Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
217 results about "Non steady state" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sound Modification System And Method
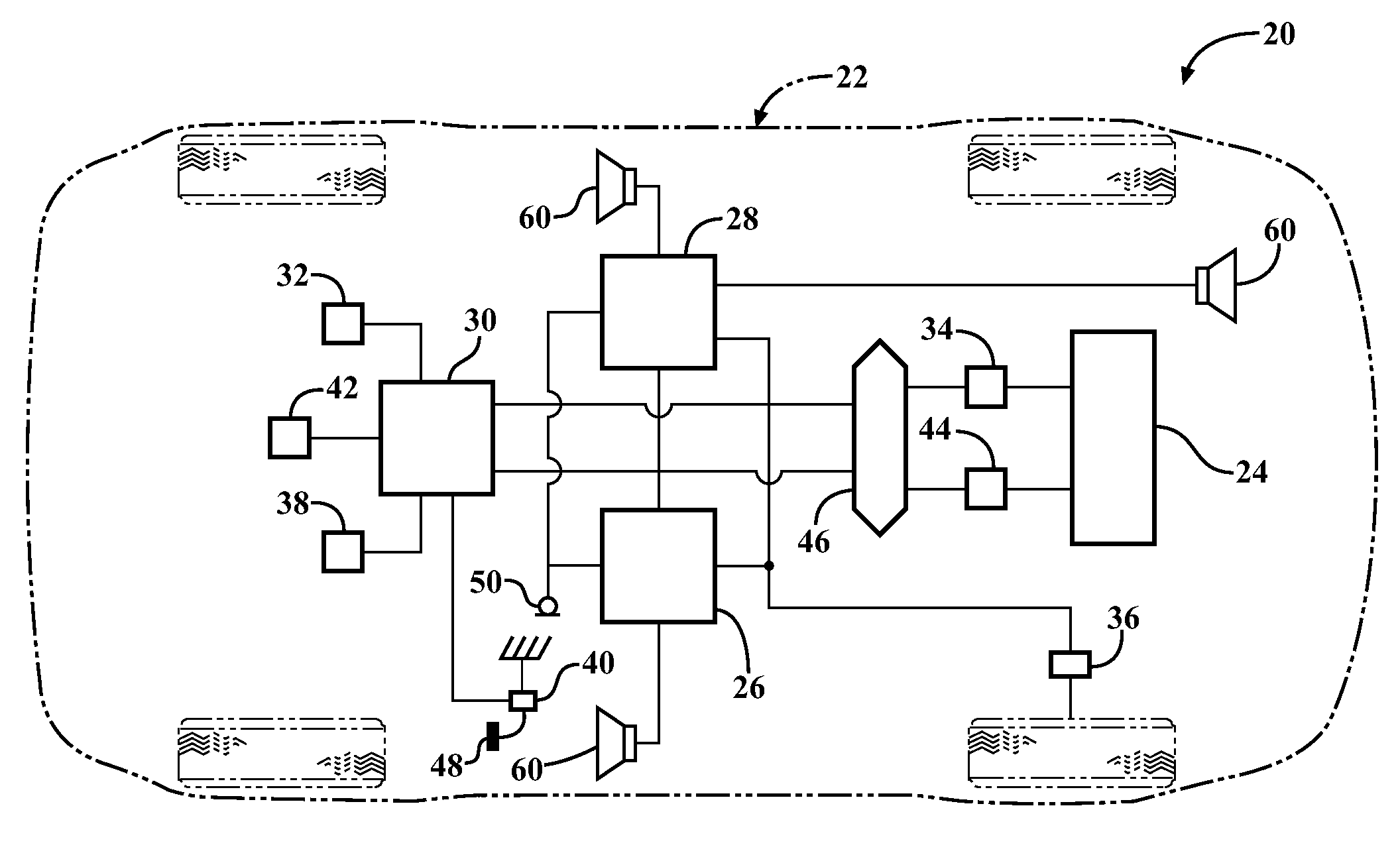
