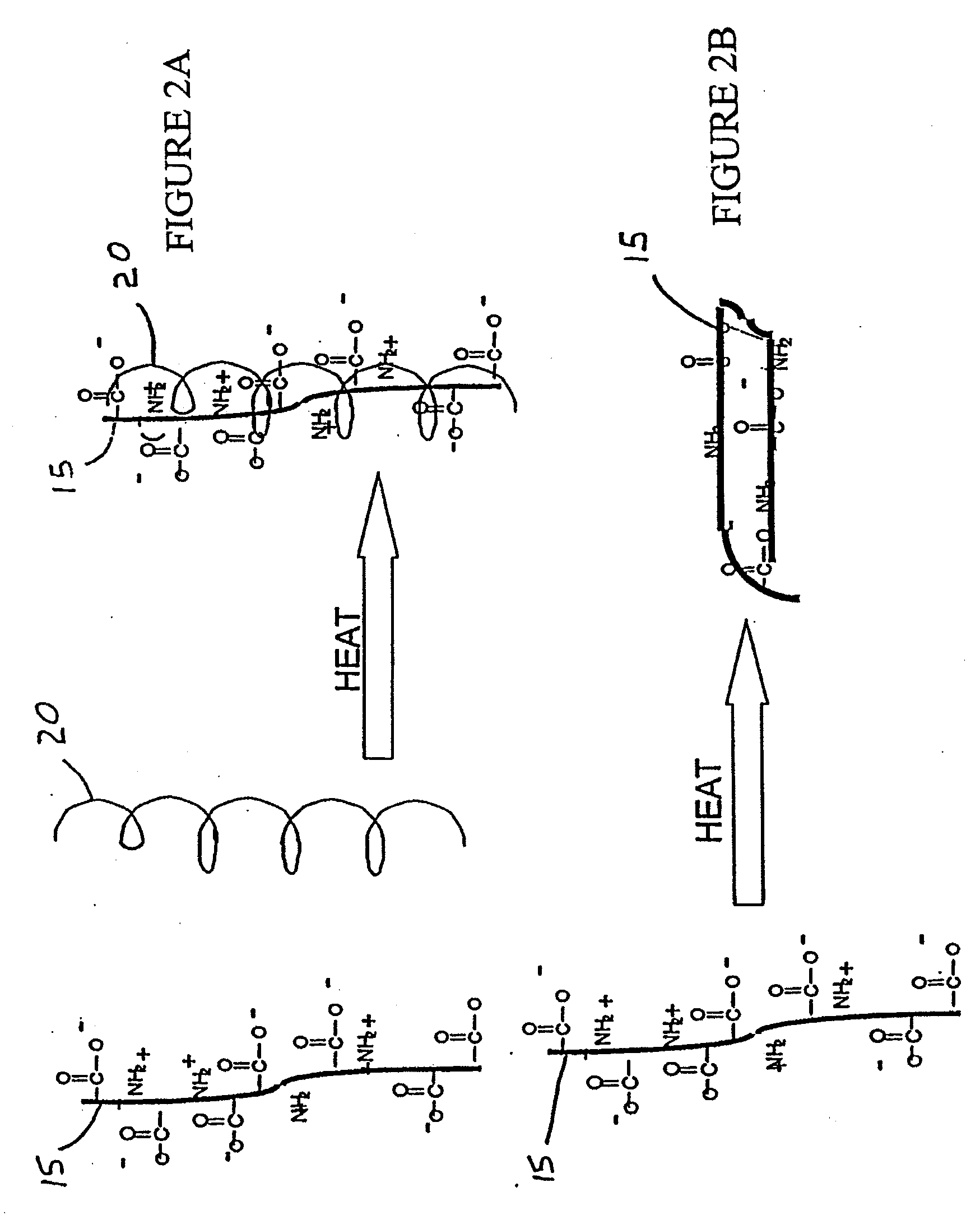
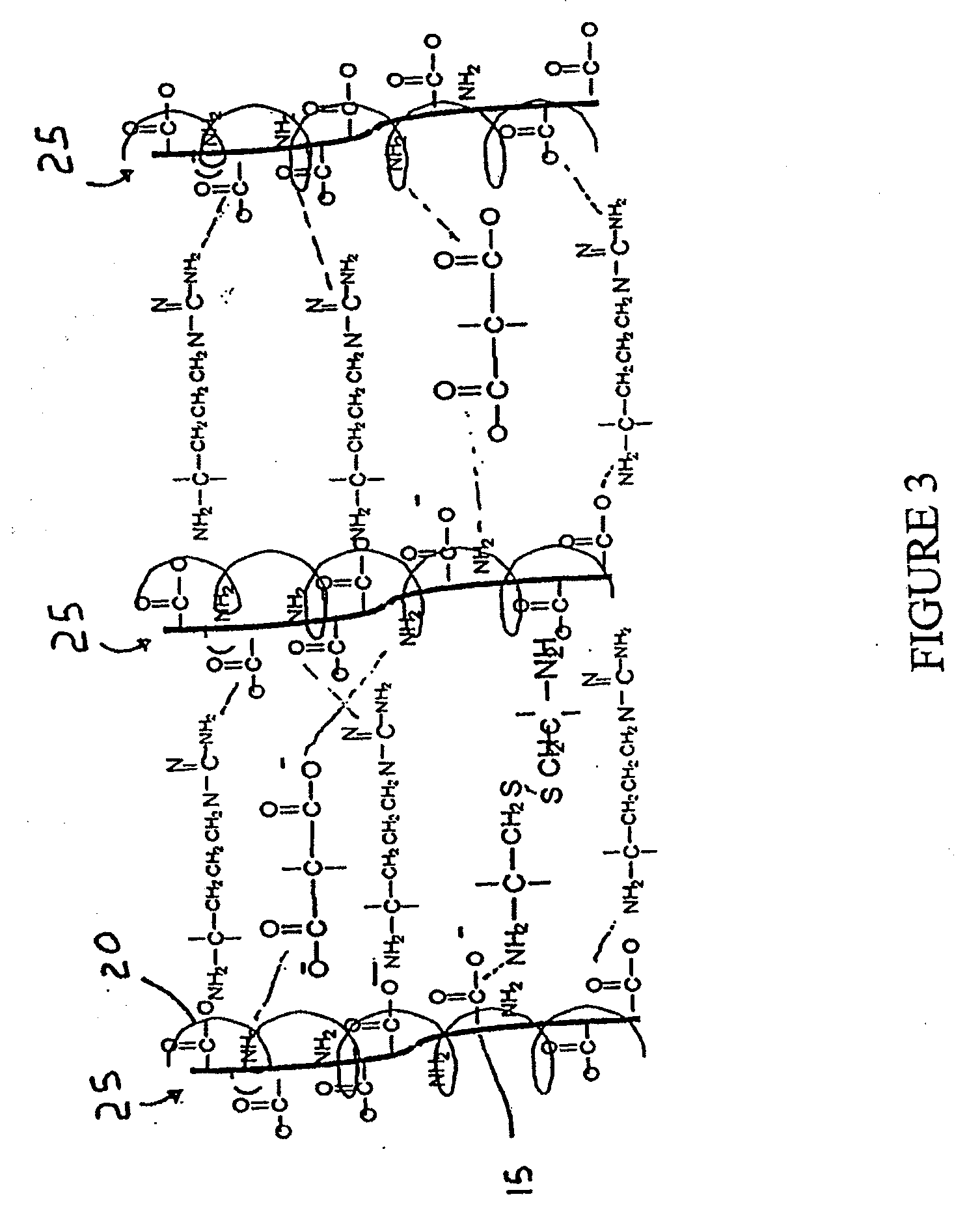
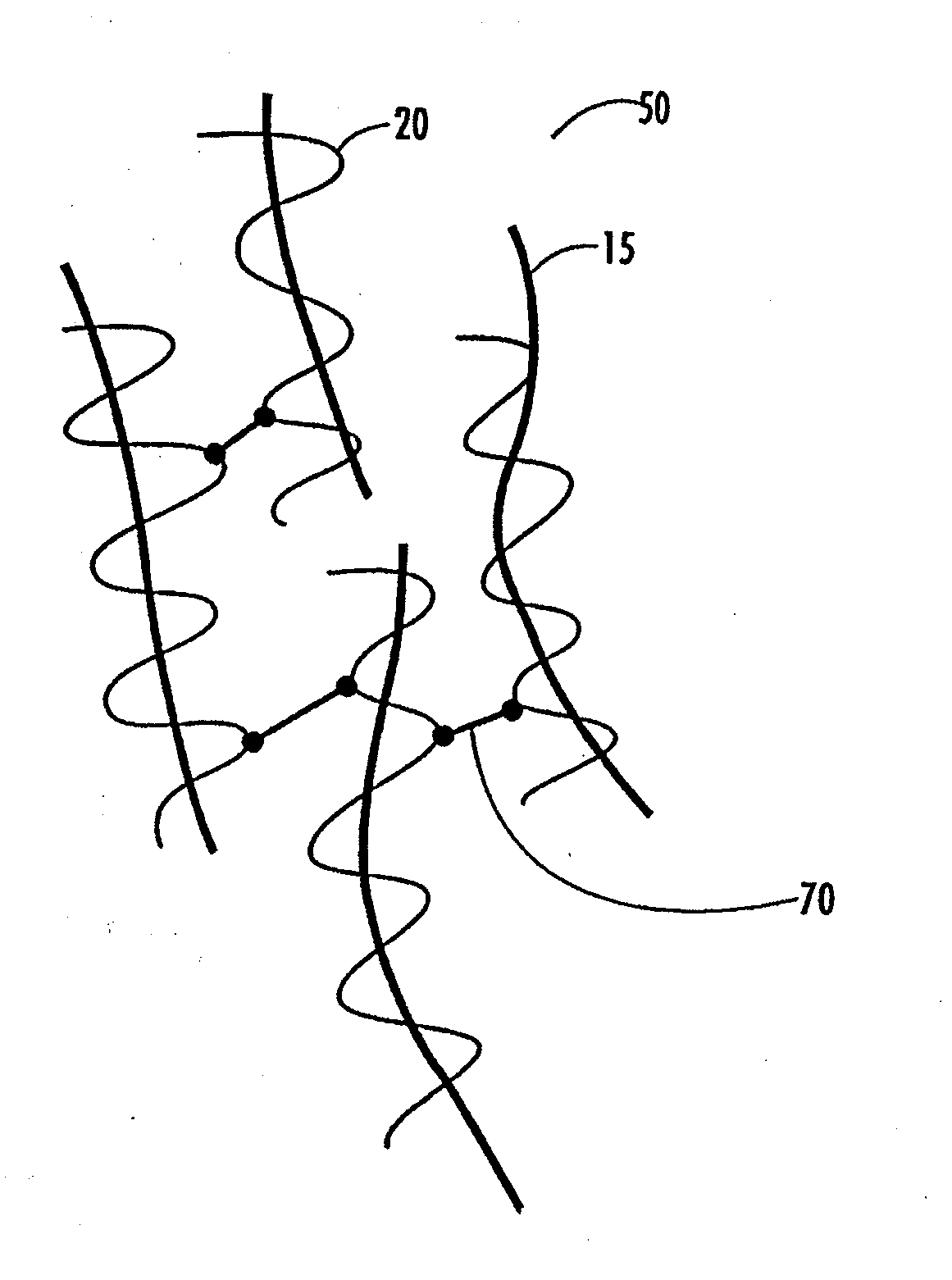
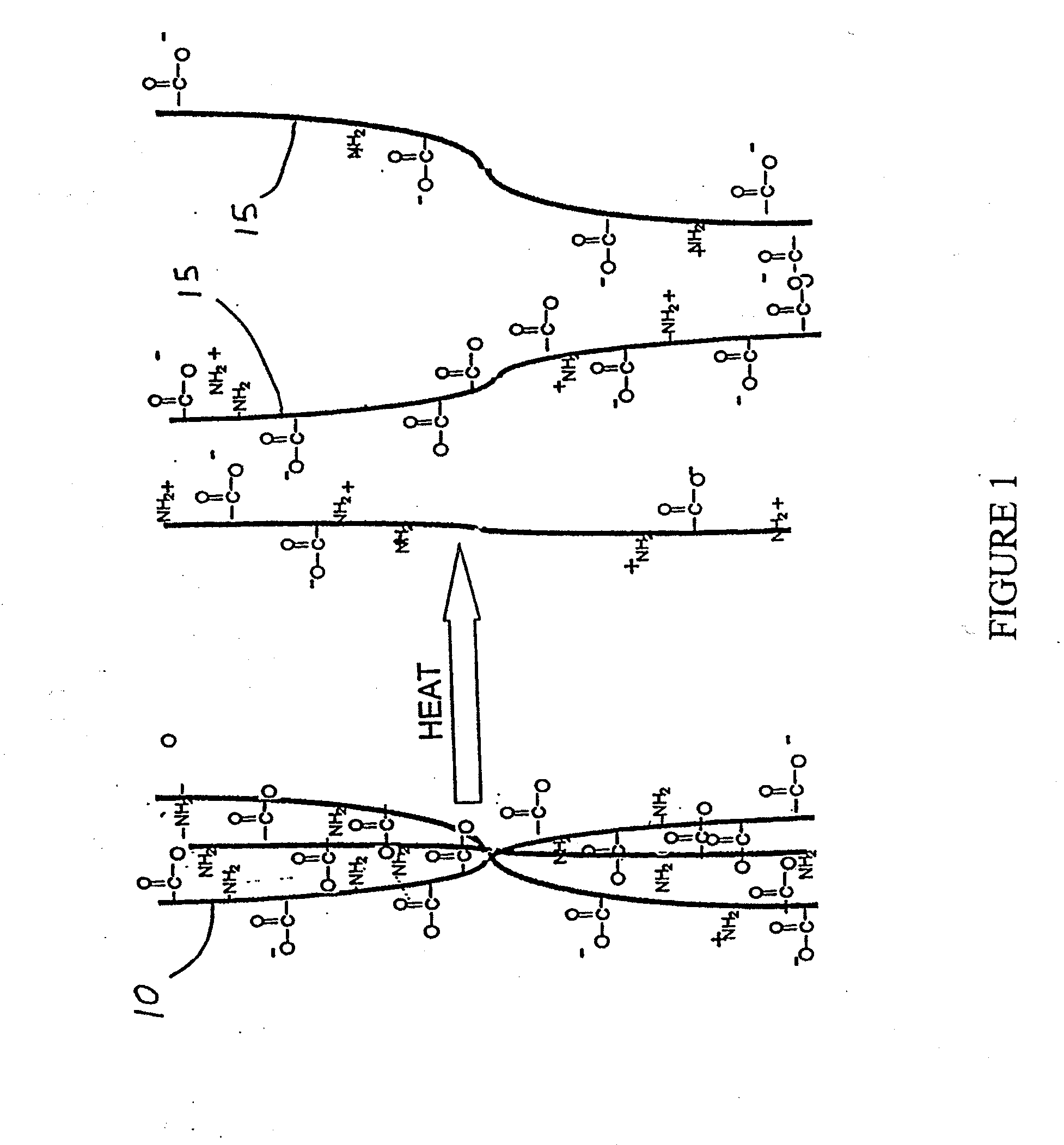
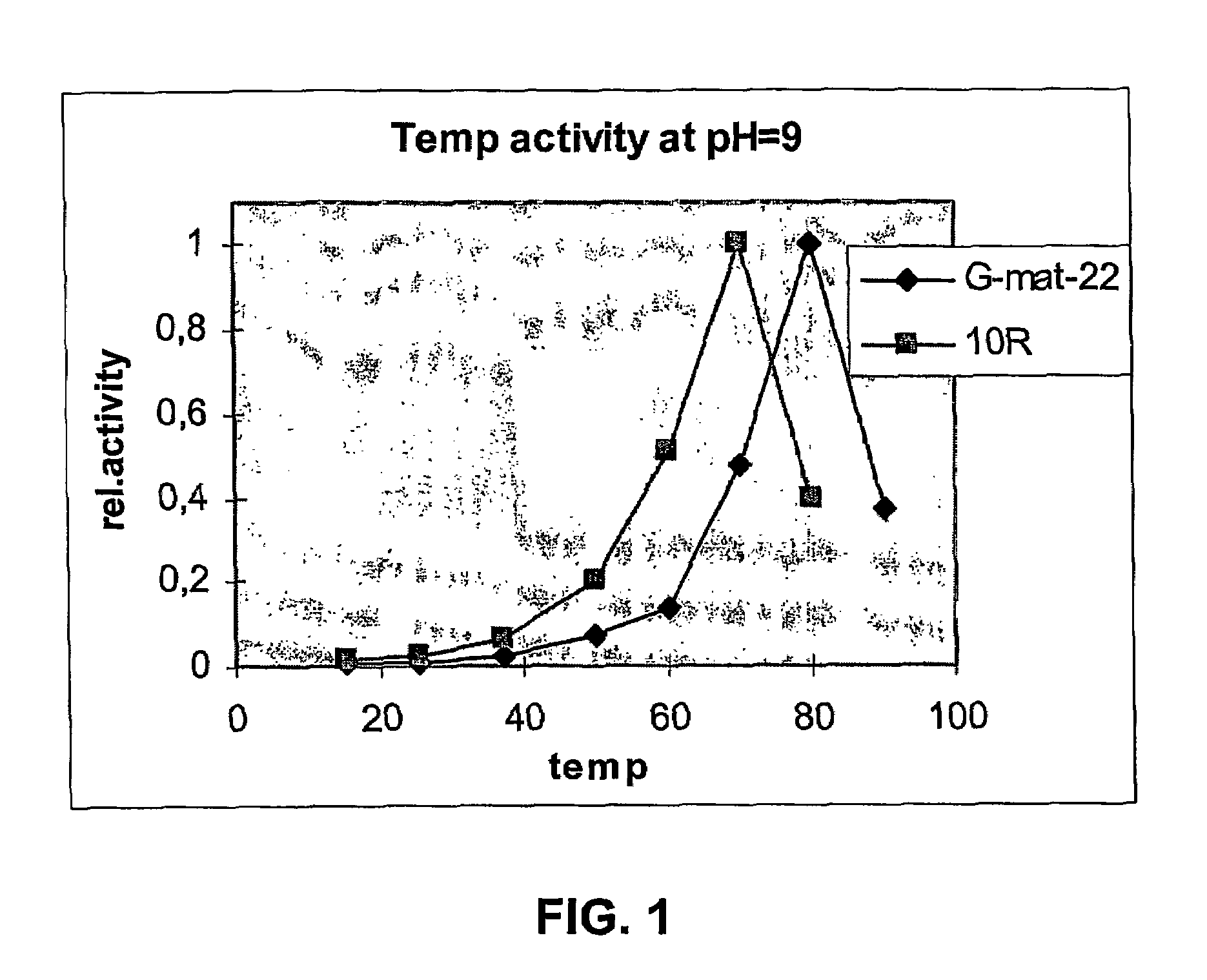
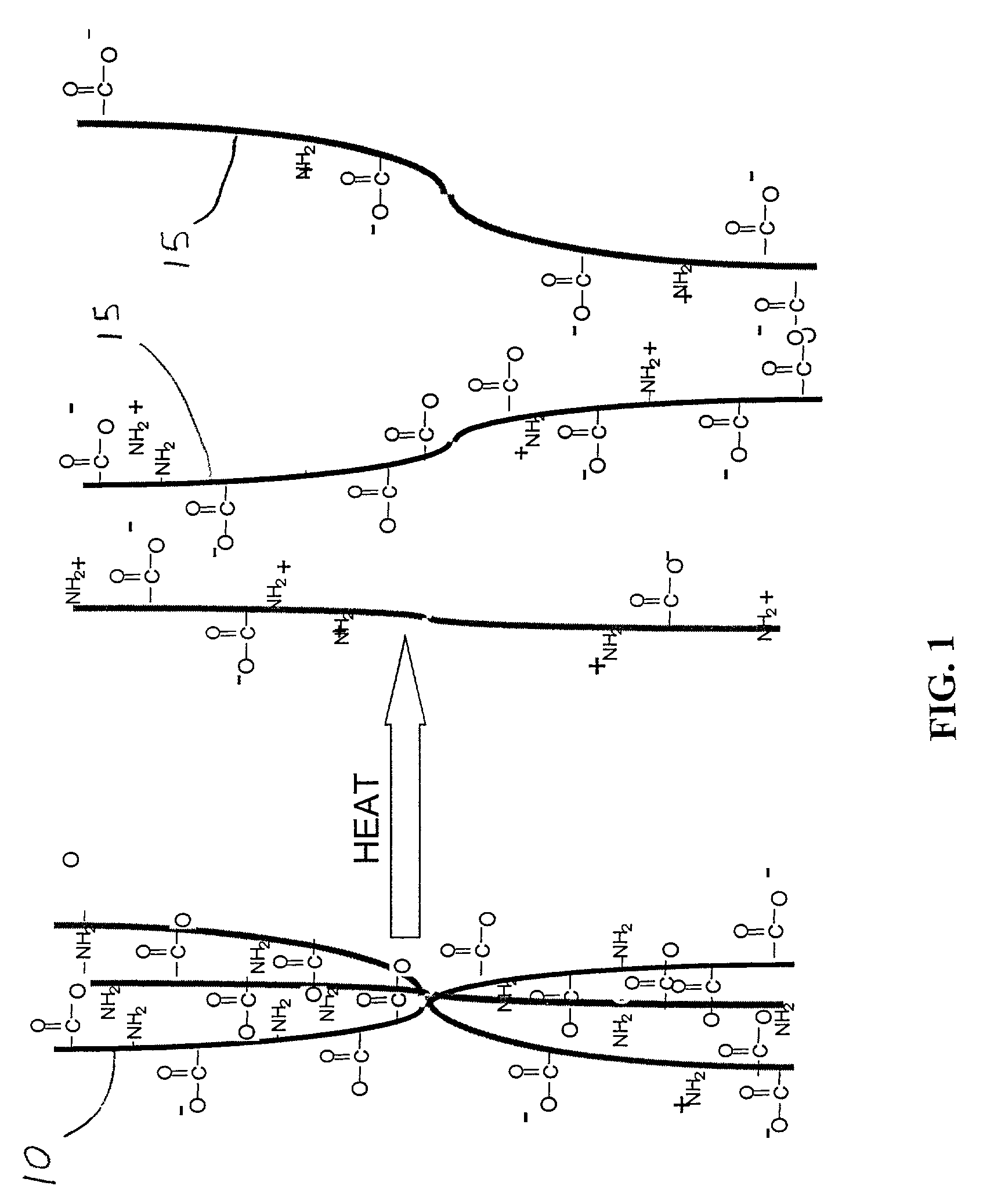
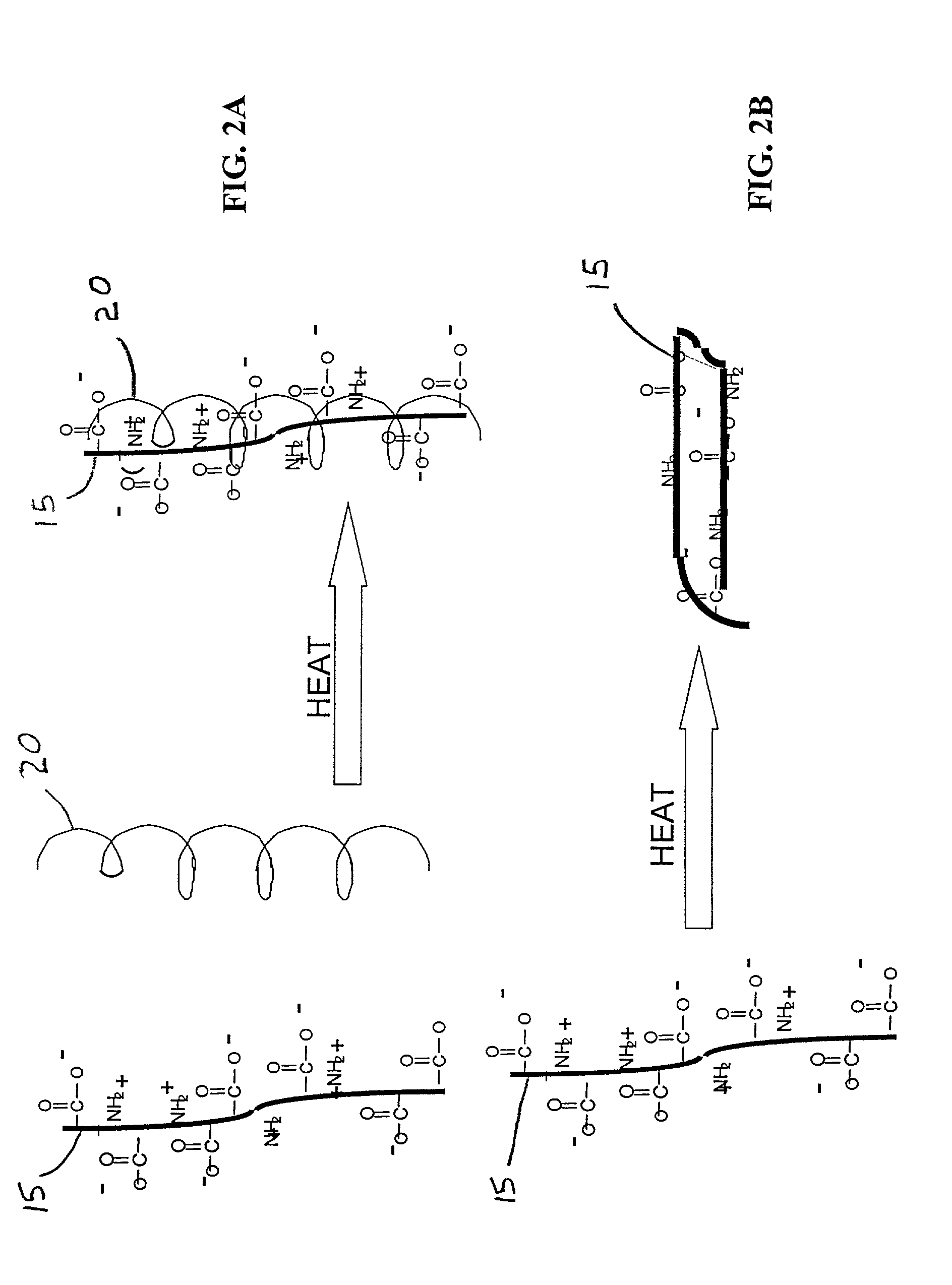
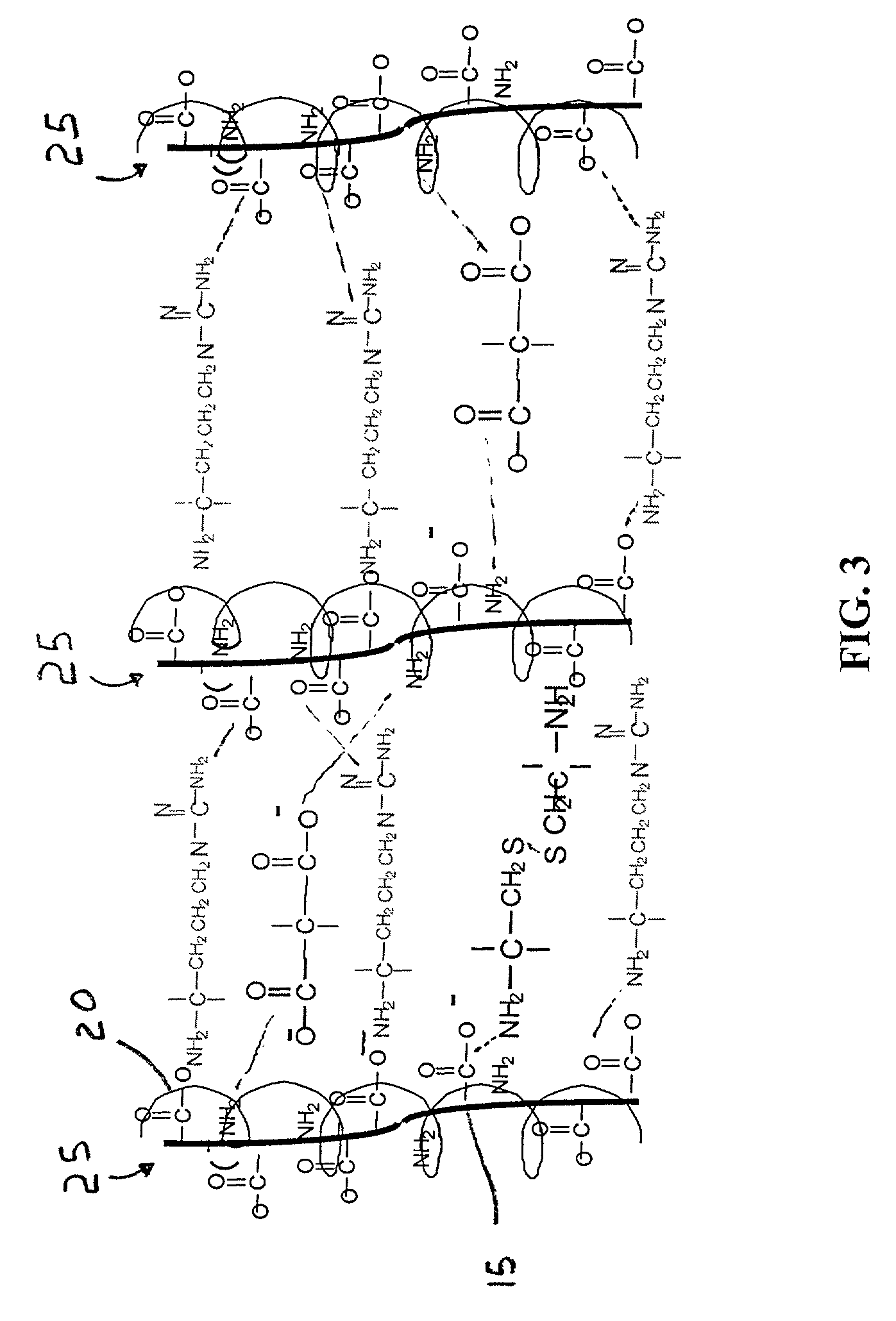
