicine-releasing speed of each pellet is uniform and the medicine effect is steady. The capsule has the advantages of even and extensive distribution of the medicine in vivo subsequent to the administering, sufficient absorption of the medicine, small stimulation to gastrointestinal tracts and good reproducibility of the medicine-releasing rule, and improves, while maintaining the properties of rapid effecting and long half-life of the cetirizine, the pharmacokinetic properties of the pseudoephedrine hydrochloride to administer twice per day instead of having to administer four times per day, so that both reach the optimal cooperative curative effect.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Cetirizine pseudoephedrine sustained-release capsules
InactiveCN101181244AAvoid molding qualityHigh yieldOrganic active ingredientsCapsule deliveryCetirizine HydrochlorideSustained Release Tablet
The invention relates to a cetirizine pseudoephedrine sustained-release capsule preparation. The interior of the capsule is provided with two tablets with different drug releasing speeds, namely, the cetirizine hydrochloride immediate-release tablet and the pseudoephedrine hydrochloride sustained-release tablet. The preparation has simple preparation process, and the ordinary tablet press machine can complete the process by only one time of tablet pressing; when taken, the cetirizine hydrochloride and the pseudoephedrine hydrochloride can be released simultaneously, so as to better achieve the purpose of combined treatment.
Owner:BEIJING SUNHO PHARMA
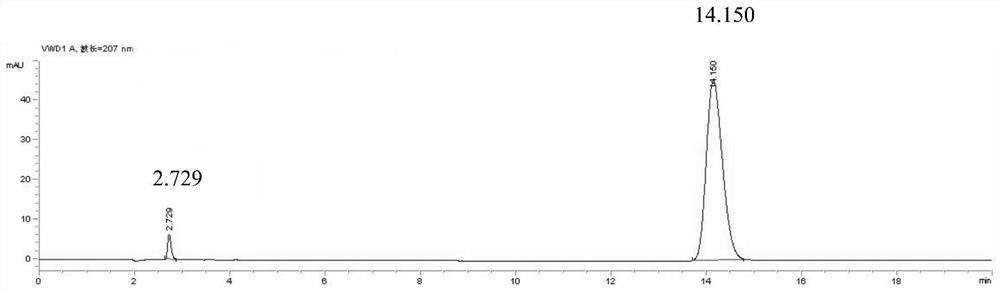
Method for determining ephedrine hydrochloride content in lung-clearing inflammation pill by high-performance liquid phase
The invention relates to a method for determining ephedrine hydrochloride content in a lung-clearing inflammation pill by a high-performance liquid phase, wherein the chromatographic condition mobile phase is acetonitrile and 0.02mol / L of monopotassium phosphate solution with pH2.7; the volume ratio is 4:96; the detection wavelength is 207nm; the flow speed is 1.0ml / min; the chromatographic column is Dikma 5mumC184.6*250mm, and the column temperature is 30 DEG C. The separating effect of ephedrine hydrochloride and pseudoephedrine hydrochloride peak is good under a flow phase condition; the quantity of the theoretical plates is 6976 according to the ephedrine hydrochloride peak, and 1g of the product contains ephedrine hydrochloride (C10H15NO.HCl) being not less than 0.65mg. According to the determination method disclosed by the invention, the controllability of content standard of the drug is improved; the internal content of the product is ensured, and the method has significance in the aspects of promoting product sales, increasing the market competitiveness of the product and ensuring the medication safety of a patient.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Anti-coronavirus traditional Chinese medicine granules and preparation method and application thereof
ActiveCN111888434AShorten recovery timeShorten the course of the diseaseAntiviralsPharmaceutical non-active ingredientsNaringinViral illness
Owner:SHANDONG BUCHANG PHARMA
Cold resistance compound rimantadine preparation
InactiveCN1689565AEffective preventionEffective therapeuticOrganic active ingredientsAntiviralsPseudoephedrine HydrochlorideCold resistance
The present invention relates to cold resisting compound rimantadine preparation. The preparation is composition containing rimantadine hydrochloride, acetaminophen, pseudoephedrine hydrochloride, chlorpheniramine and its pharmaceutically acceptable salt. The composition is used in treating and remitting cold.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
A bioadhesive nasal gel product of compound rupatadine and its preparation method
ActiveCN106265664BRapid relief of various symptomsRelieve various symptomsOrganic active ingredientsAntipyreticMetizolineXylometazoline hydrochloride
The invention discloses a compound rupatadine bioadhesion nasal gel product and a preparation method. The compound rupatadine bioadhesion nasal gel product is characterized by being prepared from, 10-100 parts of rupatadine and salts thereof, 5-20 parts of xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, 1-4 parts of furacilin, 300-5000 parts of polymer gel materials, 2-200 parts of a biological adhesive, 180-200 parts of a buffer salt, 800-1600 parts of a wetting agent and 20000 parts of sterile water for injection. The wetting agent is dissolved into 2 / 3 of the sterile water for injection to obtain a solution A; the polymer gel material is stirred and added into the solution A to obtain a gel system B; the biological adhesive is dispersed into the gel system B to obtain a gel system C; the rupatadine and salts thereof, the xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, the furacilin and the buffer salt are successively added into 1 / 3 of the sterile water for injection, and sonication is performed to obtain a solution D; the solution D is dispersed into the gel system C, and stirring is performed to obtain the final product.
