Cell-wall degrading enzyme variants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Pectate Lyase Variant (M1691, F198V)
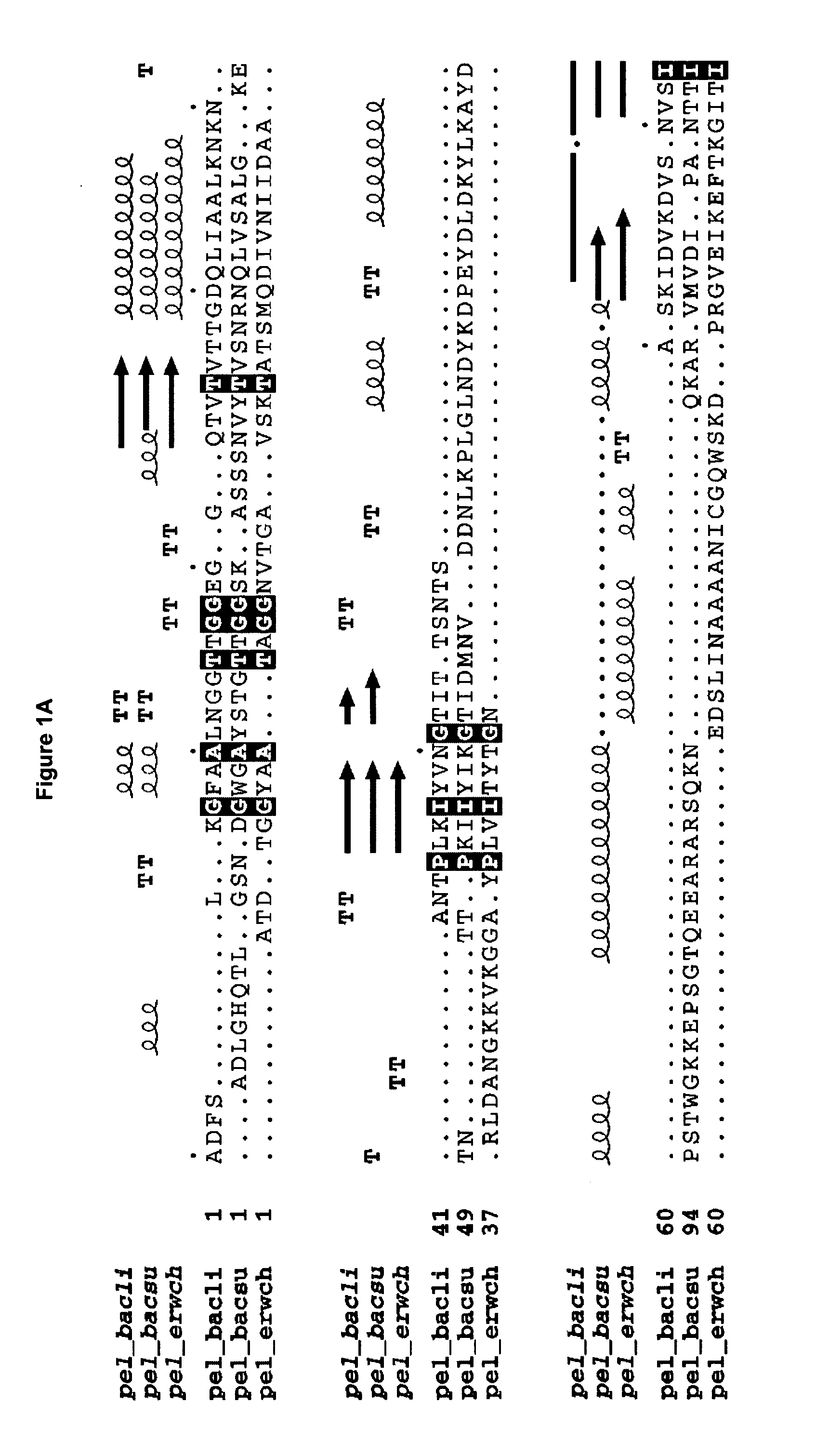
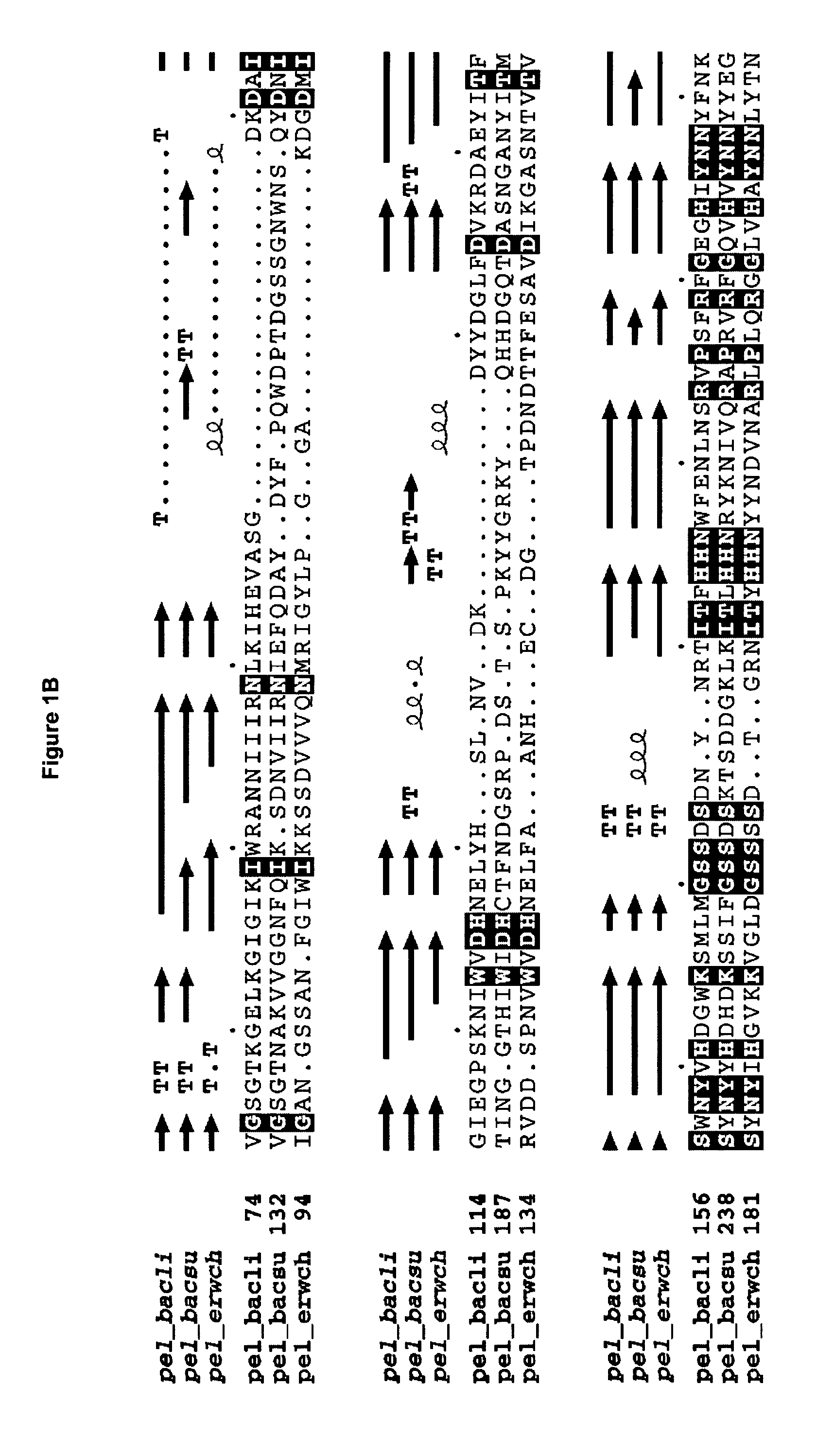
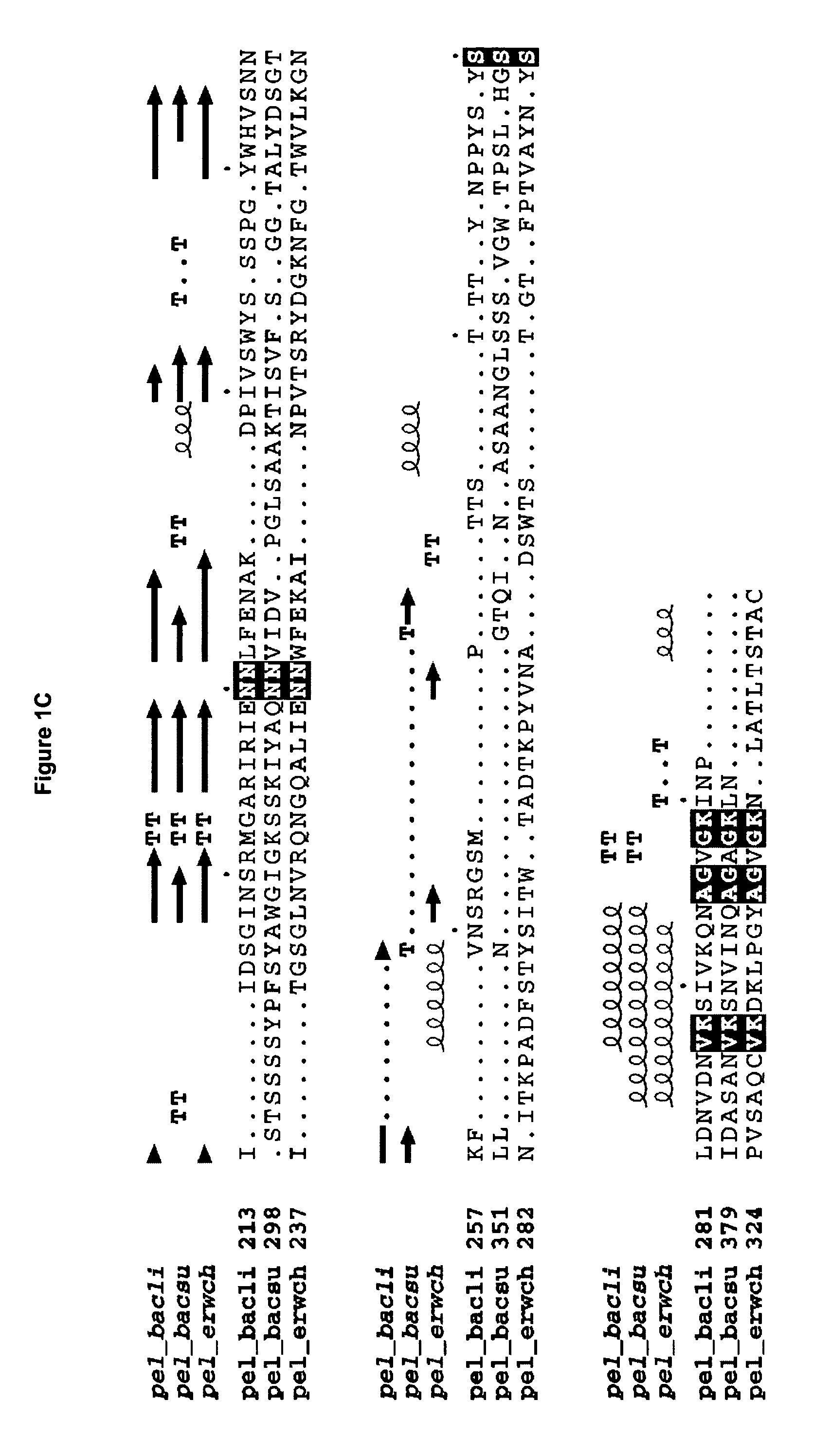
[0267] The wild-type B. licheniformis pectate lyase encoded by SEQ ID NO: 1 is expressed in B. subtilis from a plasmid denoted pMB541, see Materials and Methods. This plasmid contains a fusion of the signal sequence from B. licheniformis alpha-amylase and the gene encoding the mature protein of B. licheniformis pectate lyase (SEQ ID NO: 2, wild-type pectate lyase), the expression of which is directed by the B. licheniformis alpha-amylase promoter. Further, the plasmid contains the origin of replication, ori, from plasmid pUB110 and the cat gene from plasmid pC194 conferring resistance towards chloramphenicol. A specific mutagenesis vector with a 1.2 kb pUC fragment inserted in the unique PstI restriction site located between the nucleotide sequence coding for the signal sequence and the mature, was prepared. The important features of this vector, denoted pCA134 include an origin of replication derived from the pUC plasmids, the c...