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Polar amino acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
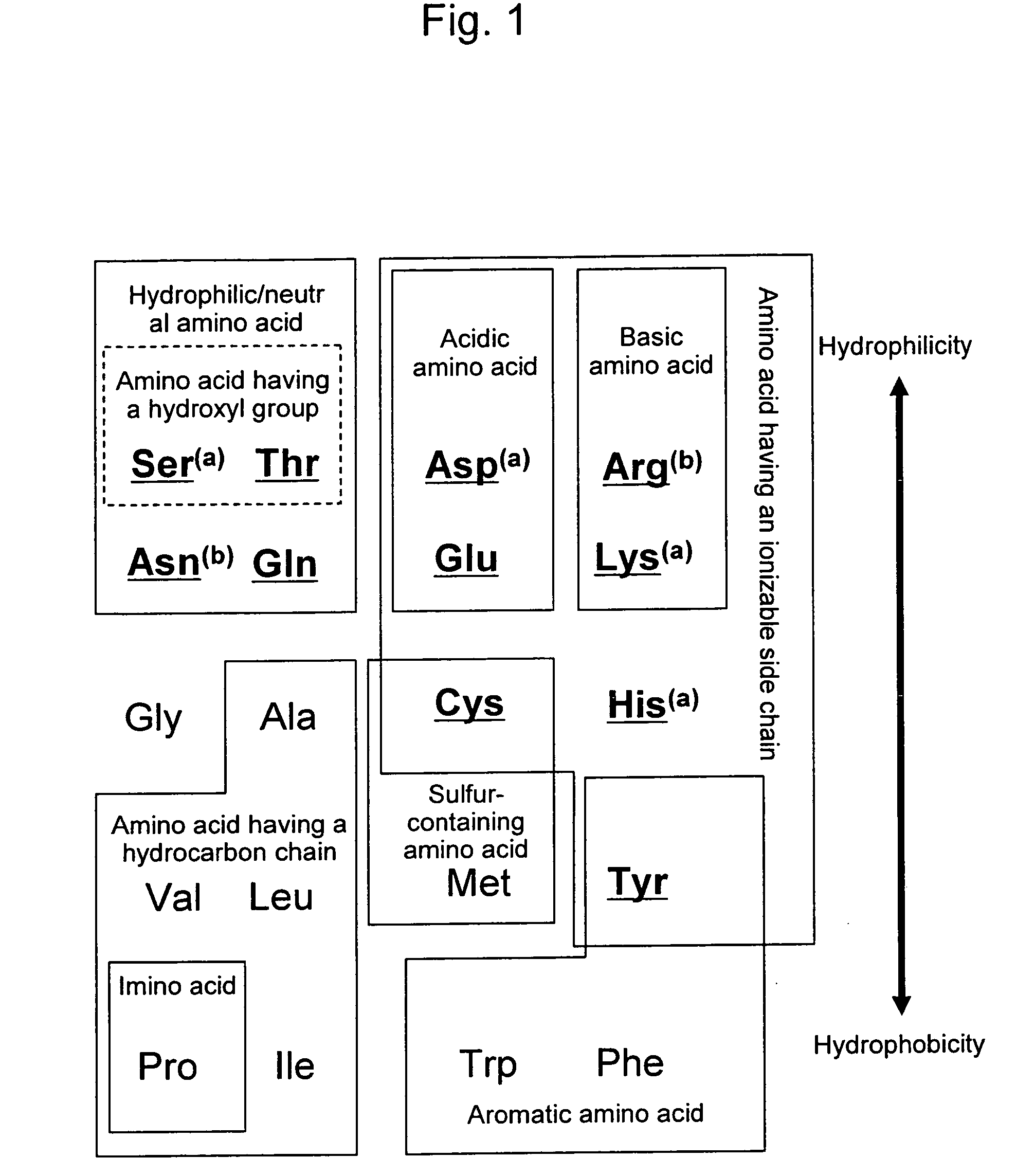
Polar amino acids are the ones which are charged( either positively: Lysine,Arginine,Histidine or negatively: Aspartate,Glutamate) and contain a hydroxyl(-OH) or Amide group in the side chain. Another polar molecule is the sulphur containing cysteine molecule.