Owner:重庆市人民医院
Quality control method of pinellia ternate syrup
PendingCN111830172AEliminate distractionsEasy to separateComponent separationPhosphoric acidPinellia tuberifera
The invention relates to a quality control method of pinellia ternate syrup. The method comprises the following steps of preparation of a test solution, taking a pinellia ternate syrup sample to be detected, adding a sodium hydroxide aqueous solution, distilling, collecting the distillate by using a hydrochloric acid aqueous solution, filtering, and taking the subsequent filtrate to prepare the test solution; preparing a reference substance solution, taking ephedrine hydrochloride and pseudoephedrine hydrochloride as reference substances, and adding a solvent for dissolving to prepare the reference substance solution; injecting the test solution and the reference solution into a liquid chromatograph for determination; wherein a mobile phase of the liquid chromatograph is an acetonitrile-0.3wt%-0.7 wt% phosphoric acid aqueous solution with the volume ratio of (3.5-7):(93-96.5). According to the method disclosed by the invention, a good separation effect of ephedrine hydrochloride and pseudoephedrine hydrochloride is realized, other substances are not interfered, and meanwhile, the method disclosed by the invention is strong in specificity, high in accuracy and good in reproducibility.
Owner:广州白云山潘高寿药业股份有限公司
Preparation method of pseudoephedrine hydrochloride sustained-release preparation
InactiveCN108992394AStable drug releaseGood slow release functionPowder deliveryOrganic active ingredientsMagnetic stirrerChemistry
The invention provides a preparation method of a pseudoephedrine hydrochloride sustained-release preparation. The preparation method comprises the following steps: dissolving pseudoephedrine hydrochloride in 95% ethanol to obtain a solution A, and adding hydroxypropyl beta-cyclodextrin and mannitol into the solution A to obtain a solution B; dissolving polylactic acid and polyethylene glycol 200 in acetone to obtain a solution C, mixing the solution B with the solution C to obtain a solution D, transferring the solution D to a magnetic stirrer, continuously stirring the solution D for 12 hours, lowering the temperature of the solution D to 0-1 DEG C within 2 hours, allowing to stand still for 12 hours, and maintaining the temperature of the solution D at 0-1 DEG C during the standing period; heating the solution D after standing till for 12 hours, continuously stirring when the temperature