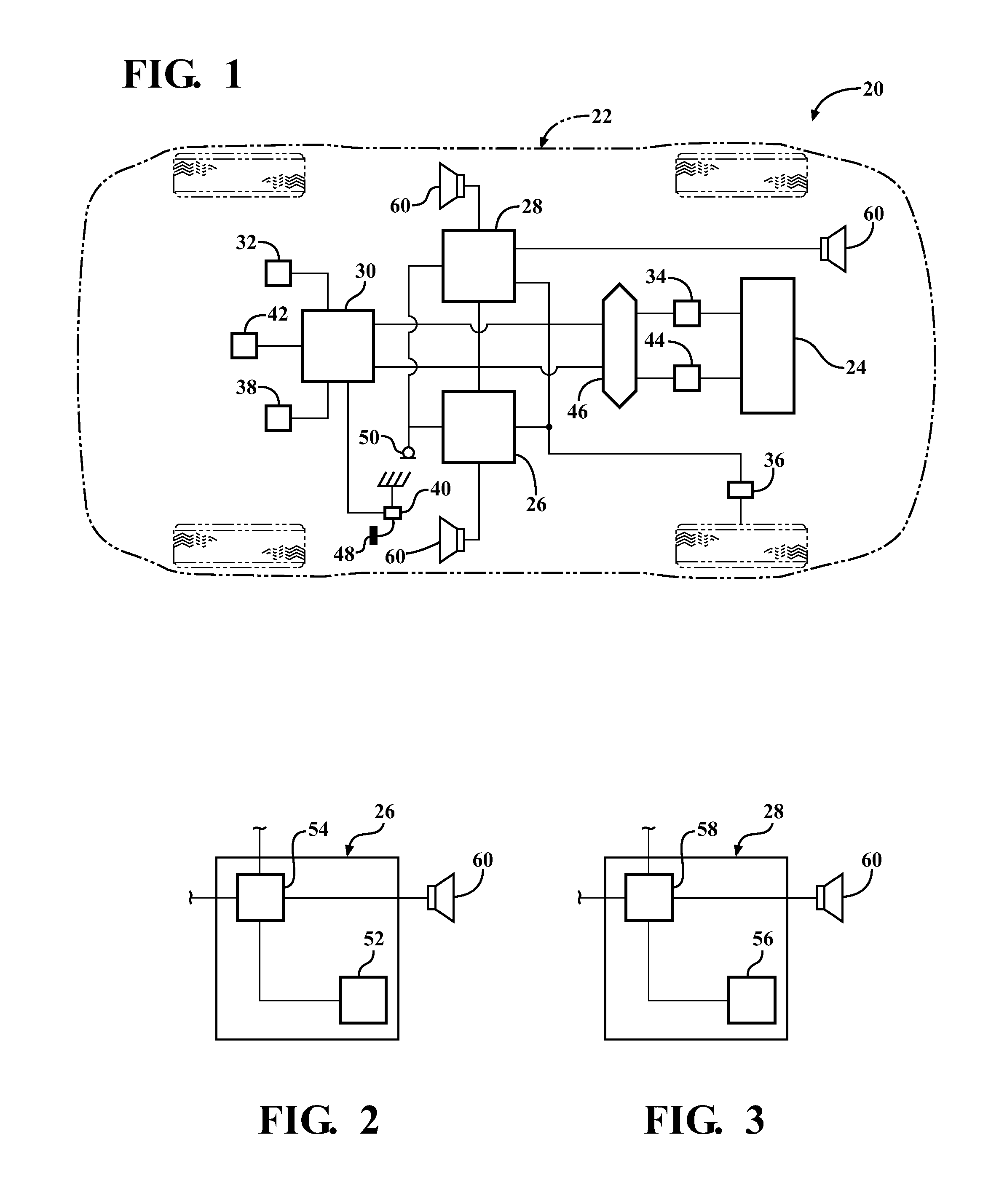
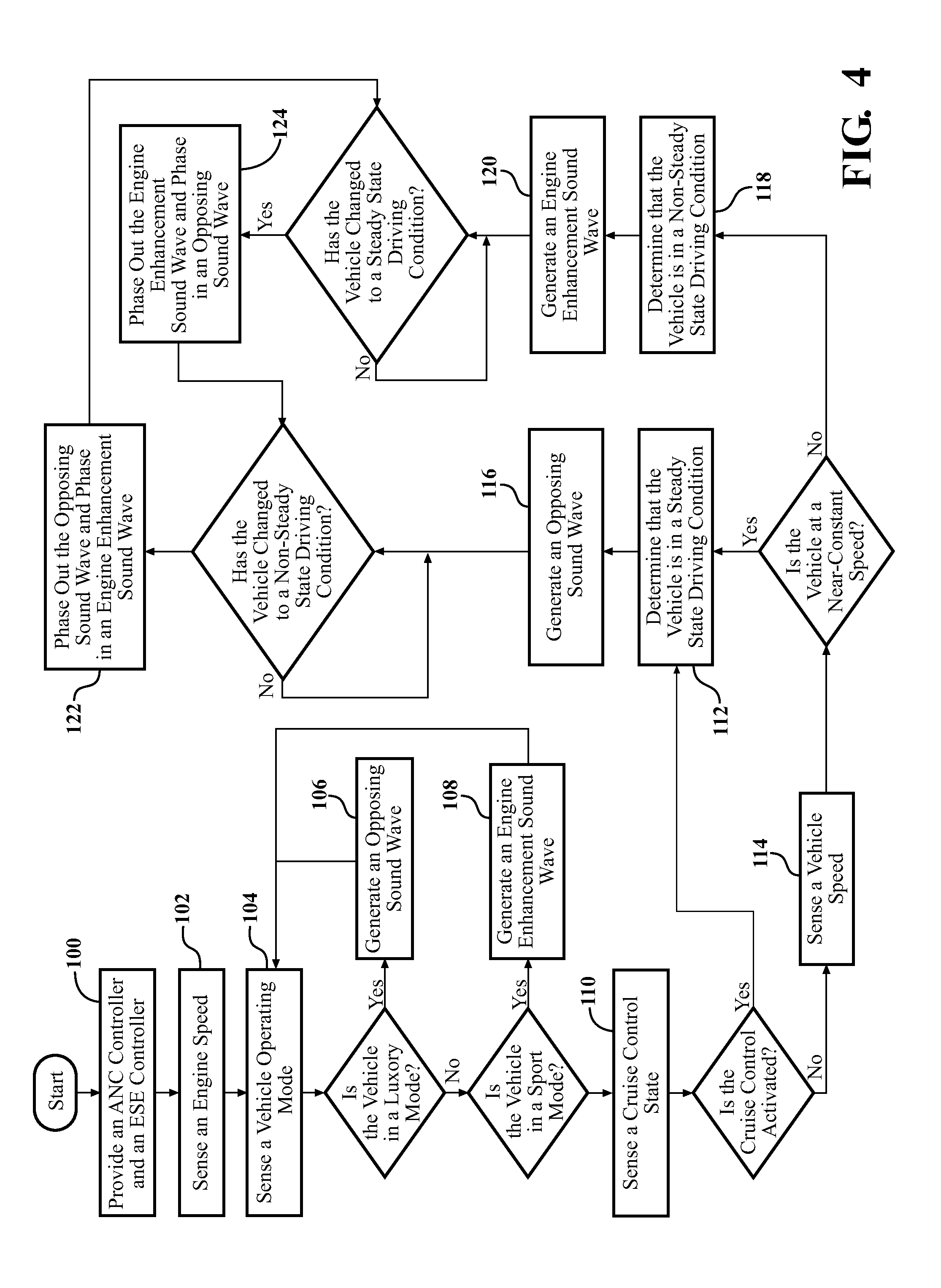
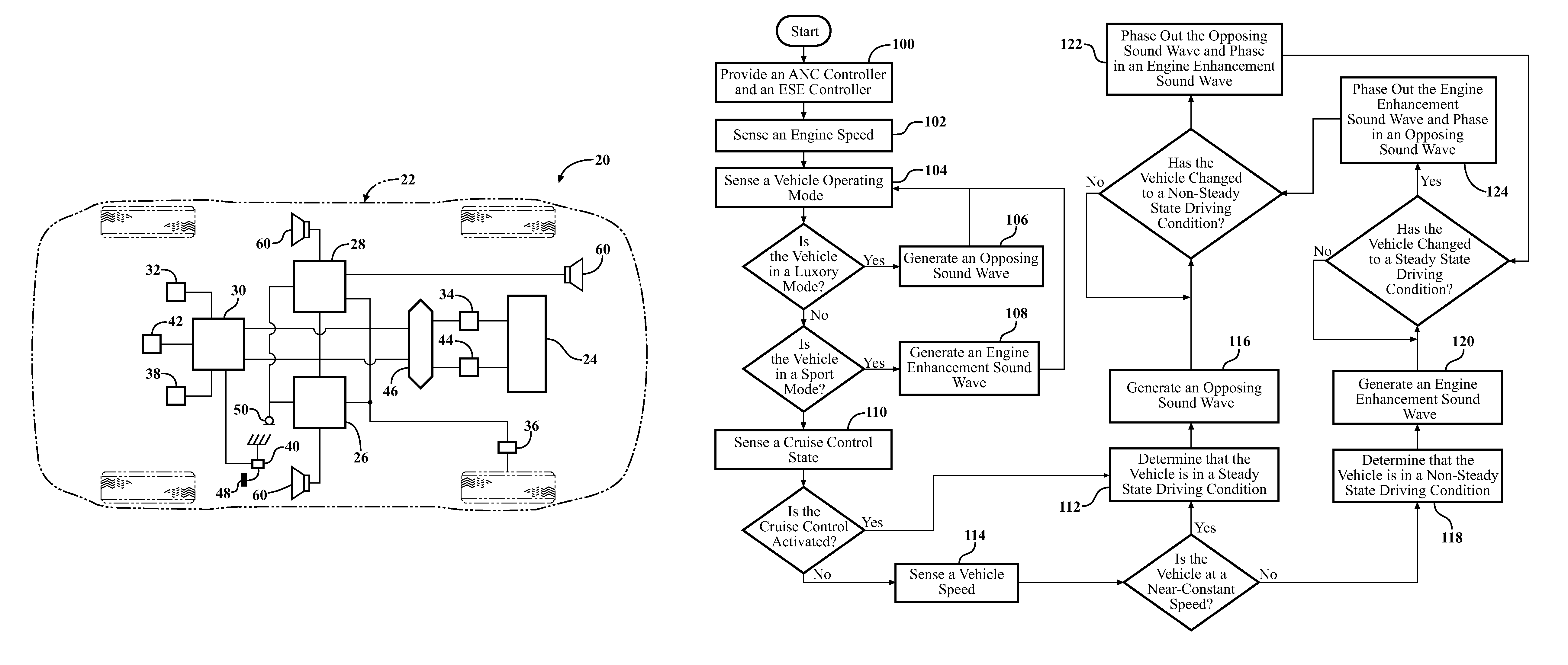