Diagnostic agent for liver function
InactiveUS6071245ALessen the burden on the bodyEasy to useRadioactive preparation carriersRespiratory organ evaluationSide effectGlycerol
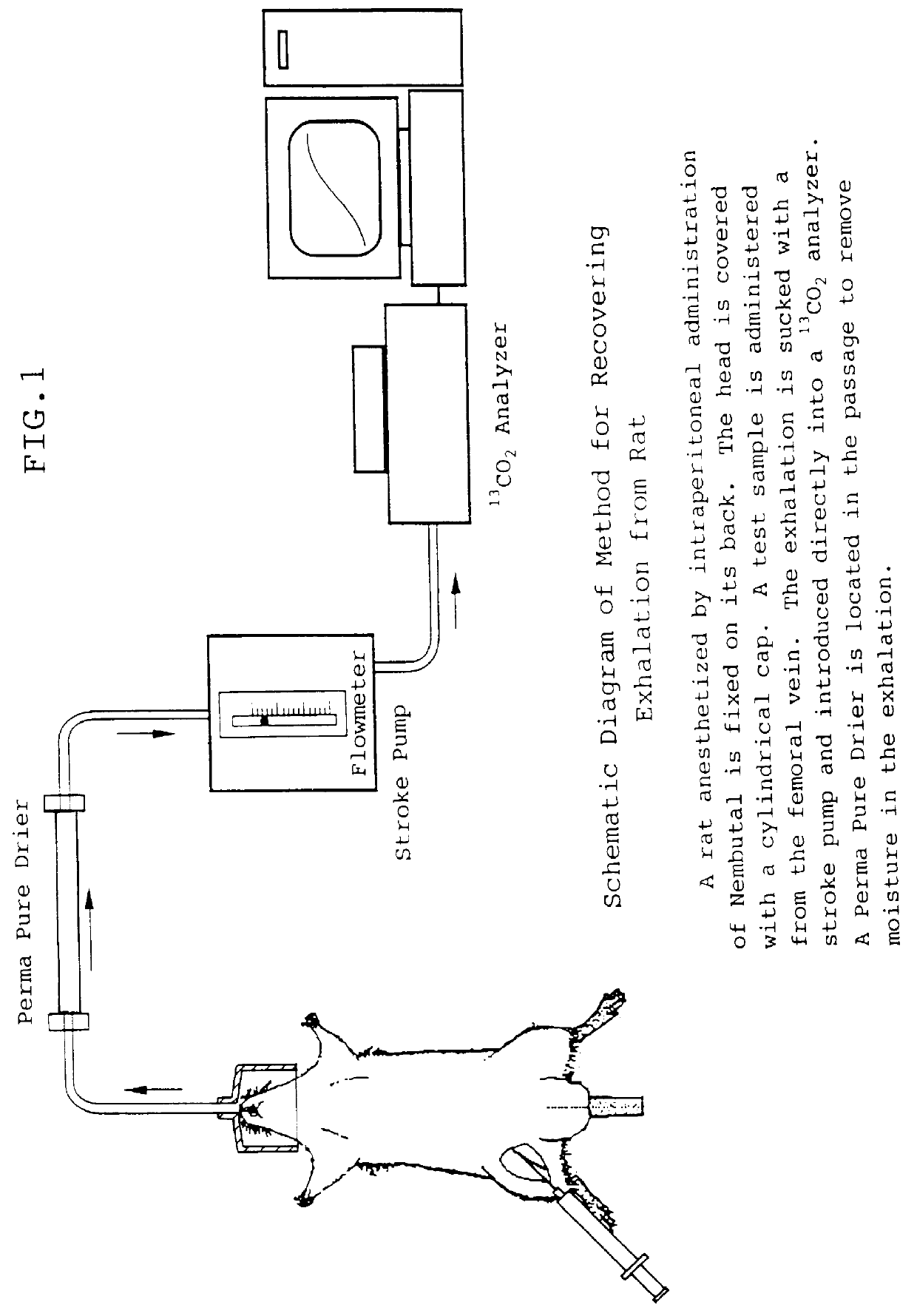
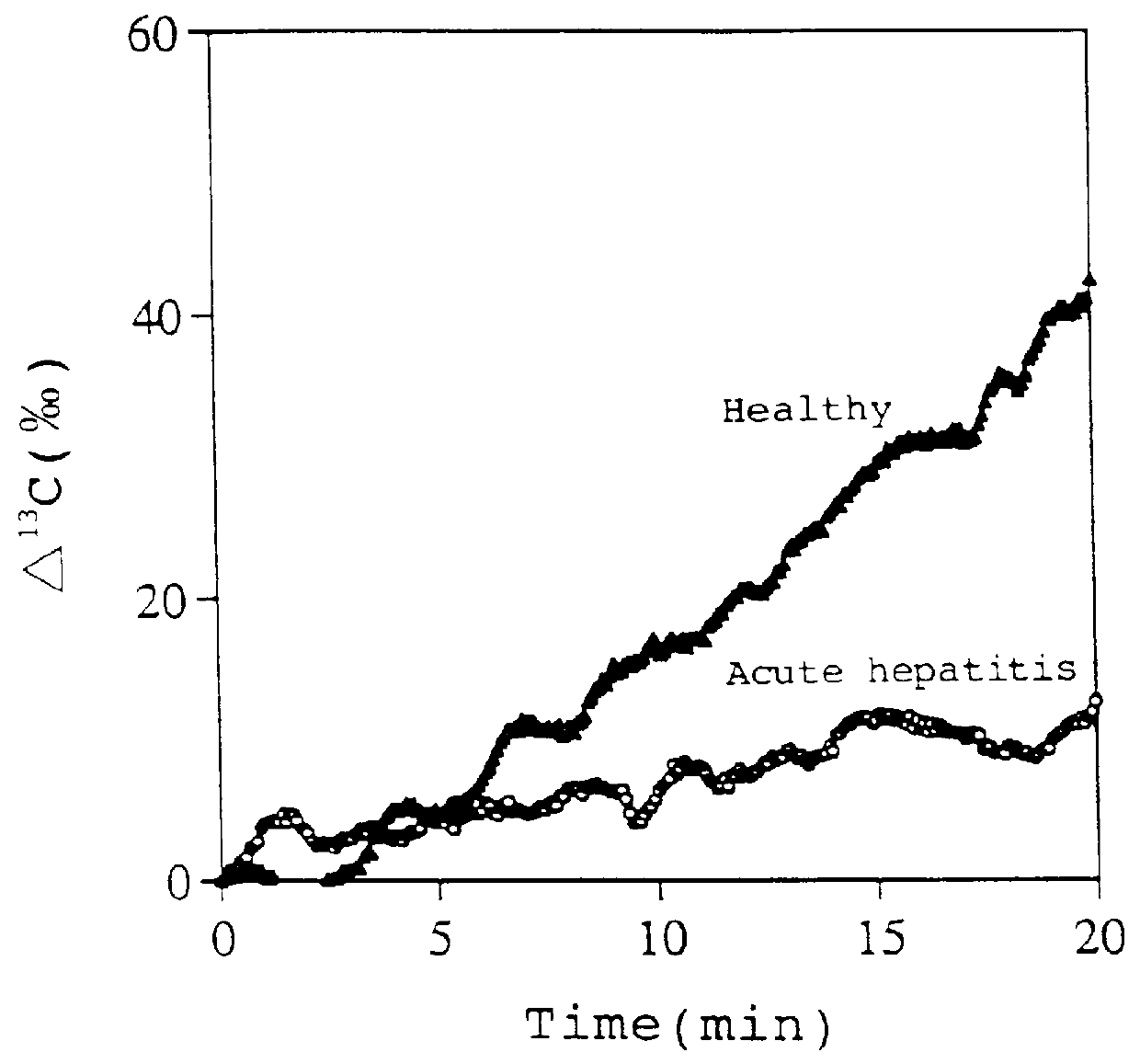
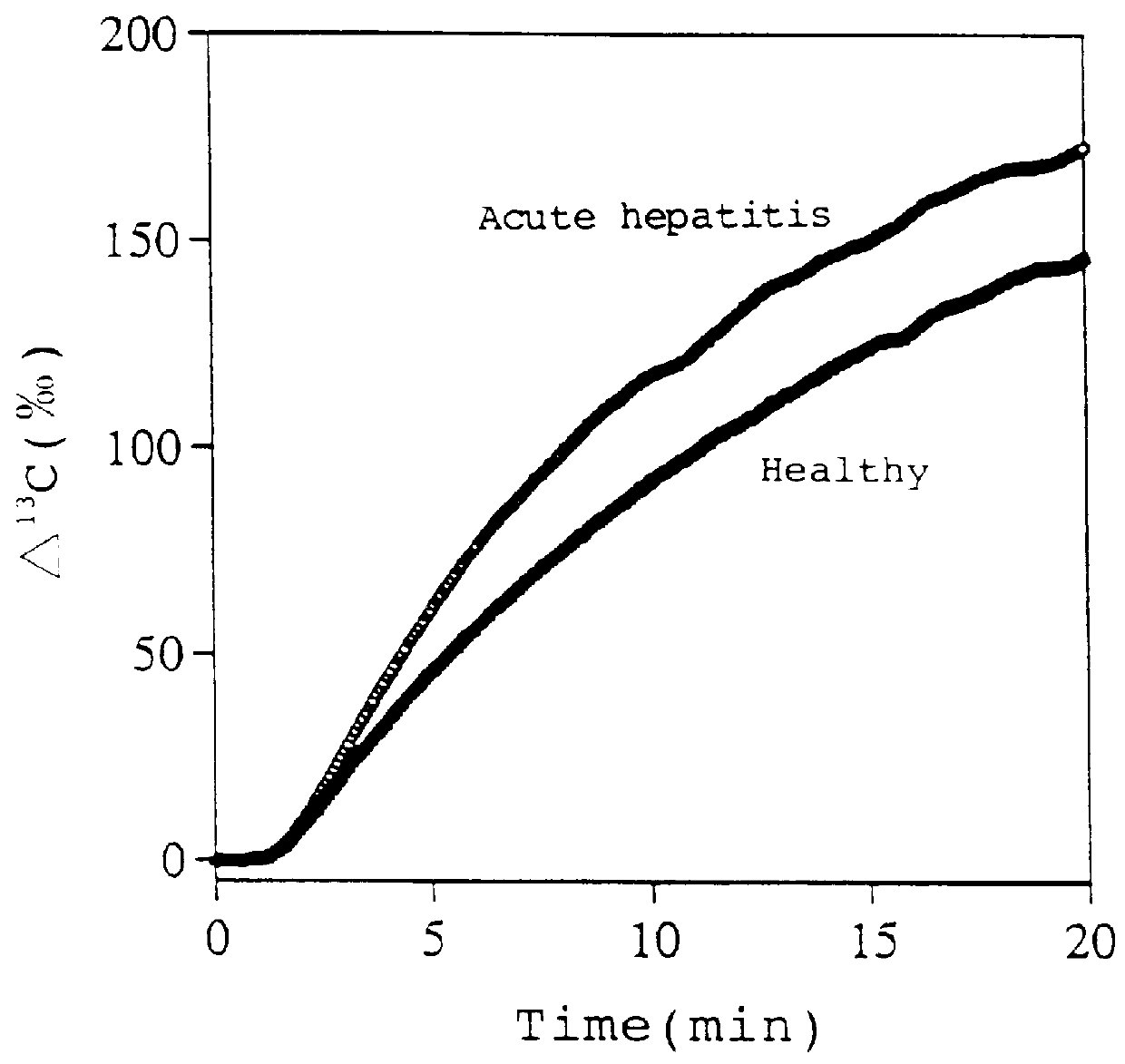
The present invention relates to a diagnostic agent for liver function, comprising a compound labelled with 13C at least at one specific position selected from the group consisting of the following (a) to (f): (a) galactose, glucose or xylose labelled with 13C at least at one specific position or a starch composed of glucose units labelled with 13C at least at one specific position; (b) a polar amino acid, heterocyclic amino acid, isoleucine or valine labelled with 13C at least at one specific position; (c) a carboxylic acid constituting the glycolytic pathway or the citric acid cycle, labelled with 13C at least at one specific position; (d) a fatty acid labelled with 13C at least at one specific position; (e) a glyceride labelled with 13C at least at one specific position; and (f) glycerol labelled with 13C at least at one specific position. According to the present invention, a diagnostic agent for liver function which imposes less physical burden on a subject, can give accurate test result immediately, and can be used safely without side effects is provided. The diagnostic agent of the invention is useful for evaluating the liver function of a subject at the time when the test is carried out.