of the solution D is raised to 15-18 DEG C, controlling the temperature of the solution D at 15-18 DEG C when stirring, and preparing the pseudoephedrine hydrochloride sustained-release preparation with a low-temperature spray drying method after continuously stirring for 12 hours. According to the invention, the dosage of a capsule wall material is moderate, the drying temperature of materials is low, and the prepared drug-loading preparation is uniform in size and stable in drug release, and has the characteristic of slow release.
Owner:刘丽
Dampness-resolving and toxin-vanquishing composition quality control method based on near infrared spectroscopy
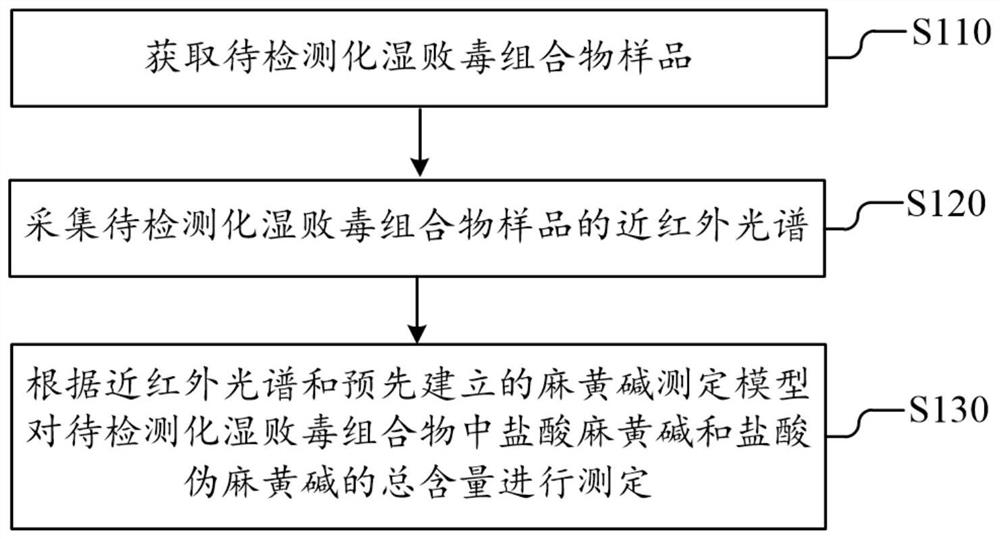
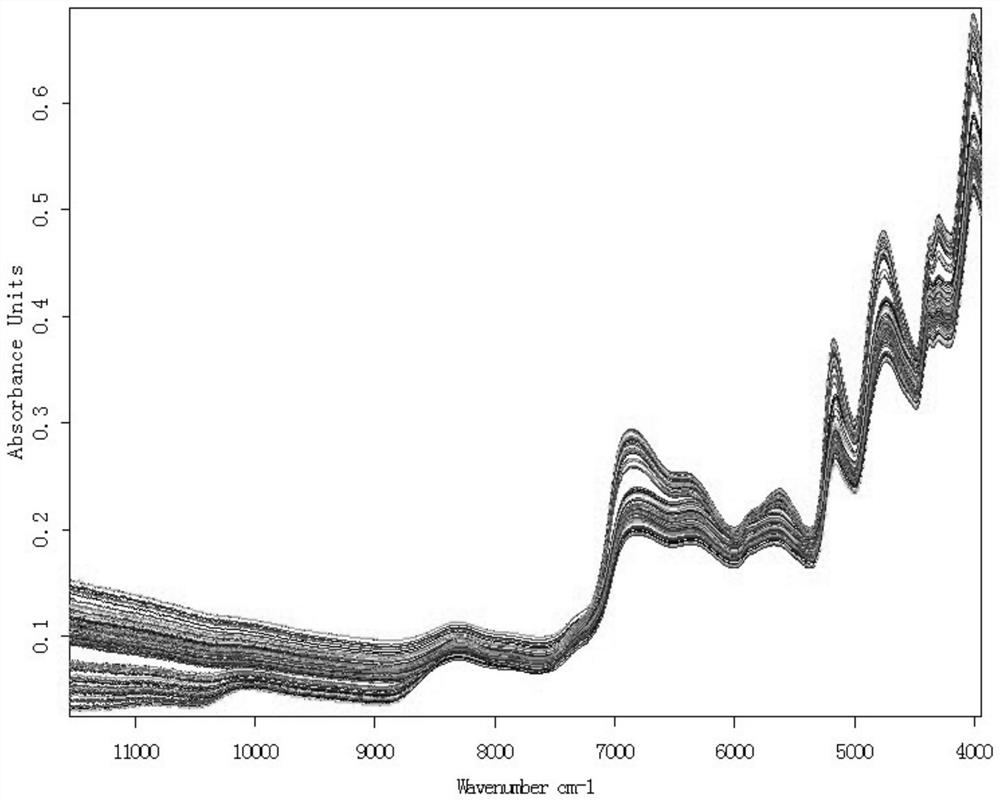
PendingCN112326592AConducive to the whole process controlShort cycleMaterial analysis by optical meansNear infrared spectraToxin
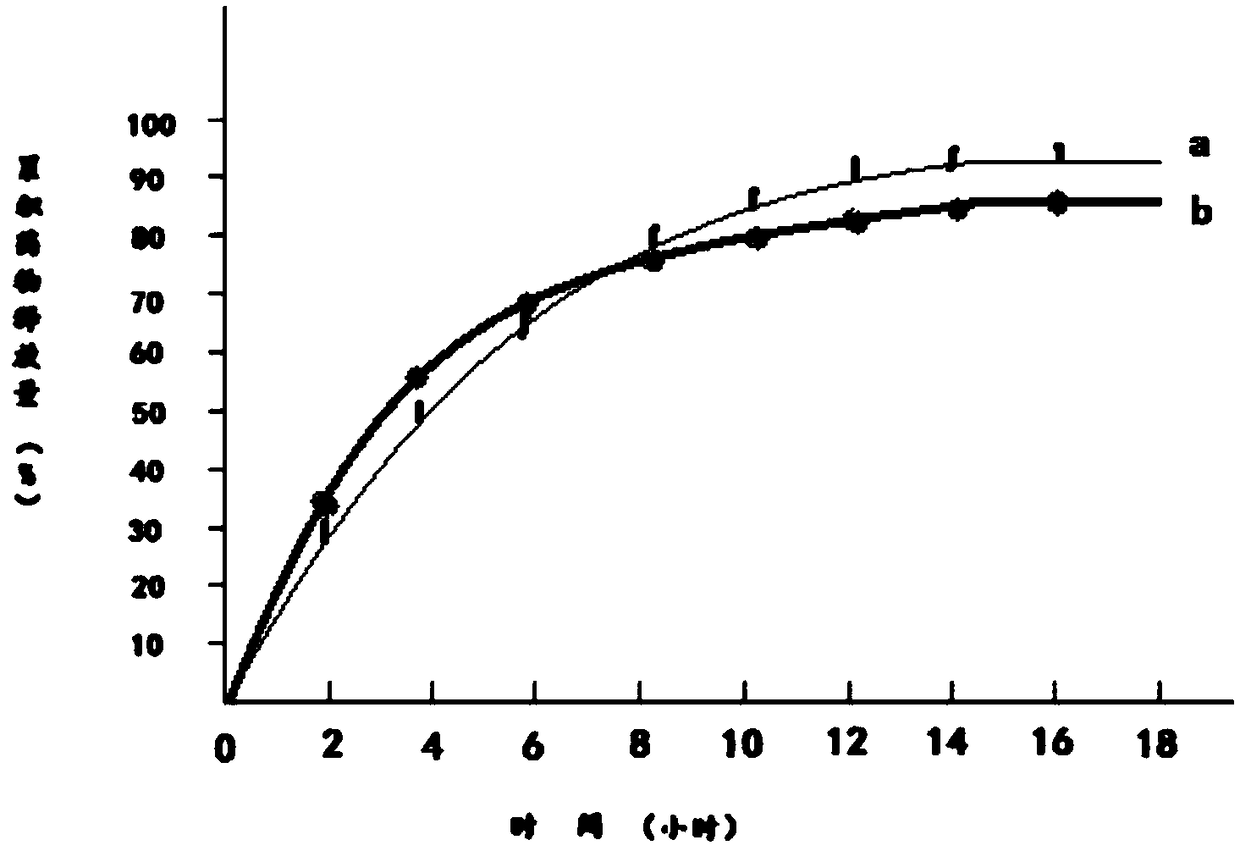
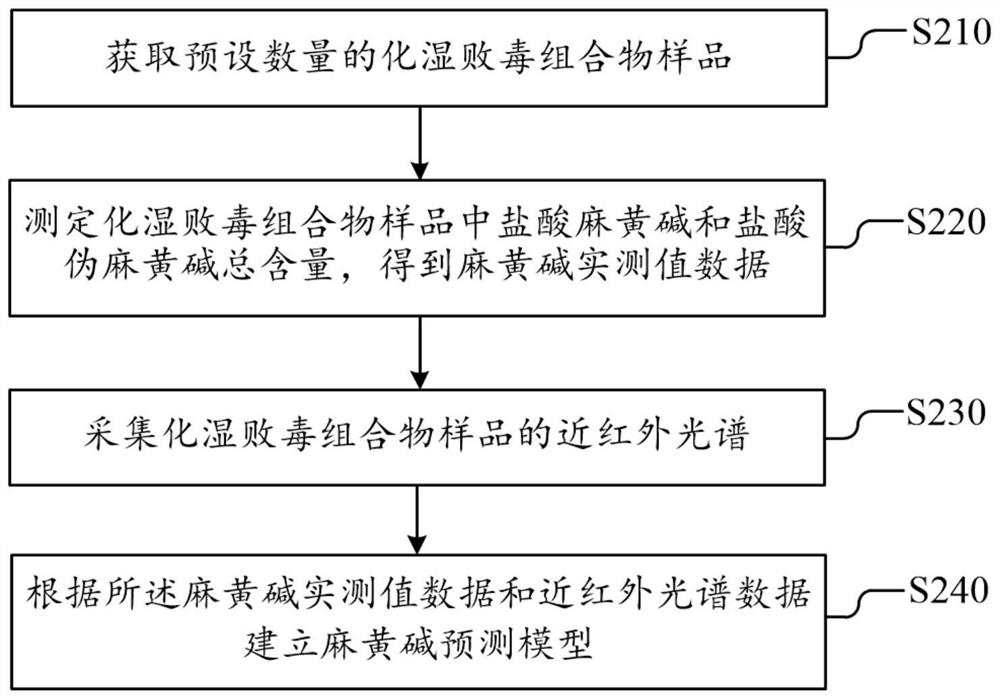
The invention discloses a dampness-resolving and toxin-vanquishing composition quality control method based on near infrared spectroscopy, which comprises the following steps: acquiring a dampness-resolving and toxin-vanquishing composition sample to be detected, collecting the near infrared spectrum of the dampness-resolving and toxin-vanquishing composition sample, and determining the total content of ephedrine hydrochloride and pseudoephedrine hydrochloride in the dampness-resolving and toxin-vanquishing composition to be detected by using a pre-established ephedrine determination model, and judging whether the quality of the dampness-resolving and toxin-vanquishing composition is qualified or not according to the determination result. The establishment method of the ephedrine determination model comprises the following steps: acquiring a sample, determining the total content of actual ephedrine hydrochloride and pseudoephedrine hydrochloride in the sample, collecting near infraredspectrum data of the sample, and establishing an ephedrine determination model according to the two types of data. The determination method provided by the invention is short in period, high in speedand high in accuracy, can realize on-line monitoring, is beneficial to controlling the whole process of the dampness-resolving and toxin-vanquishing composition, and is beneficial to improving the stability and reliability of product quality.
Owner:GUANGDONG YIFANG PHARMA
Orally administrable pharmaceutical formulation