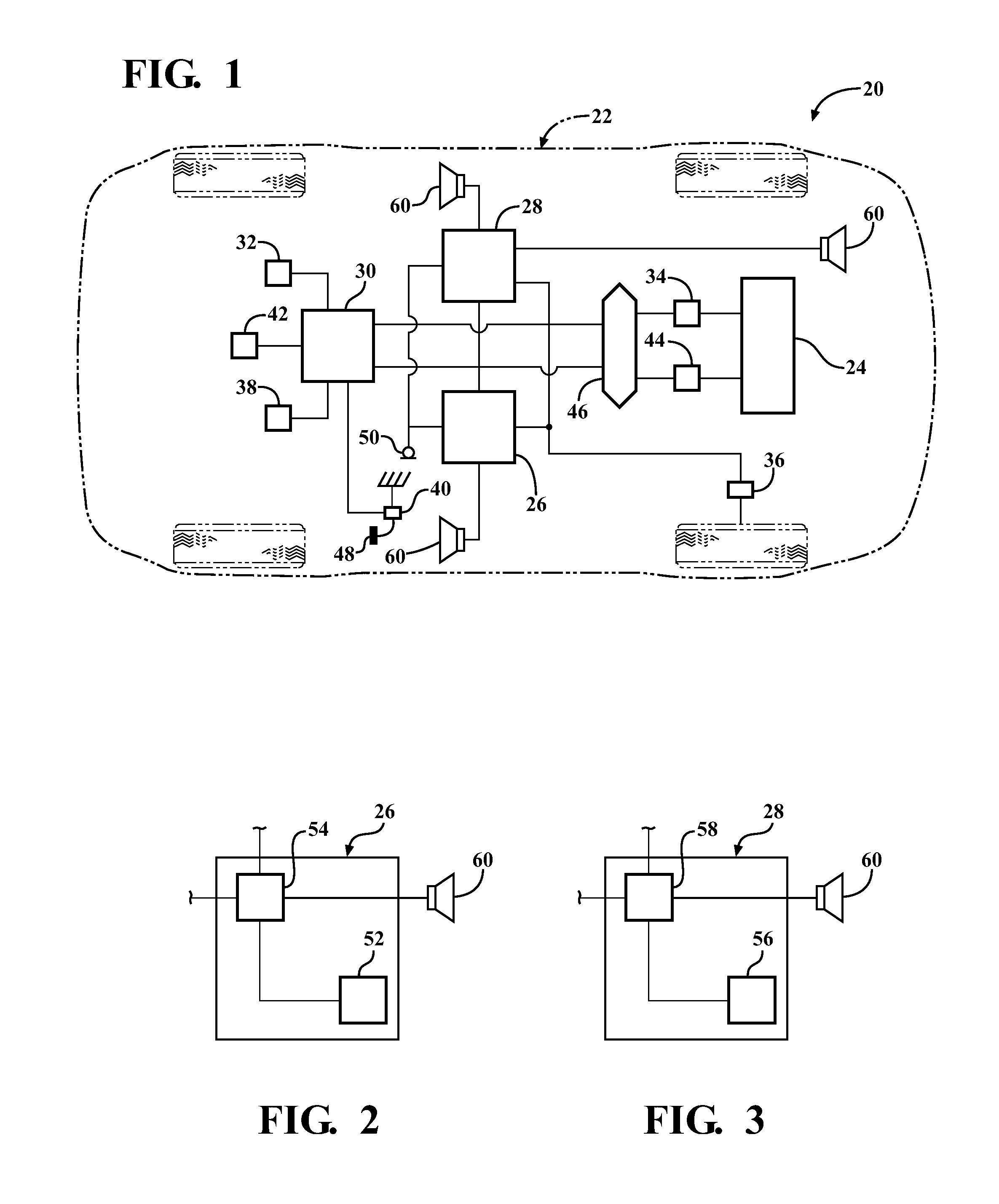
InactiveUS20120257763A1Minimize other noiseOptimal passenger compartment