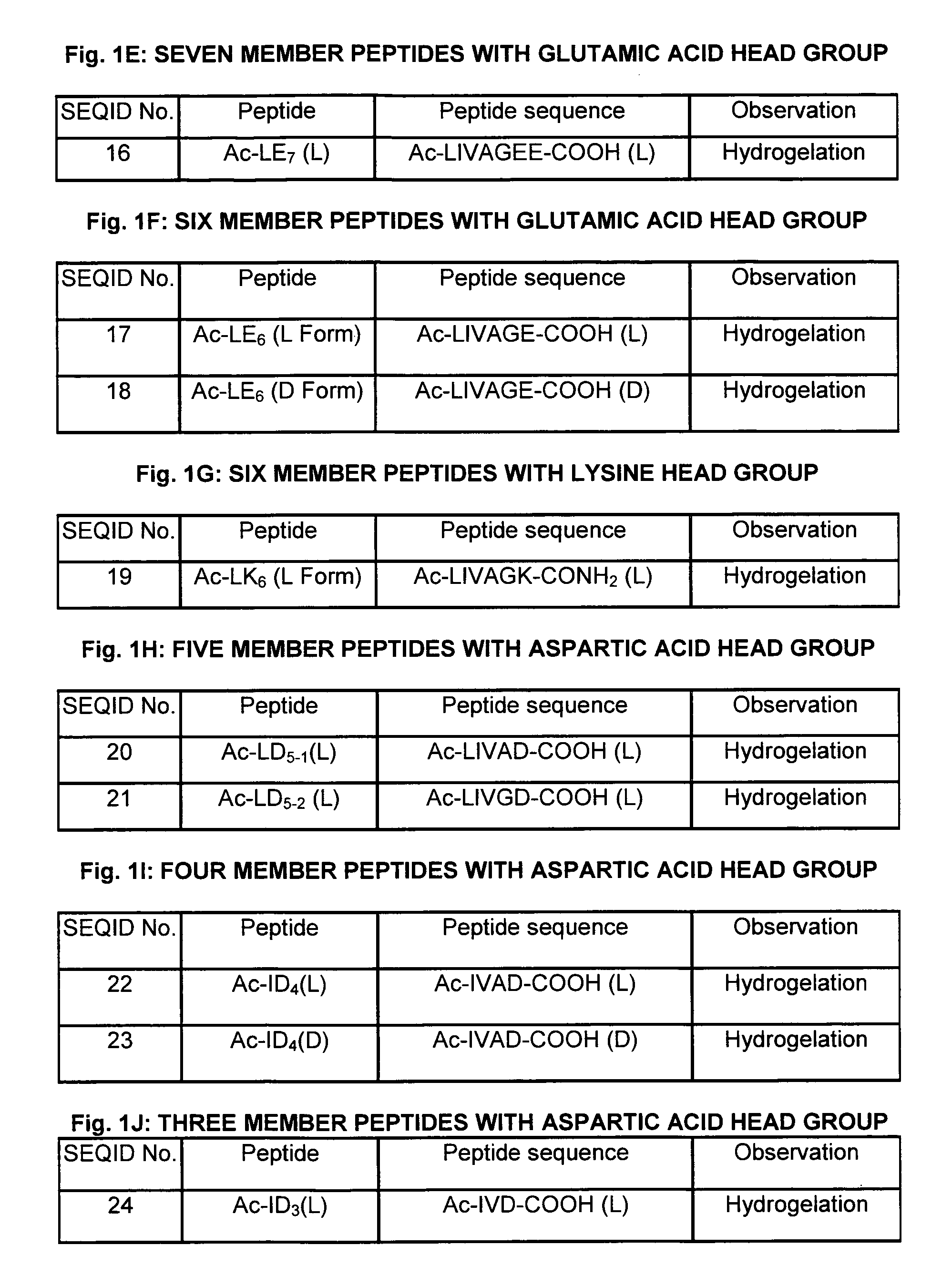
Owner:TOKYO GAS CO LTD
Methods and Compositions for Regenerating Connective Tissue
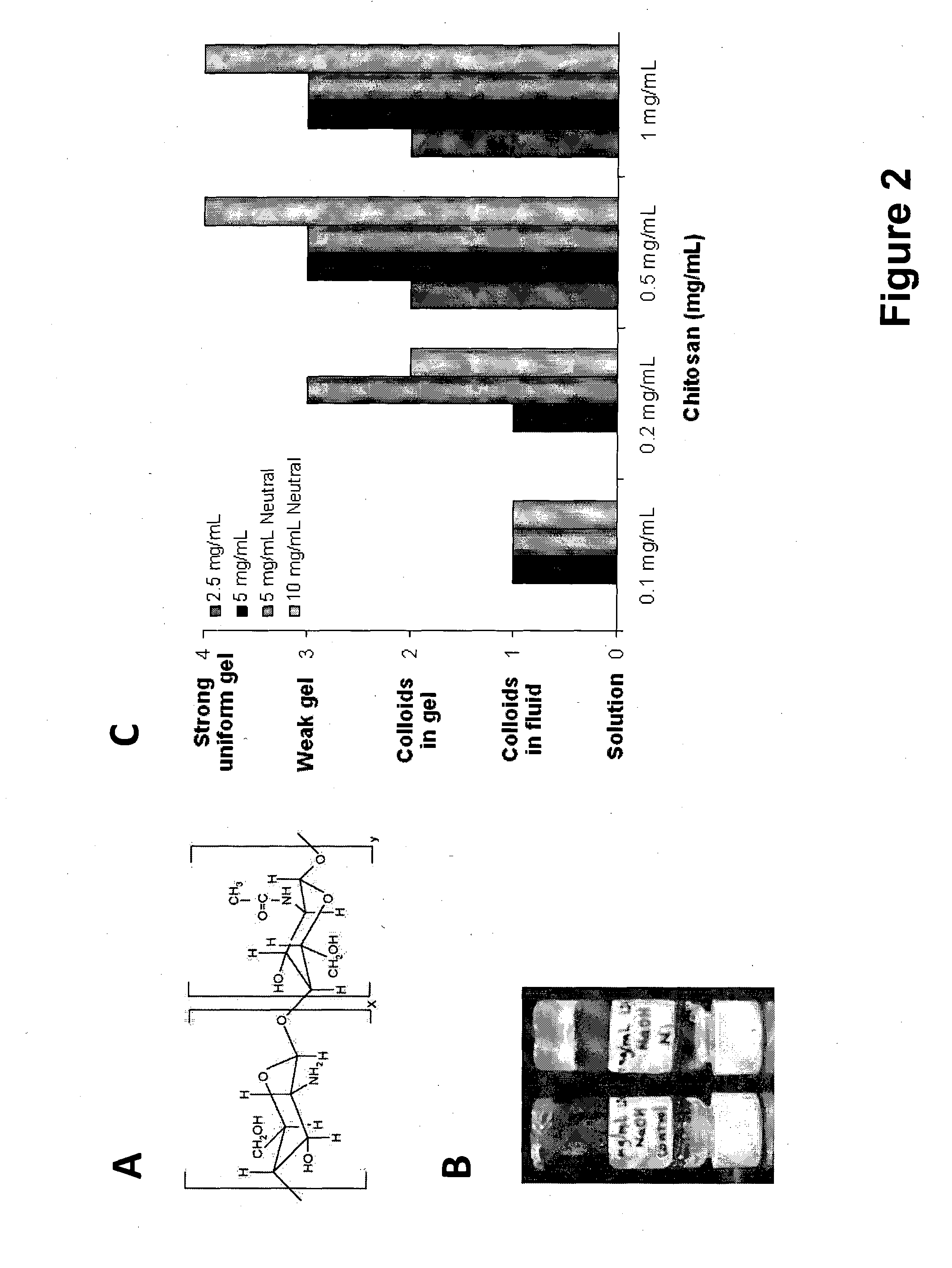
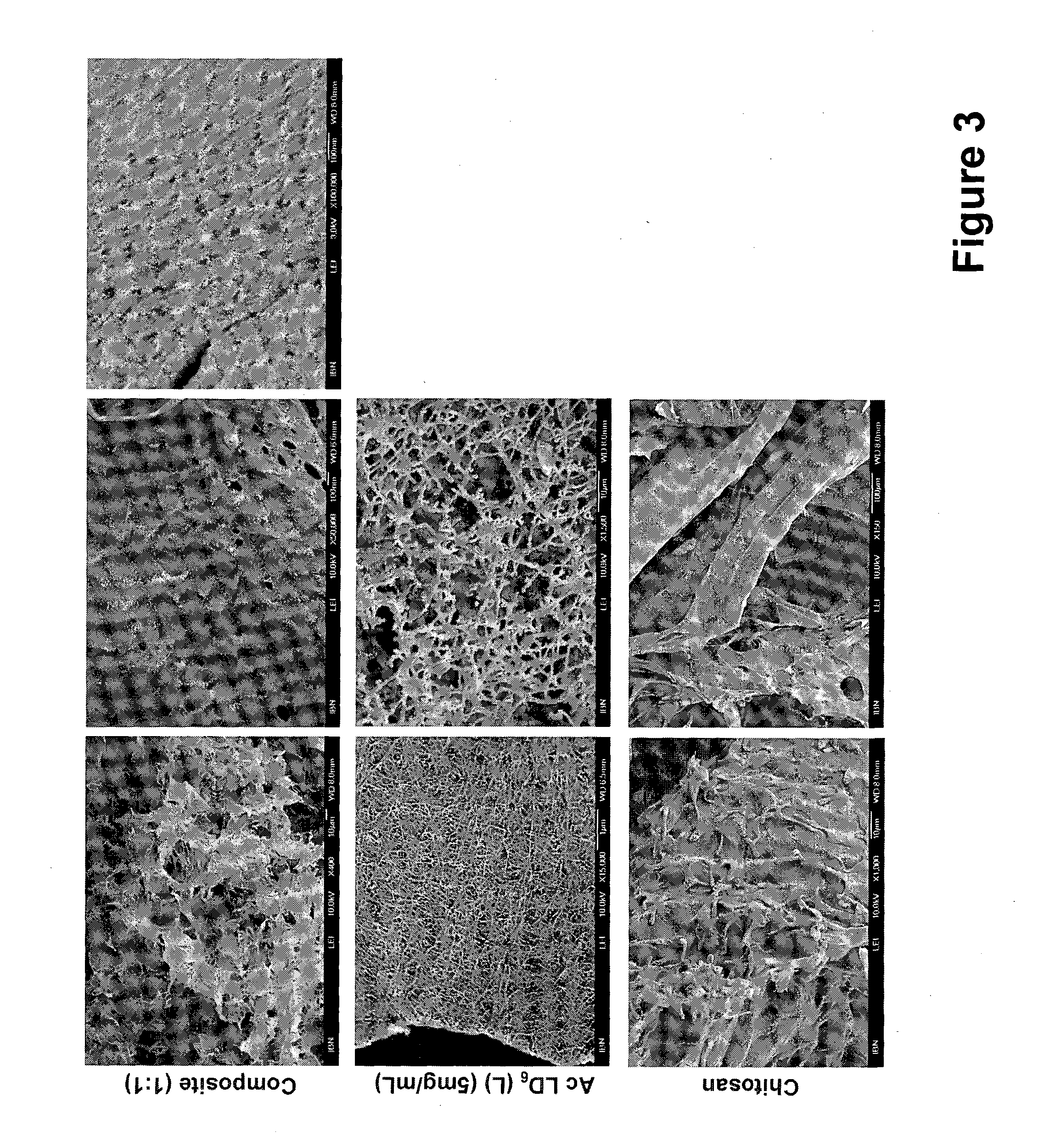
Connective tissue regenerative compositions and methods of repairing and regenerating connective tissue using such compositions are provided. The compositions generally comprise a bioactive hydrogel matrix comprising a polypeptide, such as gelatin, and a long chain carbohydrate, such as dextran. The hydrogel matrix may further include polar amino acids, as well as additional beneficial additives. Advantageously, the compositions include further components, such as osteoinductive or osteoconductive materials, medicaments, stem or progenitor cells, and three-dimensional structural frameworks. The compositions are useful for regenerating connective tissue, and can be administered to an area having injury to, or a loss of, connective tissue, such as bone, cartilage, tendon, and ligament.
Owner:PIONEER SURGICAL TECH INC
Methods and compositions for regenerating connective tissue
InactiveUS20080145404A1Halting progressionAntibacterial agentsPowder deliveryProgenitorLigament structure
Owner:PIONEER SURGICAL TECH INC
Atherosclerosis vaccine
InactiveUS20040002111A1Peptide preparation methodsLipid/lipoprotein ingredientsAntigenComplementarity determining region
The present invention relates to an antigenic composition capable of eliciting antibodies by interacting with alphabeta chains of a T cell receptor (TcR), which composition is comprised of a peptide-aldehyde conjugate. The aldehyde portion may be a dialdehyde, such as malondialdehyde (MDA), or a monoaldehyde, such as 4-hydroxynonenal (4-HNE), while the peptide portion preferably comprises at least one lysine residue. The antigenic composition according to the invention is capable of recognizing and interacting with a TcR having a complementarity-determining region 3 (CDR3) of alpha10 and beta6 chains that comprises a cluster of charged and polar amino acids. The invention also relates to a method of producing a vaccine against atherosclerosis by screening of a library of candidate compounds for their ability to bind to a conjugate of oxidized LDL and a dialdehyde as well as to such a vaccine as such.
Owner:CARDIOVAX
Conjugates of insulin-like growth factor-1 and poly(ethylene glycol)
InactiveUS20060154865A1Maintain good propertiesAntibacterial agentsNervous disorderInsulin-like growth factorPolyethylene glycol
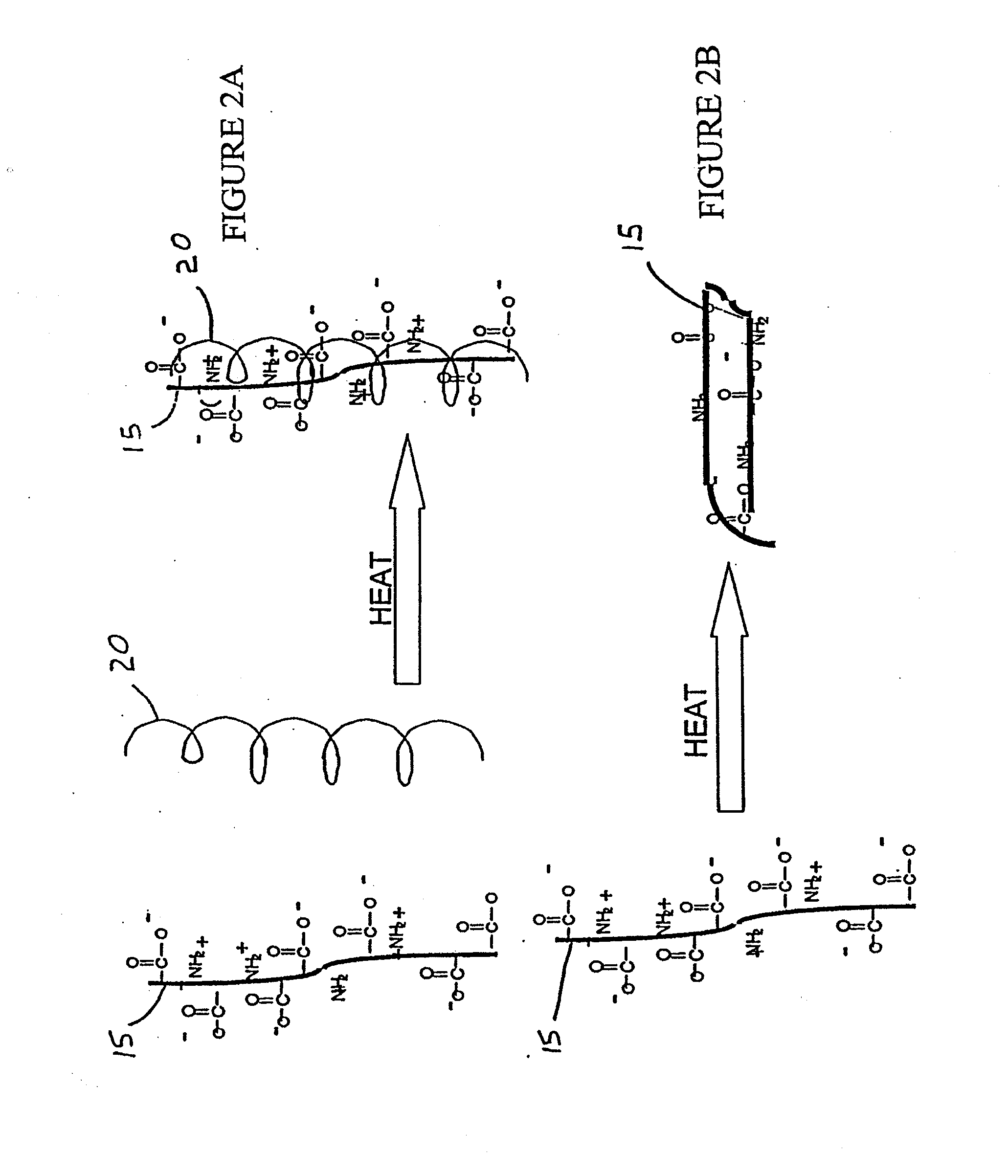
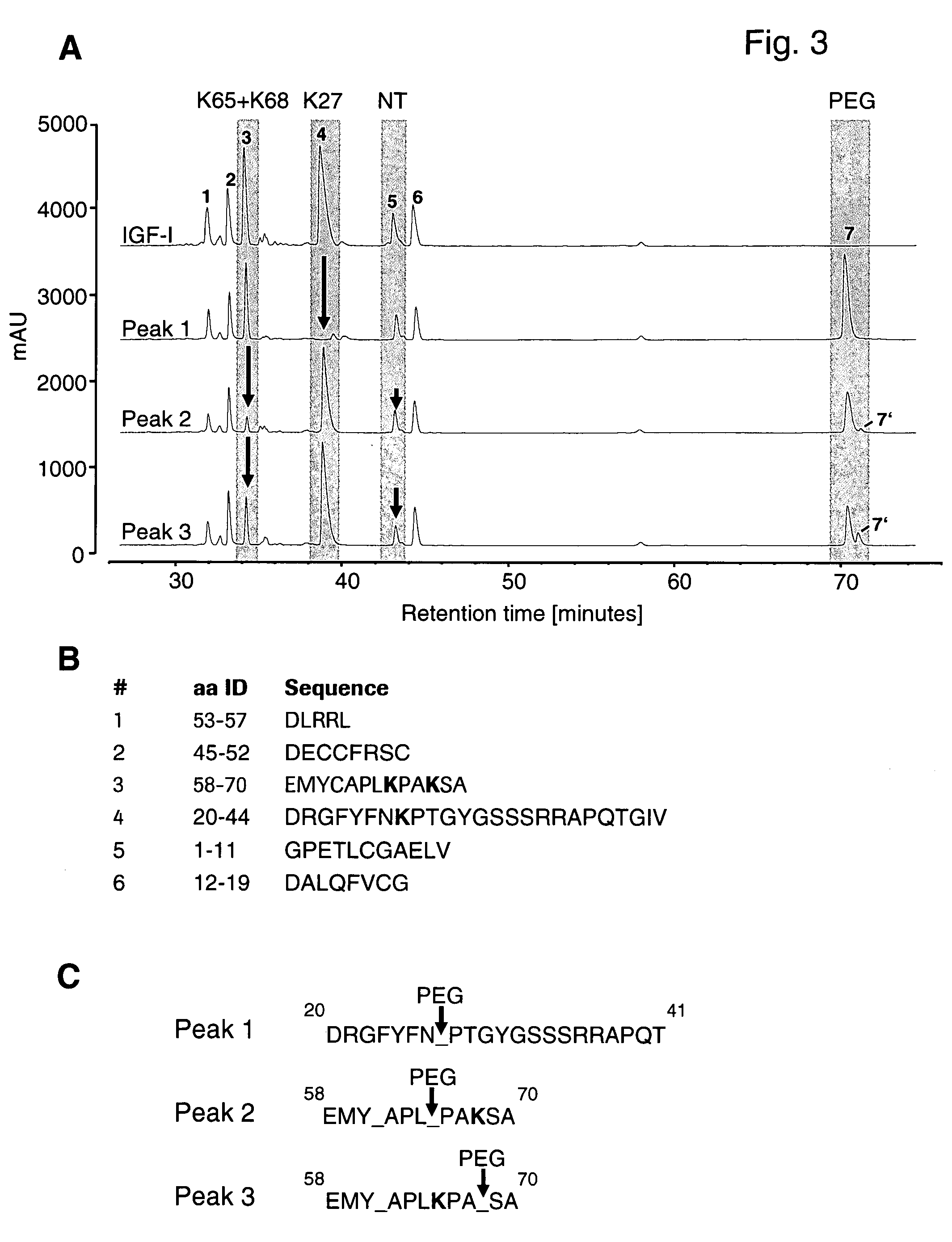
A conjugate consisting of an insulin-like growth factor-1 (IGF-I) variant and one or two poly(ethylene glycol) group(s), characterized in that said IGF-I variant has an amino acid alteration at up to three amino acid positions 27, 37, 65, 68 of the wild-type IGF-I amino acid sequence so that one or two of said amino acids is / are lysine and amino acid 27 is a polar amino acid but not lysine, is conjugated via the primary amino group(s) of said lysine(s) and said poly(ethylene glycol) group(s) have an overall molecular weight of from 20 to 100 kDa is disclosed. This conjugate is useful for the treatment of neurodegenerative disorders like Alzheimer's Disease.