example 2
Fermentation, Purification and Characterization of Bacillus licheniformis Pectate Lyase Variant M169I, F198V
[0272] The clone obtained as described in Example 1 was grown in 25×200 ml BPX media with 10 microliters / ml of kanamycin in 500 ml two baffled shake flasks for 5 days at 37° C. at 300 rpm.
[0273] 140 ml of shake flask culture fluid were diluted to 1000 ml with ion free water and applied to S-Sepharose (50 ml column equilibrated with 25 mM sodium acetate buffer pH 5.5). The pure pectate lyase variant was eluted using a NaCl gradient.
[0274] The pectate lyase variant gave a single band in SDS-PAGE of 35 kDa, exhibited 23 APSU units per mg protein, and a molar extinction coefficient of 57750.
[0275] The buffer of the pure enzyme was changed by size chromatography on a high load Superdex S200 column equilibrated with 0.1M EPPS buffer pH 8.0. DSC (Differential Scanning Calorimetry) was performed using a temperature increase of 1° C. per minute. The pure pectate lyase variant unfo...
example3
Construction, Fermentation, Purification and Characterization of Further Bacillus licheniformis Pectate Lyase Variants
[0276] By using the methods described in Example 1 and 2, the Bacillus licheniformis pectate lyase variants (relative to SEQ ID NO: 2) of Table I below were prepared and subjected to DSC (Differential Scanning Calorimetry) at pH 10 or pH 8 using a temperature increase of 1° C. per minute. The wild-type Bacillus licheniformis pectate lyase (SEQ ID NO: 2) has a DSC unfolding temperature of 60° C. (pH 10) and 70° C. (pH 8).
TABLE IDSC unfoldingtemperature(° C.)Variant no.Substitutions relative to SEQ ID NO: 2pH 10pH 81M169I + F198V + E189H672M169I + F198V + S72I723M169I + F198V + F144V + M167I70.14M169I + F198V + S72I + M265K75.95M169I + F198V + S72I + G203V74.76M169I + F198V + S72I + K83H75.77M169I + F198V + S72T668M169I + F198V + M167I65.69M169I + F198V + S72I + L82I +76.8I102F + L129F + V160F10M169I + F198V + T55P70.811M169I + F198V + S269P68.512D282H + N283P + D2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degradation properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com