InactiveUS6926906B2Difficult to extractMinimize the possibilityOrganic active ingredientsBiocideVegetable oilAdditive ingredient
The present invention relates to a pharmaceutical formulation for oral administration through a soft gelatin capsule drug delivery device, wherein the pharmaceutical formulation has Pseudoephedrine HCl as the active pharmaceutical ingredient. The active pharmaceutical ingredient is embedded into an oily matrix, also the formulation comprises viscosity imparting agents, a surfactant; a suspending agent; and a suspension medium. The viscosity-imparting agents are partially hydrogenated vegetable oil and colloidal silicon dioxide, the surfactant is lecithin, the suspending agent is yellow beeswax, and the suspension medium is soybean oil. In one preferred embodiment, the formulation consists essentially of about 60 mg by weight of Pseudoephedrine HCl, about 15-25 mg by weight of partially hydrogenated vegetable oil, about 10-20 mg by weight of yellow beeswax, about 2-8 mg by weight of lecithin, about 2-8 mg by weight of silicon dioxide; and about 150-250 mg by weight of soybean oil. Also disclosed is a process for preparing the formulation.
Owner:M S STRIDES
Quality detection method of five-flavor manna medicine bath preparation
ActiveCN102707007BRaise quality standardsQuality is easy to controlComponent separationJuniperus formosanaSilica gel
The invention discloses a quality detection method of a five-flavor manna medicine bath preparation. The five-flavor manna medicine bath preparation is made from raw materials of Juniperus formosana, ephedra, rhododendron anthopogonoide, myricaria and artemisia sieversiana according to a conventional method of pharmaceutics. On the basis of the primary standard, the thin layer chromatography of ephedra and rhododendron anthopogonoide is revised, the thin layer chromatography of Juniperus formosana and artemisia sieversiana is added. Under the simple and convenient condition of mobile phase, octyl silane bonding silica gel or phenyl bonding silica gel is used as filler, and simultaneously, the contents of ephedrine hydrochloride and pseudoephedrine hydrochloride in the ephedra are detected. The invention also provides a method for measuring the content of hyperoside in five-flavor manna preparation rhododendron anthopogonoide, thus ensuring the safety, effectiveness and controllability of product quality. By the quality detection method, the quality standard of the existing five-flavor manna medicine bath preparation is improved correspondingly.