Owner:F HOFFMANN LA ROCHE & CO AG
Proteases and methods for producing them
A secreted proteolytic polypeptide comprising at least three non-polar or uncharged polar amino acids within the last four amino acids of the C-terminus of the polypeptide, encoding polynucleotides, expression vectors comprising the polynucleotides, host cell comprising the polynucleotides, methods for producing said polypeptide, and methods for using the polypeptide.
Owner:NOVOZYMES AS
Method of stimulation hair growth
The invention provides a method of stimulating hair growth, comprising administering a therapeutic amount of a hydrogel matrix to an intradermal or subdermal site where hair growth is desired, the matrix composition comprising gelatin, such as denatured collagen, and a long chain carbohydrate, such as dextran. The matrix may further include polar amino acids, nitric oxide inhibitors and super oxide inhibitors. Injection is a preferred method of administration.
Owner:PIONEER SURGICAL TECH INC
Self-assembled composite ultrasmall peptide-polymer hydrogels
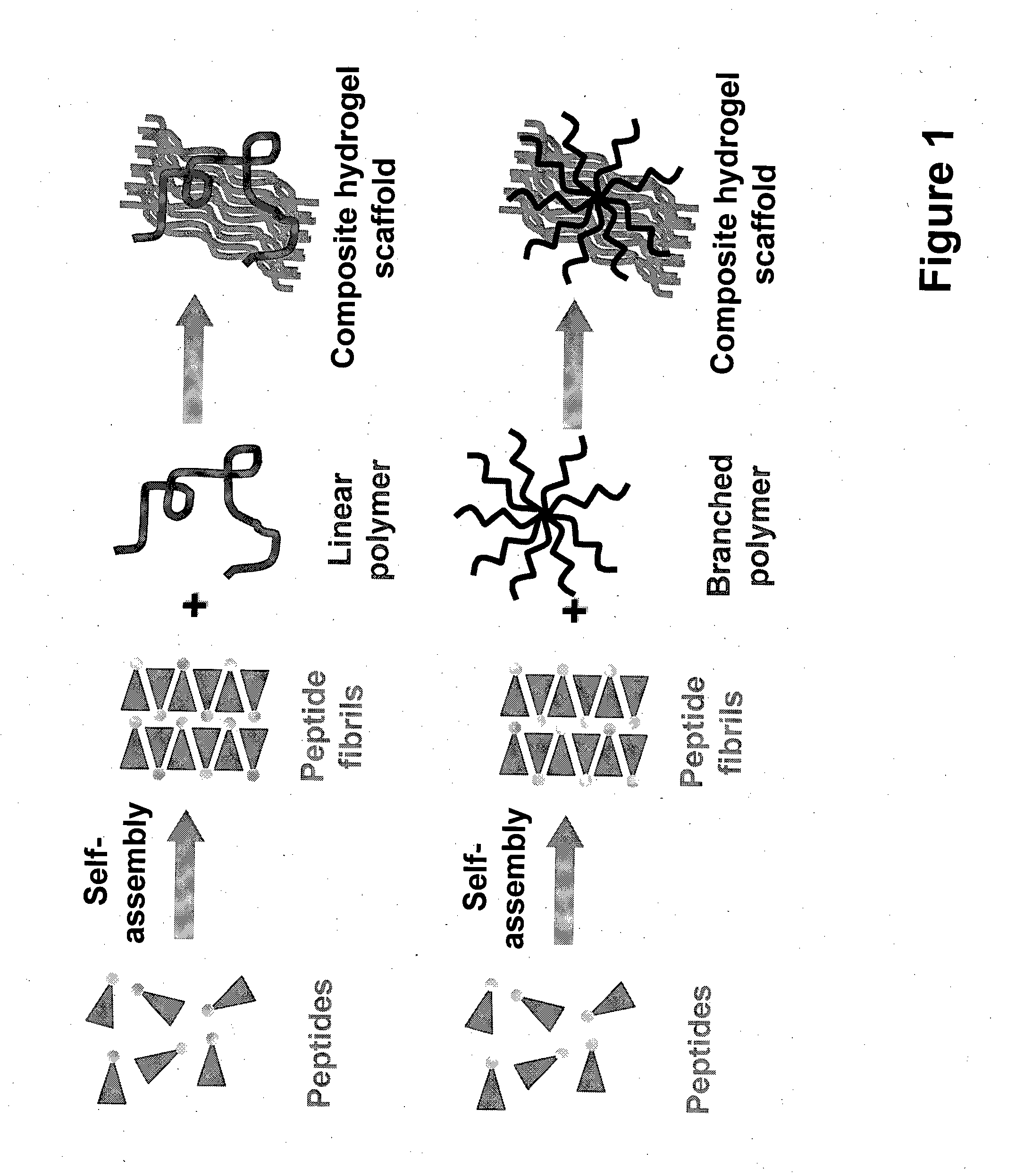
ActiveUS20140349933A1Maintain sternnessMaintain proliferationOrganic active ingredientsSpecial deliveryActive agentPolymer
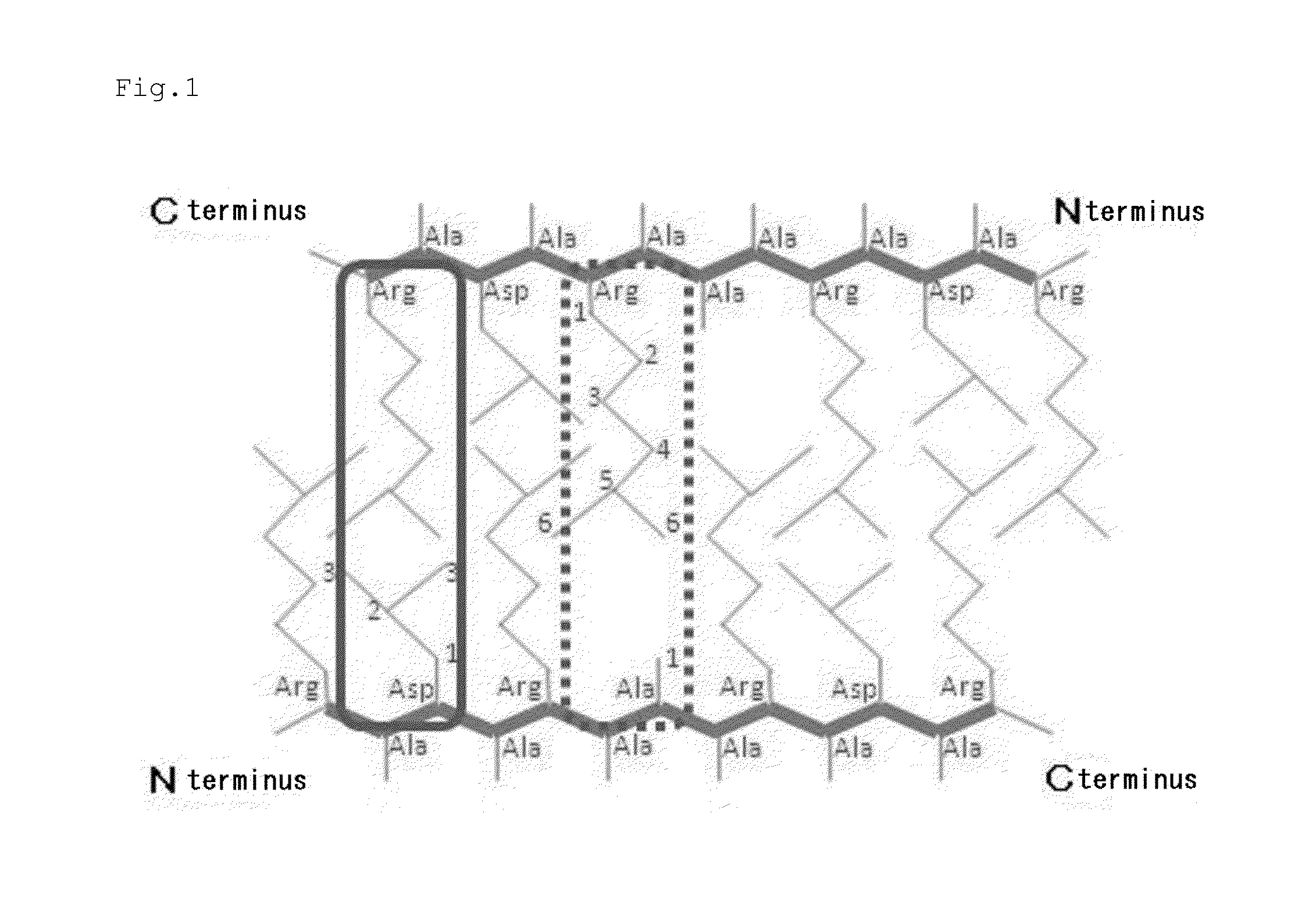
The present invention relates to composite hydrogels comprising at least one non-peptidic polymer and at least one peptide having the general formula: Z—(X)m—(Y)n—Z′p, wherein Z is an N-terminal protecting group; X is, at each occurrence, independently selected from an aliphatic amino acid, an aliphatic amino acid derivative and a glycine; Y is, at each occurrence, independently selected from a polar amino acid and a polar amino acid derivative; Z′ is a C-terminal protecting group; m is an integer selected from 2 to 6; n is selected from 1 or 2; and p is selected from 0 or 1. The present invention further relates to methods of producing the composite hydrogels, to uses of the composite hydrogels for the delivery of drugs and other bioactive agents / moieties, as an implant or injectable agent that facilitates tissue regeneration, and as a topical agent for wound healing. The present invention further relates to devices and pharmaceutical or cosmetic compositions comprising the composite hydrogels and to medical uses of the composite hydrogels.
Owner:AGENCY FOR SCI TECH & RES
Proteases and methods for producing them
A secreted proteolytic polypeptide comprising at least three non-polar or uncharged polar amino acids within the last four amino acids of the C-terminus of the polypeptide, encoding polynucleotides, expression vectors comprising the polynucleotides, host cell comprising the polynucleotides, methods for producing said polypeptide, and methods for using the polypeptide
Owner:NOVOZYMES AS
Amphiphilic linear peptide/peptoid and hydrogel comprising the same
InactiveUS20130023460A1Not be restrictCosmetic preparationsPeptide/protein ingredientsFiberCrystallography
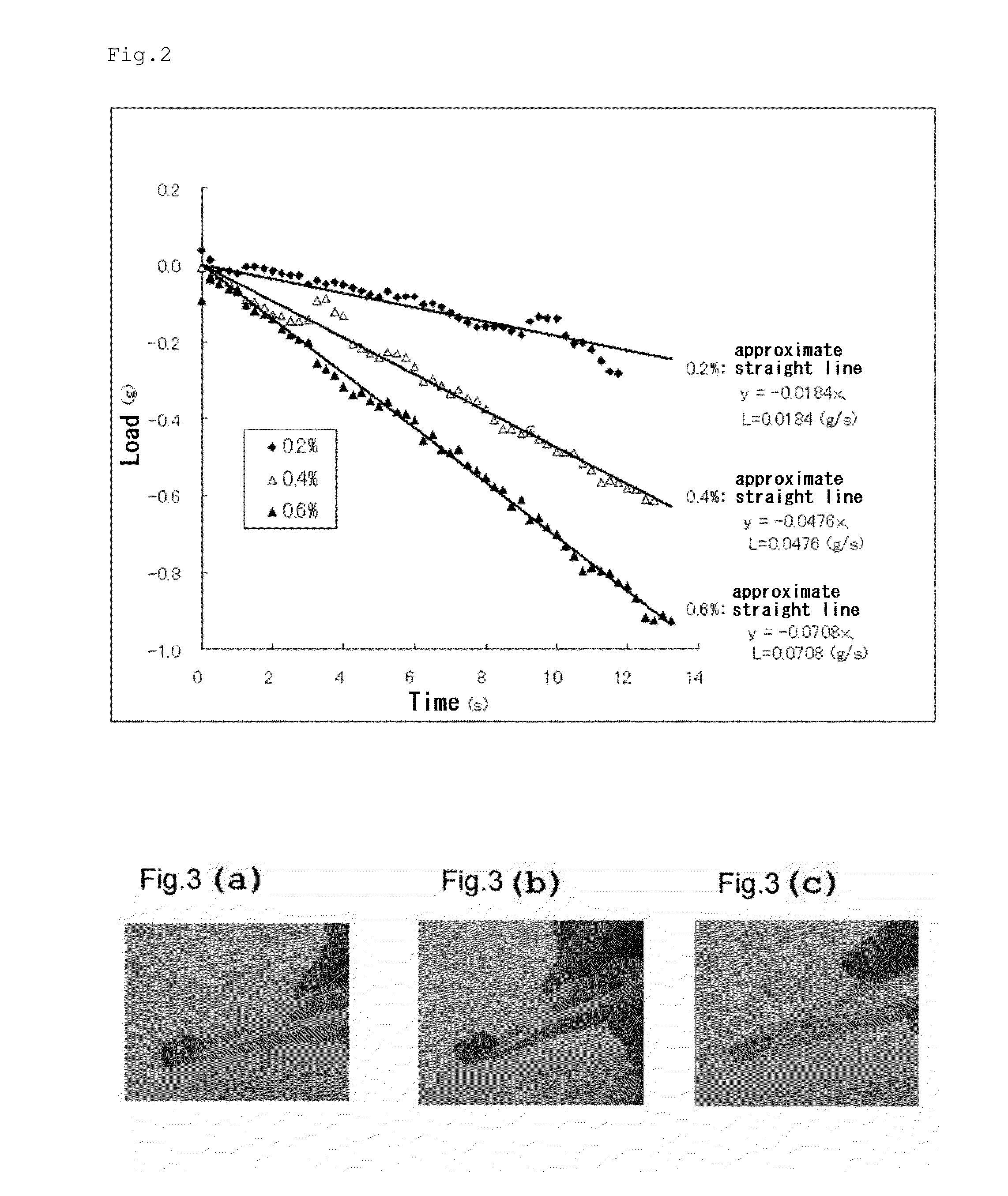
The present invention provides an amphiphilic linear peptide and / or peptoid as well as a hydrogel that includes the amphiphilic linear peptide / peptoid. The amphiphilic linear peptide / peptoid is capable of forming a hydrogel. These peptides / peptoids include short amphiphilic sequences with a hydrophobic portion of aliphatic amino acids and at least one acidic, neutral, or basic polar amino acid. The amphiphilic linear peptide / peptoid is build up of non repetitive aliphatic amino acids, which may be in the L- or D-form. A plurality of such peptides / peptoids assembles to supramolecular helical fibers and forms peptide hydrogels after assembly. A corresponding hydrogel is formed in aqueous solutions at physiological pH and is thus useful for inter alia cell culture, tissue engineering, and drug release. Such hydrogels which are rigid, biocompatible and entrapping up to 99.9% of water are also well suited for applications utilizing electronic devices.
Owner:AGENCY FOR SCI TECH & RES
Cell-wall degrading enzyme variants
InactiveUS20050250181A1Process can be usedImprove performanceBacteriaSugar derivativesEnzyme variantBiology
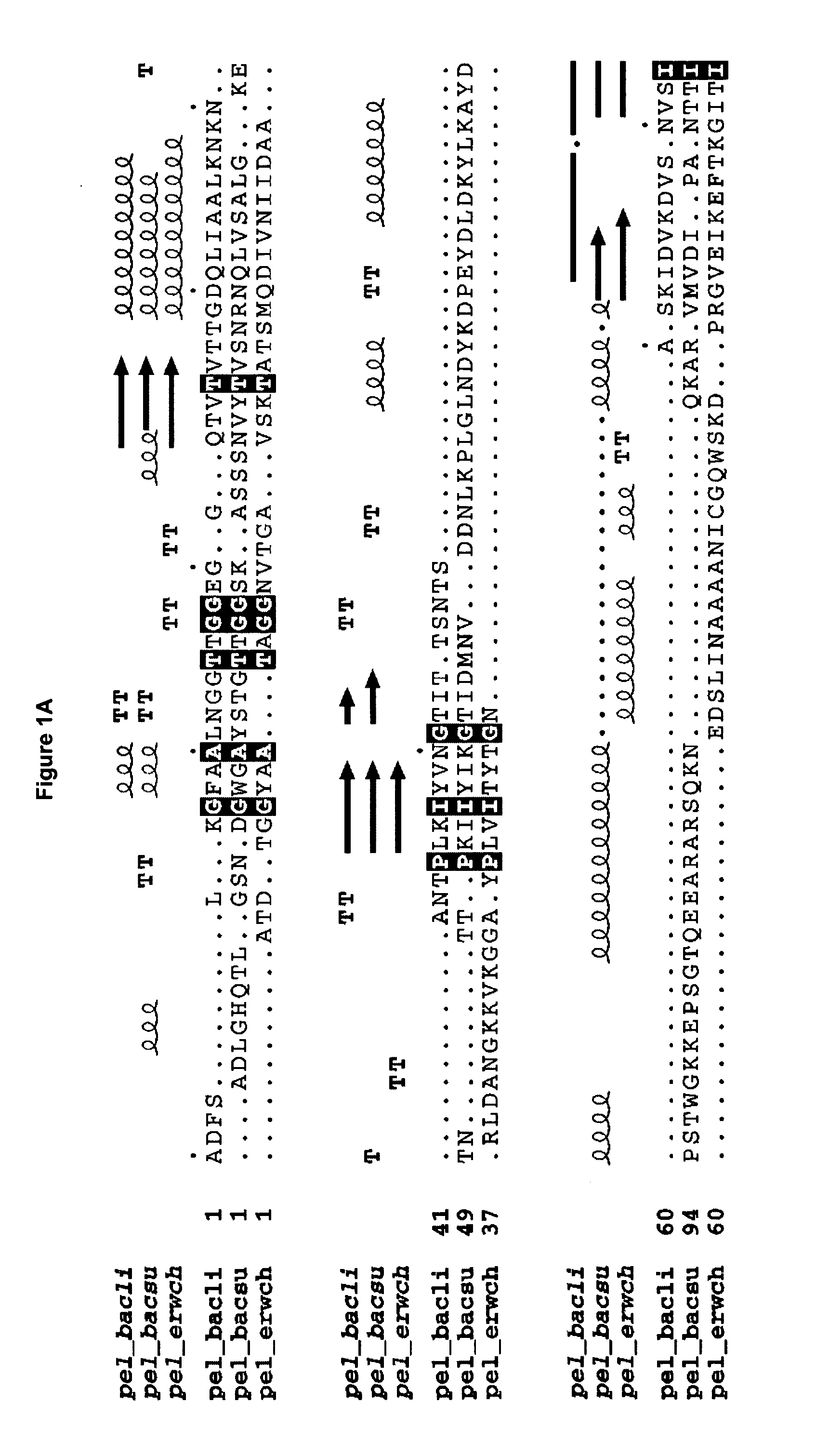
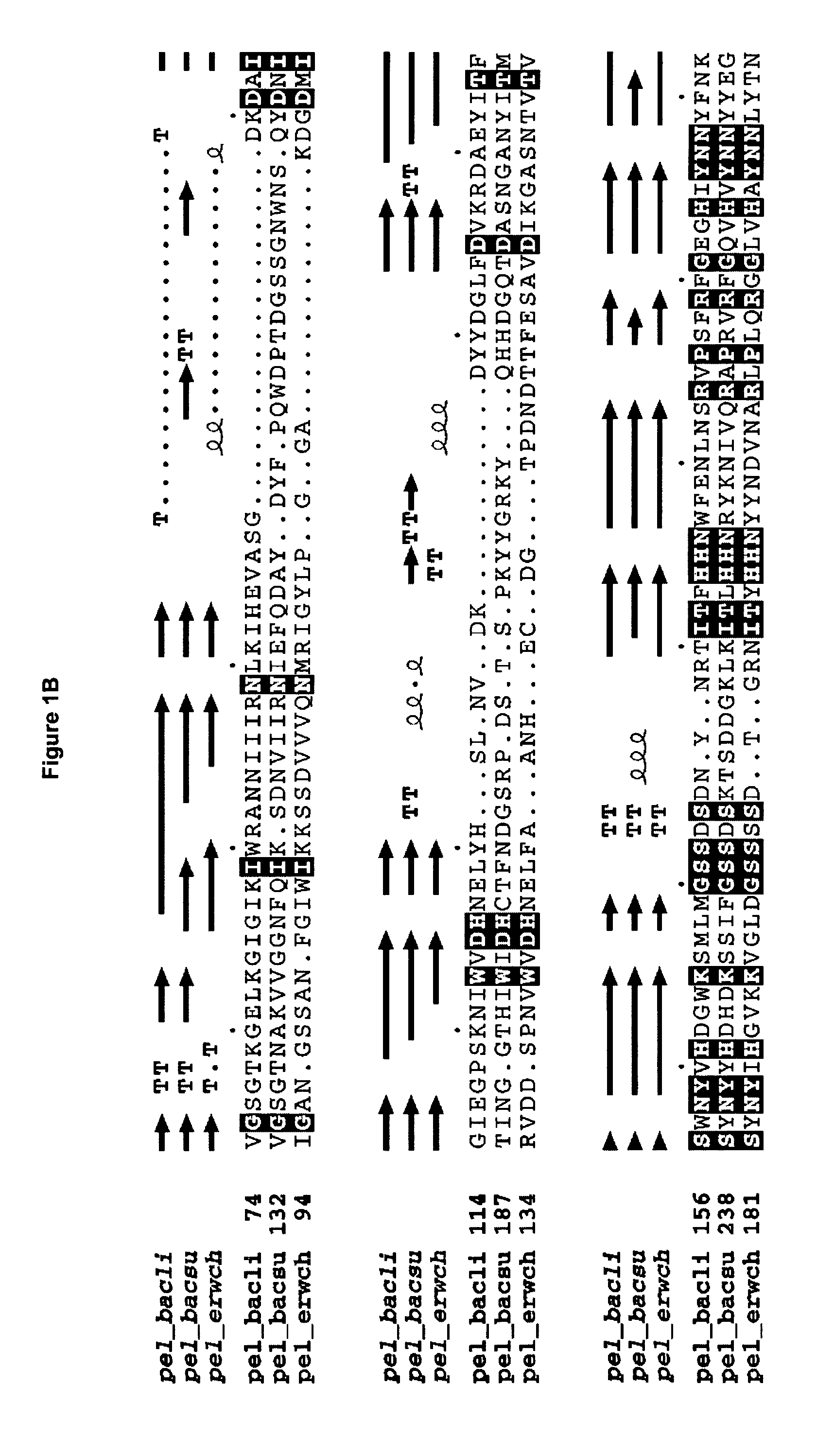
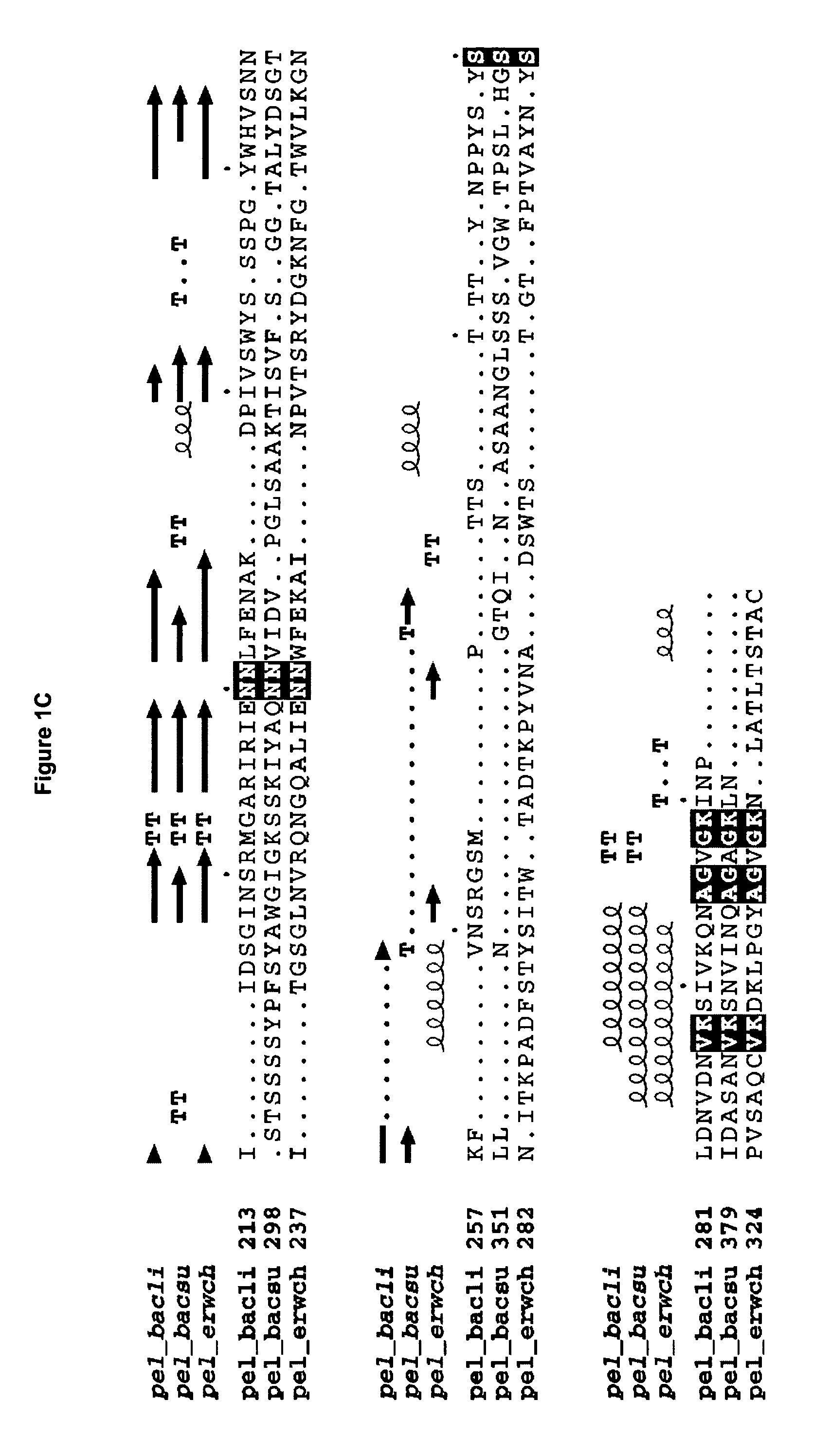
The present invention relates to variants of a cell-wall degrading enzyme having a beta-helix structure, which variant has at least one substituent in a position determined by identifying all residues potentially belonging to a stack; characterizing the stack as interior or exterior; characterizing the stack as polar, hydrophobic or aromatic / heteroaromatic based on the dominating characteristics of the parent or wild-type enzyme stack residues and / or its orientation relative to the beta-helix (interior or exterior); optimizing all stack positions of a stack either to hydrophobic aliphatic amino acids, hydrophobic aromatic or polar amino acids by allowing mutations within one or all positions to amino acids belonging to one of these groups; measuring thermostability of the variants by DSC or an application-related assay such as a Pad-Steam application test; and selecting the stabilized variants. Variants of a wild-type parent pectate lyase (EC 4.2.2.2) having the conserved amino acid residues D111, D141 or E141, D145, K165, R194 and R199 when aligned with the pectate lyase comprising the amino acid sequence of SEQ ID NO: 2 are preferred.
Owner:NOVOZYMES AS
Insulins with polar recombinant extensions
ActiveUS20180291076A1Reduce yieldHigh viscosityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusMedicine
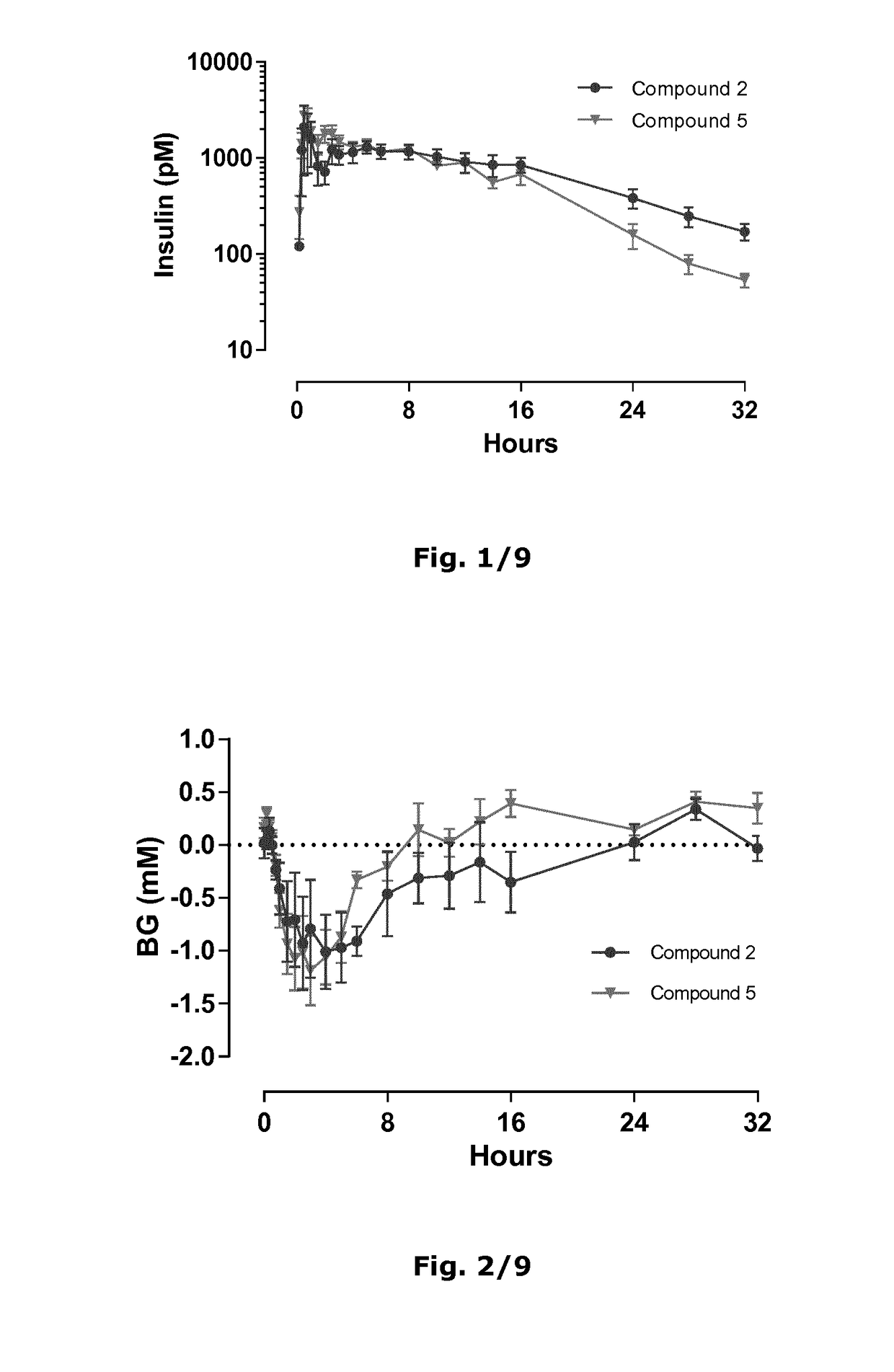
The present invention relates to novel insulins or insulins analogues that are extended with predominantly sequences of polar amino acid residues in order to improve the half-life and stability of the drug substance. The invention also provides pharmaceutical compositions comprising such drug substances, and relates to the use of such drug substances for the treatment or prevention of medical conditions relating to diabetes.
Owner:NOVO NORDISK AS
Self-assembling peptide and gel produced from the same
InactiveUS8299032B2High transparencyGood molding effectAntibacterial agentsPeptide/protein ingredientsAcidic amino acidsAqueous solution
A self-assembling peptide containing a polar amino acid residue and a nonpolar amino acid residue, wherein the self-assembling peptide contains an acidic amino acid residue and a basic amino acid residue as the polar amino acid residues, a total sum of charge of the acidic amino acid residue and charge of the basic amino acid residue in a neutral region is the number excluding 0, and the self-assembling peptide is capable of forming a beta (β)-sheet structure in which only the nonpolar amino acid residue is arranged on one face upon self-assembly in an aqueous solution.
Owner:MENICON CO LTD
Small peptide capable of resisting thrombosis and platelet aggregation
InactiveCN102391360AInhibit aggregationInhibition formationPeptide/protein ingredientsPeptidesAnti plateletValine
The invention discloses a small peptide capable of resisting thrombosis and platelet aggregation, and the sequence of the small peptide is X-Arg-Y-X-Lys, wherein X refers to nonpolar amino acid and the nonpolar amino acid includes alanine, valine, leucine, isoleucine, proline or phenylalanine; while Y refers to polar amino acid and the polar amino acid is glycine, serine, threonine, cysteine, tyrosine, asparagine, glutamine, aspartic acid or glutamic acid. An in vitro platelet aggregation test and an in vivo thrombosis test show that the small peptide can restrain platelet aggregation in a concentration dependent way and simultaneously can remarkably restrain thrombosis of artery-vein bypass in bodies of rats. The small peptide and derivatives of the small peptide can be used for resisting platelet aggregation and thrombosis in treatment. The small peptide can be used for preparing medicaments resisting thrombosis and platelet aggregation.