Owner:JINKE TIBETAN MEDICINE QINGHAI PROV
Quality control method of loquat leaf lung-moistening cough-relieving paste
PendingCN112684095AAvoid product qualityGuaranteed curative effectComponent separationBiotechnologyAster tataricus
The invention discloses a quality control method of loquat leaf lung-moistening cough-relieving paste, and relates to the technical field of traditional Chinese medicine quality detection. The method comprises identification of loquat leaves, identification of aster tataricus, identification of ephedra and determination of ephedra content. Thin-layer chromatography identification is carried out on three main components including loquat leaves, aster tataricus and ephedra in the loquat leaf lung-moistening cough-relieving paste, and meanwhile, and the contents of effective components including ephedrine hydrochloride and pseudoephedrine hydrochloride in the traditional Chinese medicinal material ephedra are measured, so that the product quality can be effectively controlled, the product curative effect is guaranteed, and great central excitation side effects of people are not easy to generate.
Owner:GUIZHOU MAQIKA PHARMA
Canopy bulk decoction, preparation method and application of canopy bulk decoction
InactiveCN109453250AHigh extraction rateEasy to prepareDispersion deliveryRespiratory disorderEphedra sinicaMedicine
The invention discloses canopy bulk decoction. The canopy bulk decoction is prepared from the following raw materials in parts by weight: 15.38 parts of fried fructus perillae, 15.38 parts of honey cortex mori radicis, 15.38 parts of fried semen armeniacae amarum, 15.38 parts of poria cocos, 15.38 parts of pericarpium citri reticulatae, 15.38 parts of ephedra sinica stapf and 7.72 parts of radix glycyrrhizae preparata. A preparation method comprises the following steps that the fried fructus perillae, honey cortex mori radicis, fried semen armeniacae amarum, poria cocos, pericarpium citri reticulatae, ephedra sinica stapf and radix glycyrrhizae preparata are weighed according to the parts by weight, placed in a decoction container and boiled twice by adding water, the water equivalent to 10 times the total mass of the raw materials is added at the first time of decoction, and filtering is performed to obtain a first filtrate; the water equivalent to 8 times the total mass of the raw materials is added in the second decoction, and filtering is performed to obtain a second filtrate; and the first filtrate and the second filtrate are combined, concentrated and subjected to volume metering to obtain the canopy bulk decoction. The canopy bulk decoction obtained by the preparation method is high in extracting rate of ephedrine hydrochloride and pseudoephedrine hydrochloride.
Owner:HUNAN XINHUI PHARMA
Compound dichlorpseudomonas sustained-release pellets and preparation method thereof
ActiveCN109966254BImprove stabilityImprove bioavailabilityOrganic active ingredientsPill deliveryHydrophilic polymersImmediate release
The invention provides a compound desloratadine / pseudoephedrine (abbreviated as compound dichloropseudoephedrine) slow-release pellets and a preparation method thereof. The preparation of the compound dichloropseudoephedrine sustained-release pellets of the present invention is to adopt the extrusion spheronization method to prepare the pellet cores containing pseudoephedrine hydrochloride, and then utilize hot-melt coating technology to coat the pellet cores with slow-release layers and desloratadine-containing pellets in sequence. It is prepared as an immediate-release layer, and the coating material does not need to use any solvent. The composition of the lipid material and the hydrophilic polymer with surface activity involved in the present invention uses the hot-melt coating technology to prepare the compound dichloropseudoelphus slow-release pellets, which can not only make full use of the hot-melt coating technology, but also has the advantages of safety, environmental protection and energy saving. , high efficiency, can improve the advantages of drug stability and bioavailability, and can release the two drugs on demand and completely, the release curve is consistent with the reference preparation, and can be produced on a large scale.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Cetirizine pseudoephedrine sustained-release capsule and preparation method thereof
InactiveCN110974807AGood reproducibilityPiece weight smallOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochlorideMagnesium stearate
The invention provides a cetirizine pseudoephedrine sustained-release capsule and a preparation method thereof. The capsule is filled with cetirizine hydrochloride quick-release pellets and pseudoephedrine hydrochloride sustained-release pellets. The cetirizine hydrochloride quick-release pellet in each capsule is prepared from the components of 5 to 8mg of cetirizine hydrochloride, 90 to 110mg oflactose, 30 to 70mg of microcrystalline cellulose, 20 to 30mg of auxiliary materials, 4 to 9mg of coating materials and 1 to 3mg of magnesium stearate. The pseudoephedrine hydrochloride sustained-release pellet is prepared from the components of 100 to 150mg of pseudoephedrine hydrochloride, 60 to 80mg of a framework material, 20 to 40mg of a retardant and 1 to 3mg of magnesium stearate. The cetirizine hydrochloride quick-release pellets and the pseudoephedrine hydrochloride slow-release pellets are mixed. The process is simple. Dissolution and release of the two medicines do not interfere with each other. The release curve reproducibility is good. The tablet weight is small, and production and split charging are facilitated.