Owner:SOUTHEAST UNIV
Self-assembling peptide and peptide gel with high strength
ActiveUS8729032B2Practical mechanical strengthAntibacterial agentsCosmetic preparationsHigh intensityAcidic amino acids
Provide are a peptide gel with practically sufficient mechanical strength and a self-assembling peptide capable of forming the peptide gel. The self-assembling peptide is formed of the following amino acid sequence: a1b1c1b2a2b3db4a3b5c2b6a4 where: a1 to a4 each represent a basic amino acid residue; b1 to b6 each represent an uncharged polar amino acid residue and / or a hydrophobic amino acid residue, provided that at least five thereof each represent a hydrophobic amino acid residue; c1 and c2 each represent an acidic amino acid residue; and d represents a hydrophobic amino acid residue.
Owner:MENICON CO LTD +1
Oxidation resistant bioactive peptide of halobios and preparation method
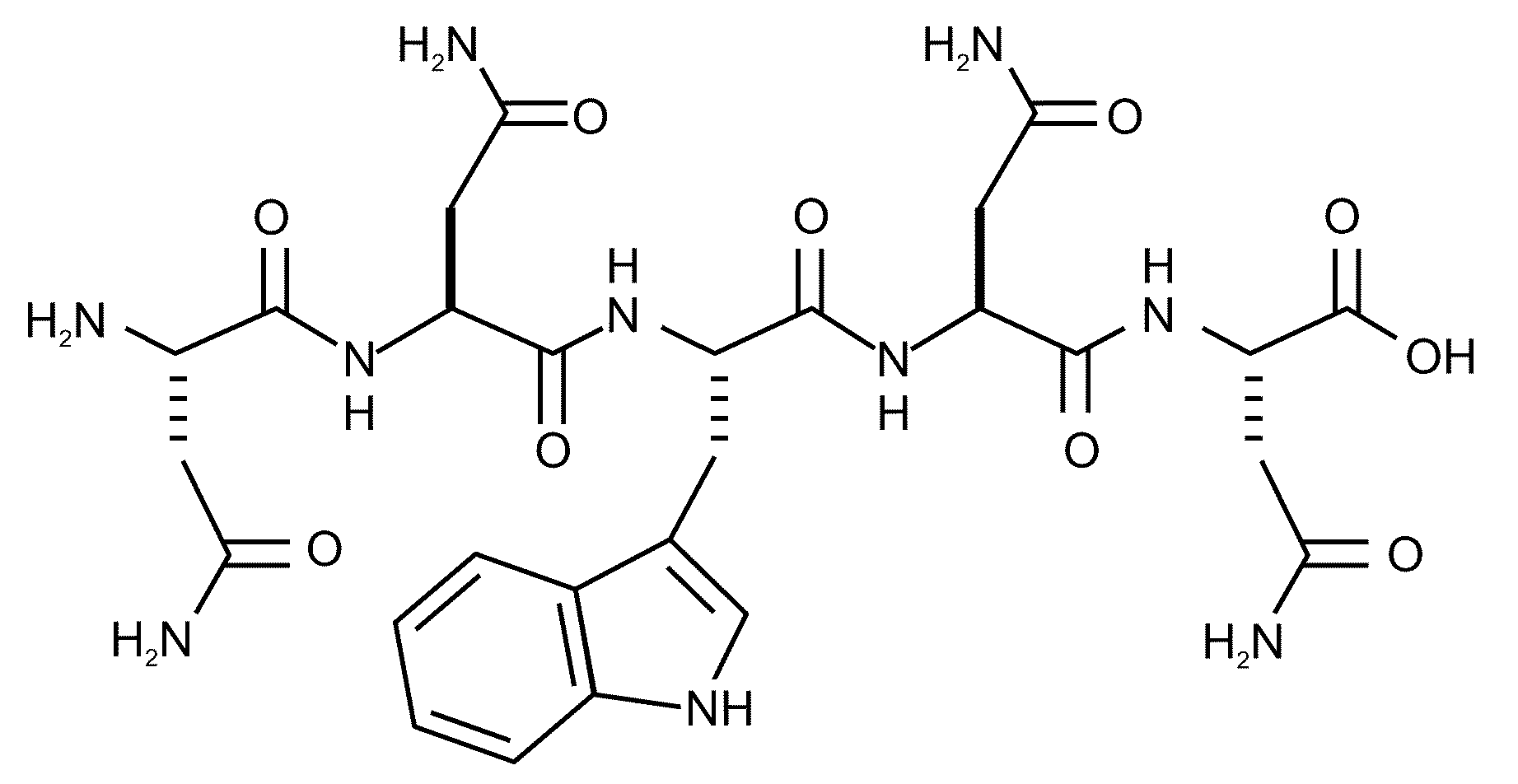
InactiveCN1660892AStrong emulsifying abilityImprove antioxidant capacityPeptide preparation methodsFermentationSolubilityOxidation resistant
An antioxidizing peptide for marine biota is a micro-molecular polypeptide consisting of 7 amino acid and containing 8 amino acid residues. Its preparing process is also disclosed. Its advantages are strong antioxidizing activity, high water solubility, and no poison and by-effect. It is suitable for food, cosmetics, medicines, etc.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Amphiphilic linear peptide/peptoid and hydrogel comprising the same
The present invention provides an amphiphilic linear peptide and / or peptoid as well as a hydrogel that includes the amphiphilic linear peptide / peptoid. The amphiphilic linear peptide / peptoid is capable of forming a hydrogel. These peptides / peptoids include short amphiphilic sequences with a hydrophobic portion of aliphatic amino acids and at least one acidic, neutral, or basic polar amino acid. The amphiphilic linear peptide / peptoid is build up of non repetitive aliphatic amino acids, which may be in the L- or D-form. A plurality of such peptides / peptoids assembles to supramolecular helical fibers and forms peptide hydrogels after assembly. A corresponding hydrogel is formed in aqueous solutions at physiological pH and is thus useful for inter alia cell culture, tissue engineering, and drug release. Such hydrogels which are rigid, biocompatible and entrapping up to 99.9% of water are also well suited for applications utilizing electronic devices.
Owner:AGENCY FOR SCI TECH & RES
Anti-bacterial peptides and methods of treating diseases using same
InactiveUS20100104607A1Reducing MazF-mediated anti-bacterial activityReduced activityAntibacterial agentsBiocideCrystallographyDisease
Isolated peptides comprising no more than ten amino acids and having anti-bacterial properties are disclosed. In one embodiment the peptides have a consensus amino acid sequence X1X2X3X4X5, wherein X1 and X5 comprise polar amino acids. In another embodiment, the peptides have a consensus amino acid sequence X1X2X3X4X5, wherein X2 and X4 are asparagine (N) residues and X3 is tryptophan (W). Compositions comprising same are also disclosed and uses thereof.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Compound gaseous-phase rust inhibitor as well as preparation method and application thereof
The invention relates to a compound gaseous-phase rust inhibitor as well as a preparation method and application thereof. The compound gaseous-phase rust inhibitor comprises the following components in percentage by mass: 25%-30% of nonpolar amino acid, 3%-5% of benzotriazole, 25%-30% of organic diacid salt and 35%-40% of benzoate. A plastic masterbatch containing the compound gaseous-phase rust inhibitor comprises the following components in percentage by mass: 30-40% of compound rust inhibitor, 50-60% of carrier plastics and 10-20% of adjuvant. Compared with the prior art, the compound gaseous-phase rust inhibitor has the advantages that nonpolar amino acid is selected by regulating and controlling the polarity of an amino acid molecule, and the compatibility of an amino acid rust inhibitor and a plastic film is improved by utilizing the good corrosion resistance of an amino acid compound, taking fumed silica as a dispersion carrier and utilizing the characteristics of the large specific surface area, strong surface adsorbability and good dispersibility of fumed silica, so that the rust inhibiting capacity of a volatile rust-inhibiting plastic film is improved.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
Method of treating chronic ulcers
InactiveUS7700660B2Successfully healingSuccessfully treatingBiocideCosmetic preparationsHydrogel matrixDiabetic ulcer
The invention provides a method of treating a chronic ulcer, such as a diabetic ulcer, comprising administering a therapeutic amount of a hydrogel matrix to the ulcer, the matrix composition comprising gelatin and a long chain carbohydrate. The matrix may further include polar amino acids, nitric oxide inhibitors and super oxide inhibitors. Injection is a preferred method of administration. The matrix may be injected into one or more locations within the ulcer, underneath the ulcer and / or around the periphery of the ulcer.
Owner:PIONEER SURGICAL TECH INC
Recombinant encephalitis B virus and application thereof
ActiveCN102964434AAvoid infectionFree from attackBacteriaViral antigen ingredientsEncephalitisBULK ACTIVE INGREDIENT
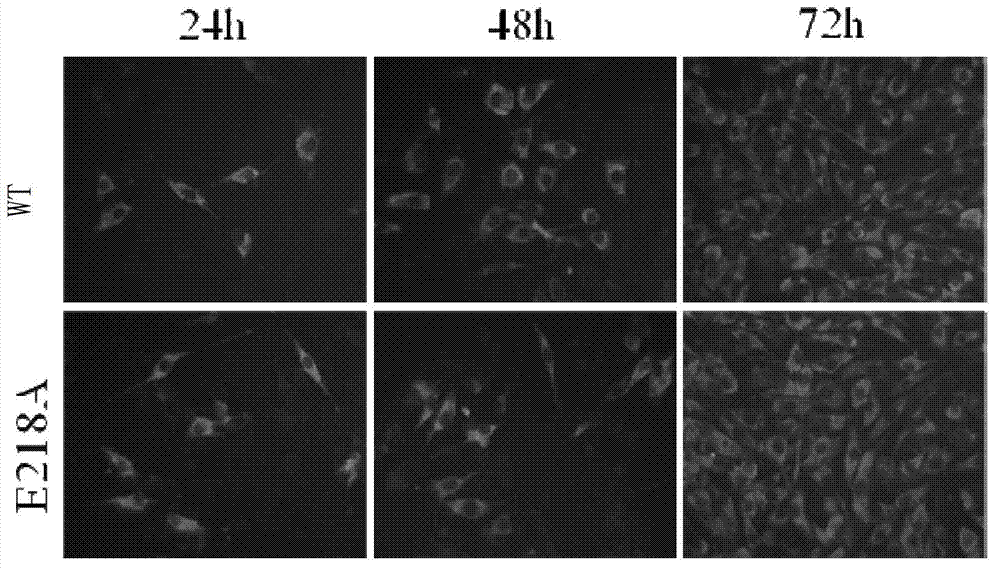
The invention discloses a recombinant encephalitis B virus and application thereof. The invention provides a protein which is obtained by mutating a 218th-site amino acid residue on an N terminal of the protein shown as a sequence 3 of a sequence table from a polar amino acid to a non-polar amino acid. The invention also discloses a gene coding the protein, a recombinant expression vector containing the gene, an expression cassette, a transgenic cell line, and a recombinant virus or recombinant bacterium. The invention also discloses an encephalitis B virus vaccine of which the active ingredient is the recombinant virus. The recombinant encephalitis B virus provided by the invention can be used as the encephalitis B virus vaccine, and has a better application prospect in prevention of the encephalitis B virus infection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Cation amphiphilic membrane targeted alpha-helix polypeptides and application thereof
ActiveCN104974227AHas anticancer activityViolent killing effectPeptide/protein ingredientsPeptidesPharmaceutical drugOrganic chemistry
The invention discloses cation amphiphilic membrane targeted alpha-helix polypeptides and an application thereof. The polypeptides comprise an amino acid sequence [X1X2X3X1X2X3X1]n-NH2, wherein NH2 represents amination of C terminal; X1 is polar amino acid with positive charges; X2 and X3 are nonpolar hydrophobic amino acids; n is a positive integer; and all amino acids are L type amino acids. According to the invention, a series of cation amphiphilic membrane targeted alpha-helix polypeptides are provided, and have anti-cancer activity; different types of hydrophobic amino acids (L, I, F, A and the like) are remarkably different in the membrane action effect; and if the concentration is higher than the key micelle forming concentration, the hydrophobic amino acids can be self-assembled into a micro-micelle to generate a violent cell killing effect, and a new basis is provided for the development of anti-cancer drugs.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
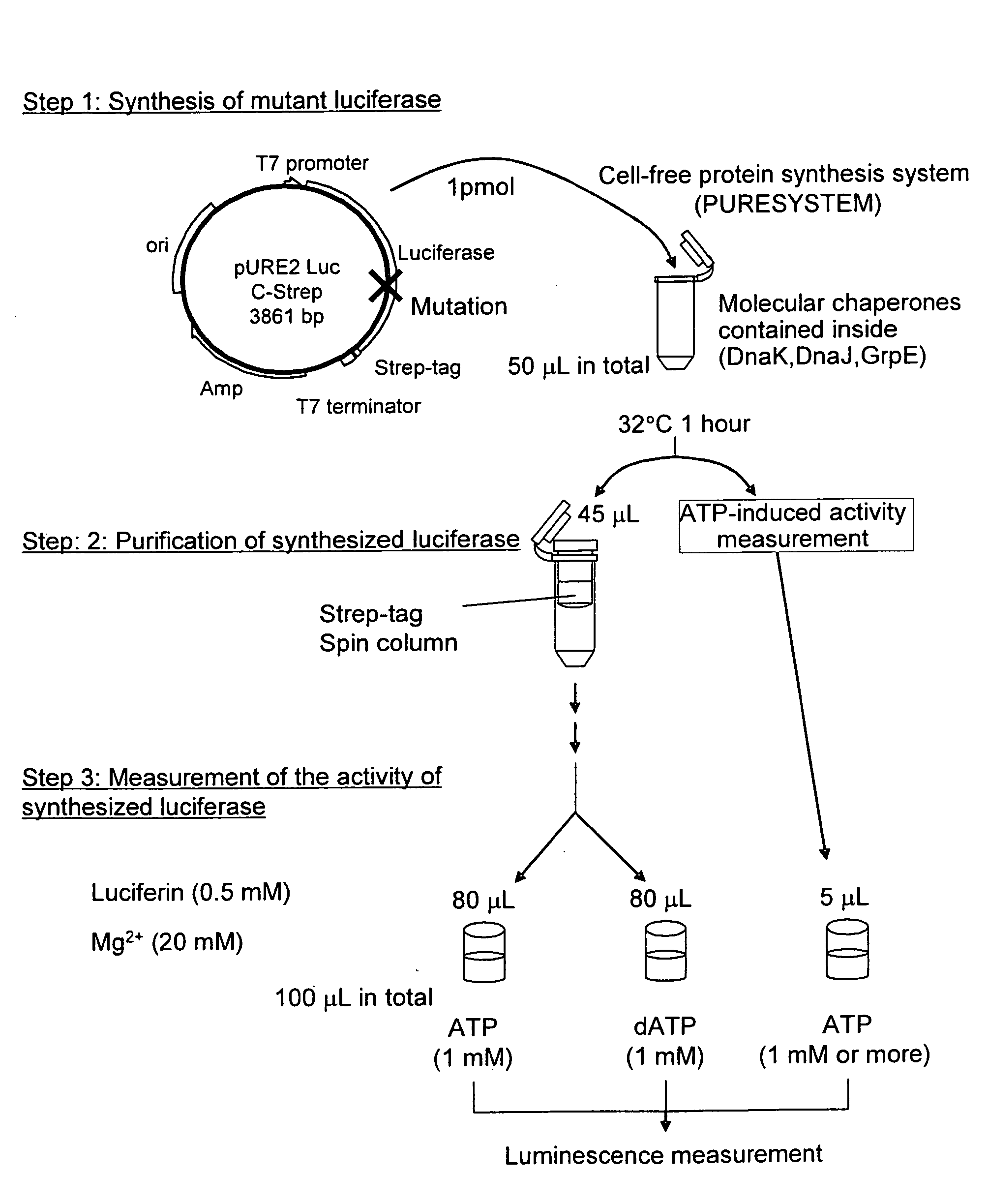
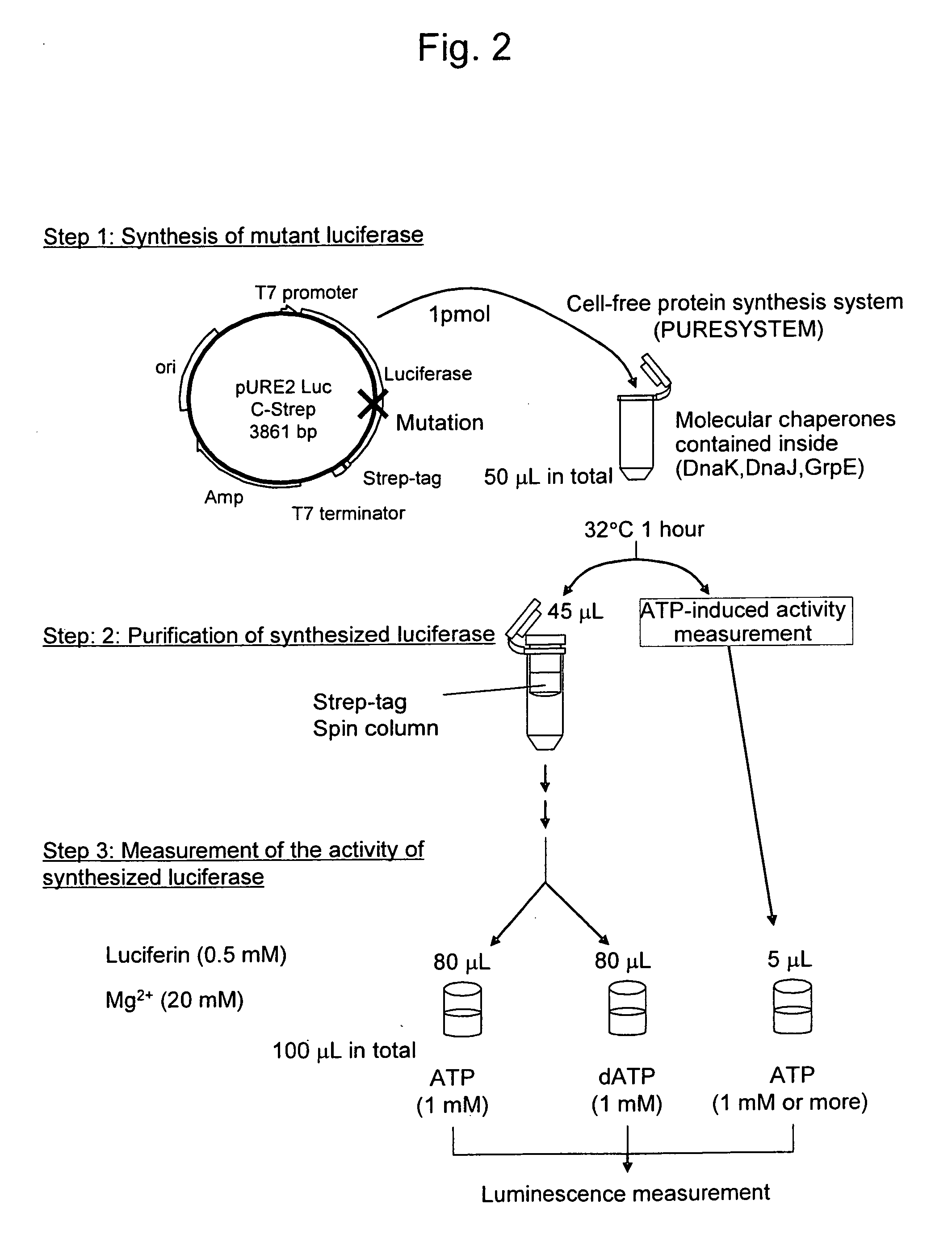
Mutant firefly luciferase
InactiveUS20090081680A1Reduce the ratioSugar derivativesMicrobiological testing/measurementFluorescenceWild type
This invention relates to: the development of a mutant firefly luciferase in order to use dATP as a DNA polymerase substrate upon pyrosequencing, such luciferase being subjected to substrate specificity modification in a manner such that the dATP-induced activity alone is decreased while the ATP-induced activity is maintained; and a mutant firefly luciferase for which the proportion of activity induced by dATP to activity induced by ATP (dATP / ATP) is lower than that for the wild-type firefly luciferase, in which an amino acid identified based on homology analysis as corresponding with the 421st amino acid (glycine) of the amino acid sequence of the wild-type North American firefly (Photinus pyralis) luciferase has been substituted with a polar amino acid.