Owner:白喜平
Cetirizine and pseudoephedrine sustained-release capsule and preparation method thereof
InactiveCN101474166BWell mixedQuick effectOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideSustained Release Capsule
The invention discloses a cetirizine pseudoephedrine slow release capsule. Cetirizine hydrochloride is prepared to quick release pellets and pseudoephedrine hydrochloride is prepared to slow release pellets, in accordance with the weight proportion that each 1000 preparations contain 5g of cetirizine hydrochloride and 120g of pseudoephedrine hydrochloride, the two pellets are uniformly mixed and encapsulated; or the two pellets are encapsulated respectively in proportion. The quick release part of the cetirizine hydrochloride and the slow release part of the pseudoephedrine hydrochloride are synthesized together subsequent to being prepared to the pellets respectively, so as to prepare the capsule with two different medicine-releasing speeds, the medicine-releasing speed of each pellet isuniform and the medicine effect is steady. The capsule has the advantages of even and extensive distribution of the medicine in vivo subsequent to the administering, sufficient absorption of the medicine, small stimulation to gastrointestinal tracts and good reproducibility of the medicine-releasing rule, and improves, while maintaining the properties of rapid effecting and long half-life of the cetirizine, the pharmacokinetic properties of the pseudoephedrine hydrochloride to administer twice per day instead of having to administer four times per day, so that both reach the optimal cooperative curative effect.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Quick-acting anti-influenza cold capsule
InactiveCN104666376AImprove the immunityReduce swelling and painAntipyreticAnalgesicsVitamin b6Nervous system
The invention relates to the field of medicines, and in particular relates to a quick-acting anti-influenza cold capsule. The quick-acting anti-influenza cold capsule is characterized by being prepared from the following raw materials in parts by weight: 50-150 parts of acetaminophen, 50-150 parts of pseudoephedrine hydrochloride, 30-50 parts of chlorpheniramine maleate, 1-3 parts of dioxopromethazine, 10-30 parts of vitamin C, 10-30 parts of vitamin B1, 10-30 parts of vitamin B2, 10-30 parts of vitamin B6, 1-3 parts of furazolidone, 50-150 parts of radix isatidis and 30-50 parts of radix bupleuri. The quick-acting anti-influenza cold capsule provided by the invention is combination of traditional Chinese medicines and western medicines, and is capable of regulating a nervous system of the human body through western medicine components to reach the effects of relieving pain, clearing heat, relieving cough, preventing asthma, and relieving the symptoms such as rhinobyon and pharyngeal swelling and pain; necessary vitamins for human metabolism and the traditional Chinese medicines such as radix isatidis are added, so that the quick-acting anti-influenza cold capsule is capable of enhancing the resistivity of a human body and treating both symptoms and root causes from improvement of the immunity, and is short in treatment course, fast in acting, and free of causing doze after being taken.
Owner:YANTAI JUXIAN PHARMA
Paracetamol pseudophedrine sulfateand clemastine fumarate tablets, film-coated tablets and preparation method thereof
ActiveCN104873497AHigh dissolution rateImprove stabilityAntipyreticAnalgesicsCoated tabletsClemastine Fumarate
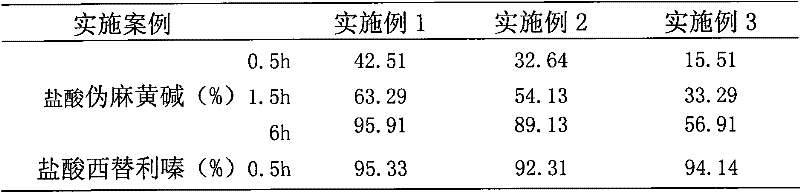
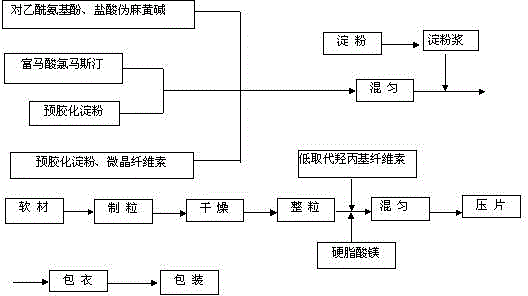
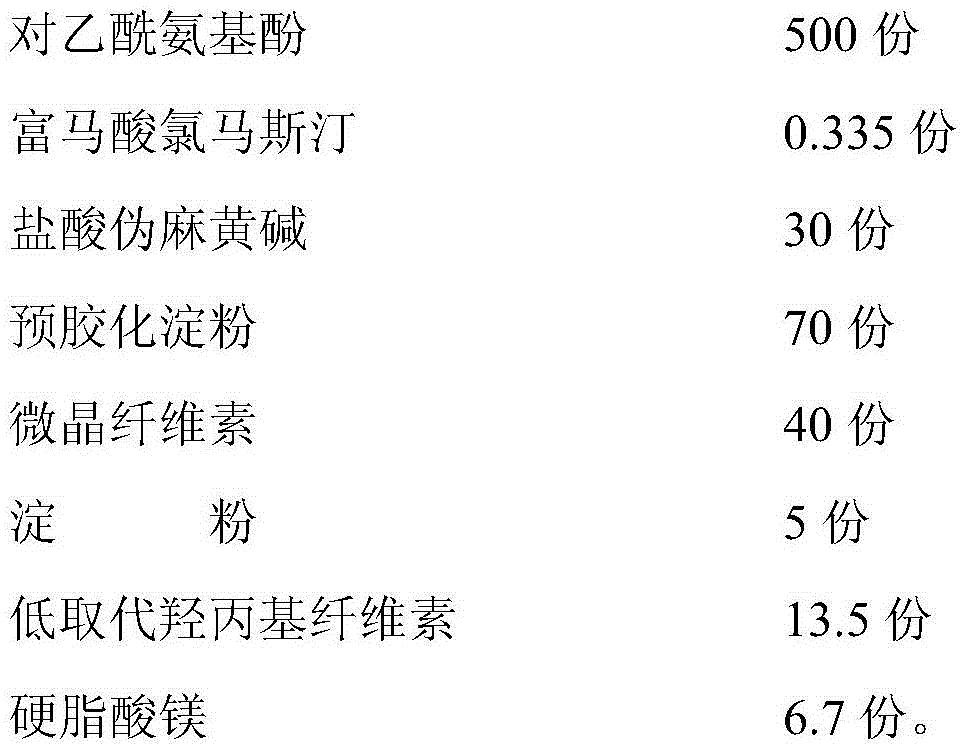
The invention discloses paracetamol pseudophedrine sulfateand clemastine fumarate tablets which are composed of the following ingredients: 400-600 parts of paracetamol, 0.3-0.4 part of clemastine fumarate, 25-35 parts of pseudoephedrine hydrochloride, 60-80 parts of pregelatinized starch, 35-45 parts of microcrystalline cellulose, 4-6 parts of starch, 12-15 parts of low-substituted hydroxy propyl cellulose and 6-8 parts of magnesium stearate. The invention also discloses paracetamol pseudophedrine sulfateand clemastine fumarate film-coated tablets and their preparation method. The paracetamol pseudophedrine sulfateand clemastine fumarate tablets have excellent dissolubility and stability and have properties of other preparations. In addition, the technology is simple and is easy for industrial mass production.
Owner:JINAN LIMIN PHARMA
Method for quantifying effective components of herba ephedrae in lung-heat-clearing and toxin-expelling granules
PendingCN113917000AAchieve separationRealize determinationComponent separationO-Phosphoric AcidGradient elution
The invention relates to the technical field of medicines, and particularly discloses a method for quantifying effective components of herba ephedrae in lung-heat-clearing and toxin-expelling granules. According to the method, a high performance liquid chromatography is adopted, and the chromatographic conditions are as follows: polar ether connected phenyl bonded silica gel is taken as a chromatographic column filler, acetonitrile is taken as a mobile phase A, a 0.2% phosphoric acid solution (containing 0.2% triethylamine) is taken as a mobile phase B, and gradient elution is carried out; the gradient elution process is as follows: the volume ratio of the mobile phase A to the mobile phase B is 1: 99 within 0-20 minutes; within 20-20.1 min, the volume ratio of the mobile phase A to the mobile phase B is gradually changed from 1: 99 to 50: 50 at a constant speed; within 20.1-25 min, the volume ratio of the mobile phase A to the mobile phase B is 50: 50; the measured effective components of ephedra are ephedrine hydrochloride and pseudoephedrine hydrochloride. The method provided by the invention is good in repeatability and high in precision, and determination results on different high performance liquid chromatographs and different chromatographic columns have no significant difference, so that the method provided by the invention is good in applicability and reproducibility and suitable for general popularization.
Owner:INST OF BASIC RES & CLINICAL MEDICINE CHINA ACAD OF CHINESE MEDICAL SCI
Capsule containing pseudoephedrine and method for preparing the same
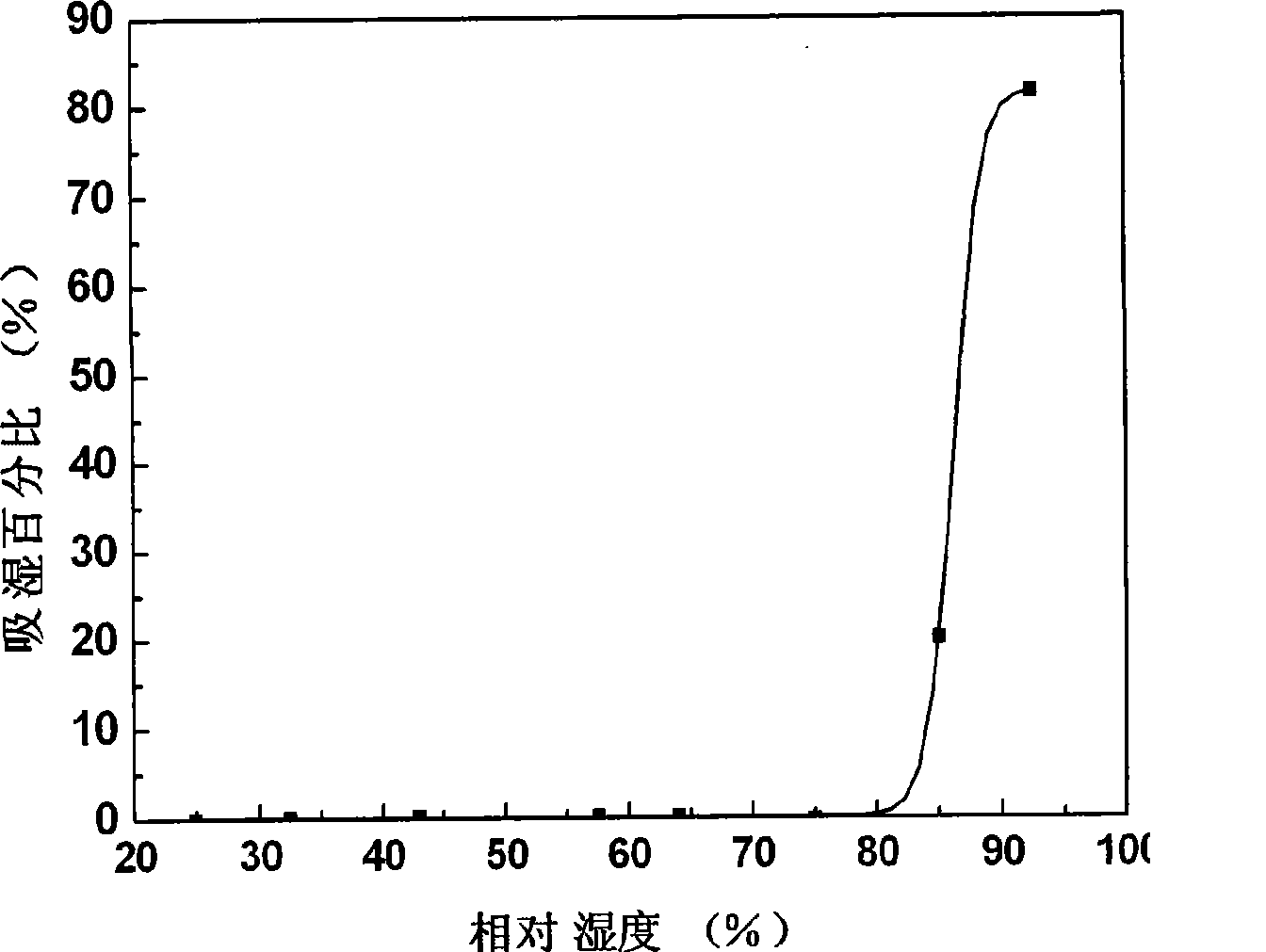
ActiveCN101152170BPrevent moisture absorption and liquefactionPrevent moisture absorption and corrosionPharmaceutical non-active ingredientsCapsule deliveryPseudoephedrine HydrochlorideIon exchange
The invention discloses a capsule containing pseudoephedrine and the preparation method, which solves the problem of hygroscopicity. The capsule containing pseudoephedrine of the invention contains cation exchange resin, which forms pseudoephedrine resin compound with pseudoephedrine hydrochloride or pseudoephedrine sulfate. The preparation procedure is as follows: pseudoephedrine hydrochloride or pseudoephedrine sulfate is proportioned and cation exchange resin is put into the solution for ion exchange reaction to produce drug resin compound; the compound is dried and pseudoephedrine resin compound is produced; the pseudoephedrine resin compound is mixed uniformly with formula components and filled into capsules with a filling engine. Compared with prior art, the invention adopts drug resin compound, which prevents moisture absorption and liquefaction of the drug, maintains the stability of the drug property, reduces the packaging cost of the capsule containing pseudoephedrine and ensures the safety and the effects of the clinical application of the drug.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Pharmaceutical composition comprising ibuprofen, pseudoephedrine and chlorpheniramine