Owner:HITACHI LTD
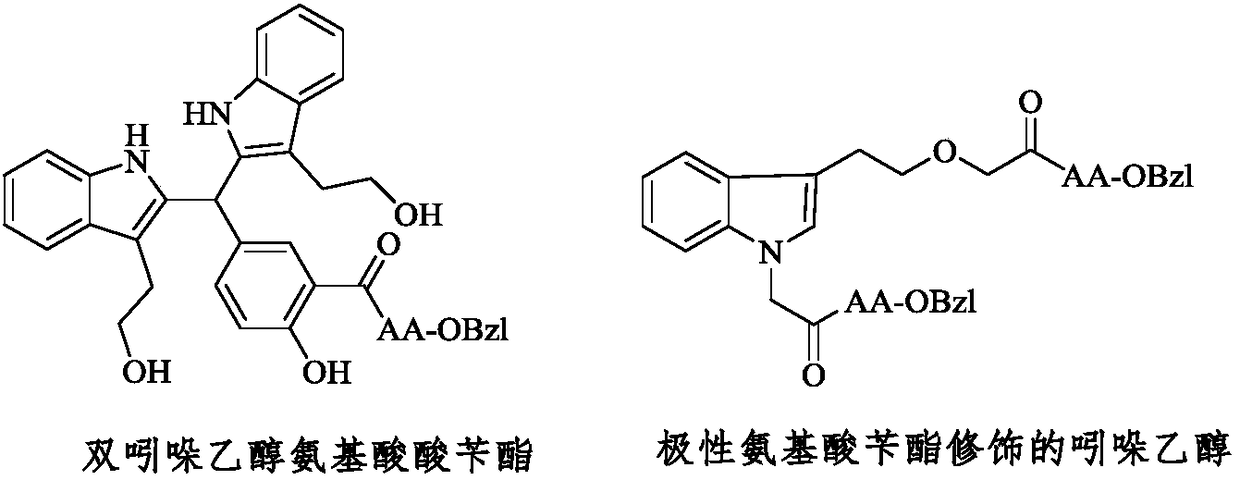
Polar amino acid-modified indole ethanol derivative and its synthesis, activity and application
The invention discloses 1-(acetyl-AA-OBzl)-3-(ethoxyacetyl-AA-OBzl)indole shown as a formula below, which is shown in the description, (wherein, AA is L-Ser residue, L-Thr residue, L-Gln residue, L-Asn residue and L-Cys(Bzl) residue), and also discloses their preparation method, antitumor activity, anti-tumor-metastasis activity, and anti-inflammatory activity. Therefore, the invention discloses their application in the preparation of antitumor drugs, anti-tumor-metastasis drugs and anti-inflammatory drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Self-Assembling Peptide and Peptide Gel with High Strength
InactiveUS20140161753A1Practical mechanical strengthCosmetic preparationsPeptide/protein ingredientsAcidic amino acidsComputational chemistry
Provide are a peptide gel with practically sufficient mechanical strength and a self-assembling peptide capable of forming the peptide gel. The self-assembling peptide is formed of the following amino acid sequence: a1b1c1b2a2b3db4a3b5c2b6a4 where: a1 to a4 each represent a basic amino acid residue; b1 to b6 each represent an uncharged polar amino acid residue and / or a hydrophobic amino acid residue, provided that at least five thereof each represent a hydrophobic amino acid residue; c1 and c2 each represent an acidic amino acid residue; and d represents a hydrophobic amino acid residue.
Owner:UNIV OKAYAMA +2
Anti-bacterial peptides and methods of treating diseases using same
Isolated peptides comprising no more than ten amino acids and having anti-bacterial properties are disclosed. In one embodiment the peptides have a consensus amino acid sequence X1X2X3X4X5, wherein X1 and X5 comprise polar amino acids. In another embodiment, the peptides have a consensus amino acid sequence X1X2X3X4X5, wherein X2 and X4 are asparagine (N) residues and X3 is tryptophan (W). Compositions comprising same are also disclosed and uses thereof.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
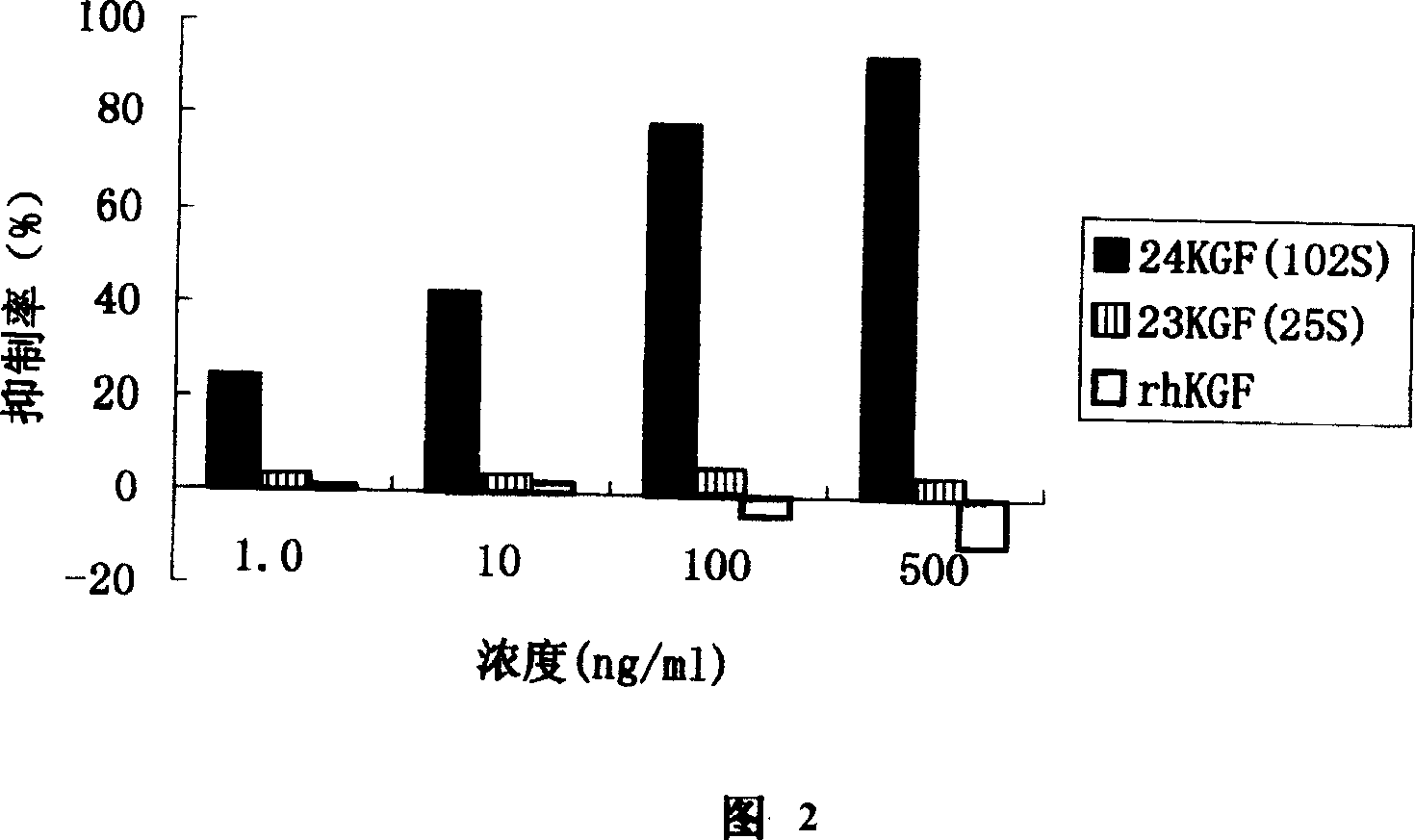
Novel human keratinocyte growth factor mutant
A novel human keratinocyte growth factor mutant features that the cysteine at its site No.102 is mutated to become non-polar amino acid and the deficient mutation takes place at its terminal N. It can prevent the generation of scar and fibrosis and promote the epiderm healing.
Owner:CHONGQING FAGEN BIOMEDICAL
Anti-pathogenic therapeutic compositions
ActiveUS20200046666A1Vitro stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsAnthraquinonesMicrobiology
Therapeutic compounds useful for the treatment of pathogens including bacteria (gram positive and gram negative, mycobacteria), some fungi and viruses. The compounds described herein may include a mixture of therapeutically-effective amounts of a polar amino acid, a CI 1 fatty acid, and an anthraquinone. The invention further provides for the administration of the therapeutic compounds to a patient (e.g., a human) suffering from an infection.
Owner:WINTERMUTE BIOMEDICAL INC
Silver impregnation liquid and silver catalyst for ethylene oxide production through ethylene epoxidation, and preparation methods thereof
ActiveCN110605116AImprove uniformityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsEthylene DibromideAmmonia
The invention belongs to the field of catalysts, and provides a silver impregnation liquid for ethylene oxide production through ethylene epoxidation, a silver catalyst and preparation methods thereof. The silver impregnation liquid for ethylene oxide production through ethylene epoxidation comprises the following components: 1) a silver-ammonia complex; 2) polar amino acid; 3) water; 4) an optional auxiliary agent; wherein based on the total weight of the impregnation liquid, the content of the polar amino acid is 0.05-60 wt%. Compared with the prior art, the uniformity of the silver catalystprepared by the method provided by the invention is improved, and the silver catalyst has higher selectivity and higher catalytic activity for the reaction of producing ethylene oxide by ethylene oxidation.
Owner:CHINA PETROLEUM & CHEM CORP +1
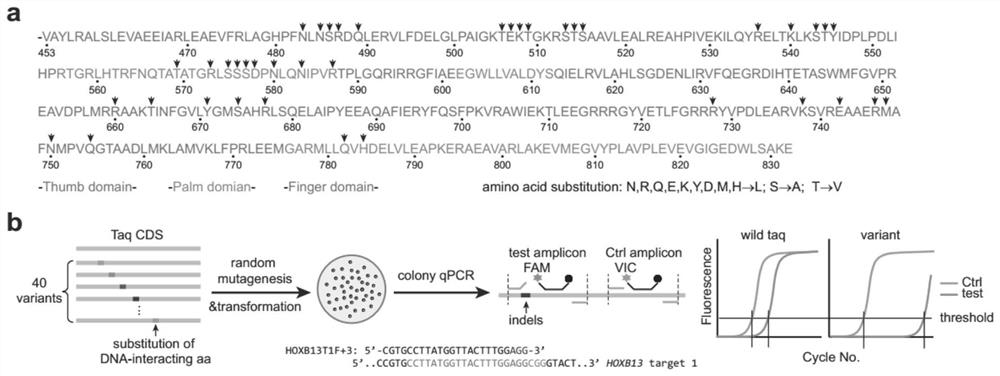
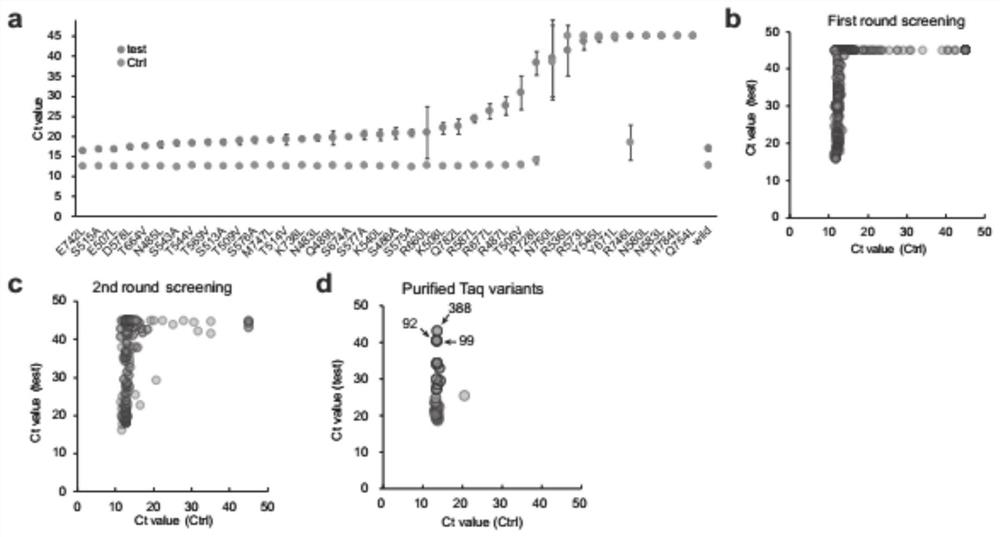
High-specificity Taq DNA polymerase variantS and application thereof in genome editing and gene mutation detection
ActiveCN112921015AImprove performanceStrong specificityMicrobiological testing/measurementTransferasesWild typeVariome
The invention provides high-specificity Taq DNA polymerase variants and application thereof in genome editing and gene mutation detection, and belongs to the technical field of biology. Semi-rational directed molecular evolution is carried out on A wild type full-length Taq DNA polymerase, so that the specificity of the polymerase is improved. All polar amino acids, directly interacting with a primer / template compound, on Taq enzyme are selected to be mutated one by one to obtain 40 Taq variants, and then extensive random mutagenesis is performed on the basis of the variants and wild type sequences to generate a Taq mutant library. A series of Taq mutants with high specificity are screened on a qPCR screening system by taking genome editing indels plasmid as a template, and the Taq mutants show great advantages in CRISPR / Cas9 editing efficiency evaluation and single cell cloning genotyping, so that the Taq mutants have good practical application value.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com