InactiveCN102264358AReduce coldRelieve headacheOrganic active ingredientsDispersion deliverySodium bicarbonateActive agent
The present invention relates to a pharmaceutical composition comprising ibuprofen as the active agent, preferably chloropheniramine maleate as an antihistaminic agent, preferably pseudoephedrine HCl as a sympathomimetic amine, and an alkaline salt, such as sodium bicarbonate, in an amount adequate to maintain the final solution's pH level between 7 and 9.
Owner:博科药物化学有限公司
Quality Control Method of Huashi Baidu Composition
ActiveCN111983106BGuaranteed stabilityQuality improvementComponent separationBiotechnologyAnthraquinones
The invention discloses a method for quality control of a dampness-removing and detoxifying composition. The dehumidification and detoxification composition mainly includes the following components: ephedra, fried bitter almonds, raw gypsum, licorice, patchouli, magnolia officinalis, bran fried Atractylodes atractylodes, fried grass nuts, pinellia, Poria cocos, rhubarb, astragalus, Tinglizi, red peony; the quality control methods of Huashibaidu composition include: (1) Determination of Huashibaidu composition by high performance liquid chromatography Total anthraquinone content, free anthraquinone content, ephedrine hydrochloride content, pseudoephedrine hydrochloride content and paeoniflorin content in the product, and calculate the combined anthraquinone content; wherein, combined anthraquinone content=total anthraquinone content-free anthraquinone content; ( 2) Identify ephedra, licorice and Magnolia officinalis by TLC. By implementing the present invention, each link in the production process of the Huashi detoxification composition can be well controlled, effectively ensuring product quality stability and controllability.
Owner:GUANGDONG YIFANG PHARMA
Double-layer sustained release tablet treating allergic rhinitis and preparation method thereof
InactiveCN105232487AQuick releaseImprove compliancePill deliveryPharmaceutical non-active ingredientsDrug adsorptionPatient compliance
The invention discloses a double-layer sustained release tablet treating allergic rhinitis. The double-layer sustained release tablet is prepared from fexofenadine hydrochloride and pseudoephedrine hydrochloride, fexofenadine hydrochloride and pseudoephedrine hydrochloride are combined for use, the allergic rhinitis can be effectively treated, nasal congestion can be effectively removed, and the rhinobyon symptom is relieved. Meanwhile, the double-layer gel skeleton sustained release technology is adopted, a fast release layer fast releases fexofenadine hydrochloride in a fast disintergration mode, and the effect can be fast achieved; a slow release layer slowly releases pseudoephedrine hydrochloride for 12 hours, stable blood concentration can be kept, and bad reactions are reduced; the number of taking times is reduced, and patient compliance is enhanced; the drug release time is prolonged, drug adsorption is improved, and bioavailability can be improved easily.
Owner:HARBIN SHENGJI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com