experienceEar treatmentGain controlDriver/operatorNoise reduction
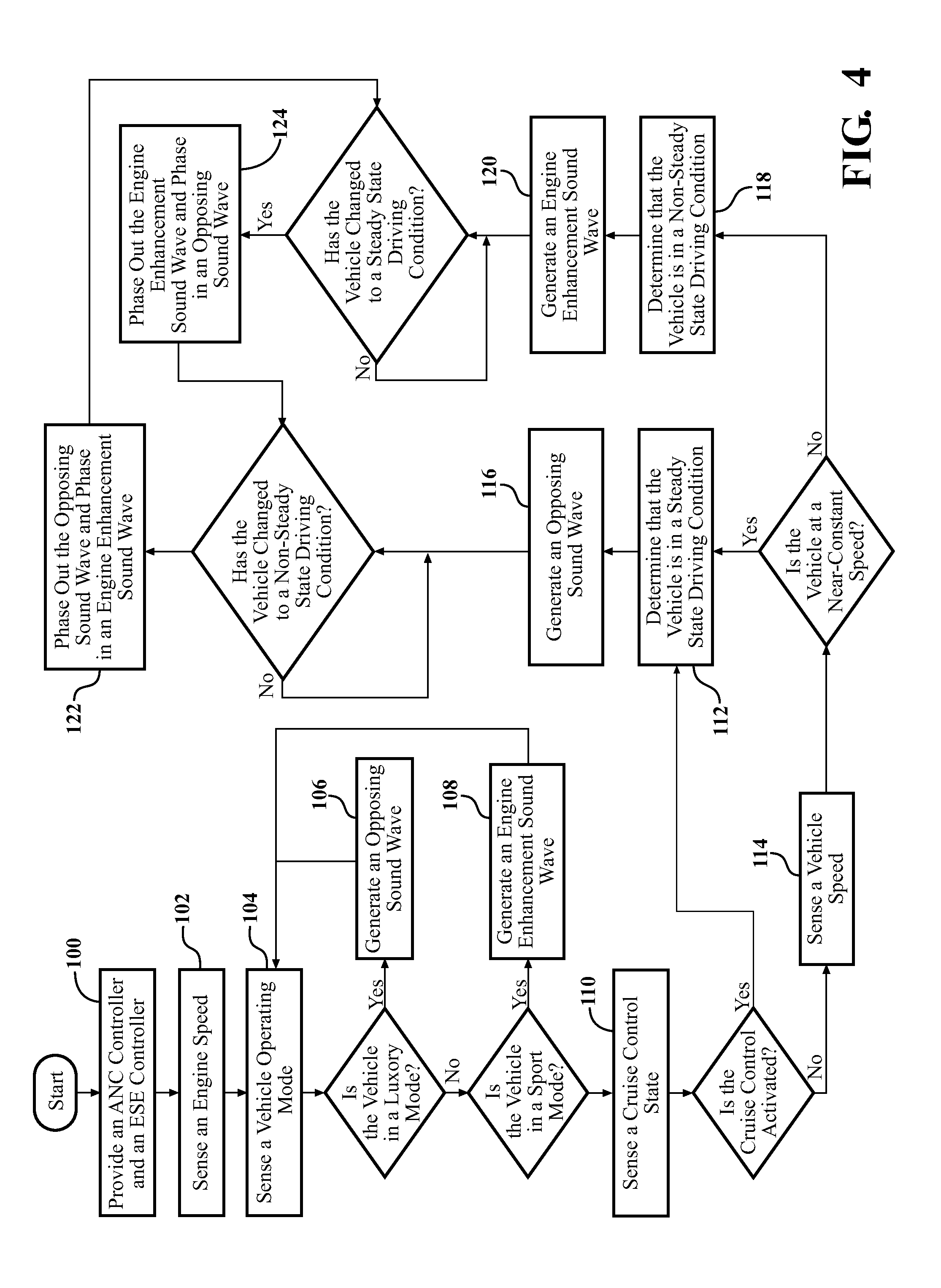
A sound modification system and a method of managing sound in a vehicle. The system includes an active noise reduction (ANR) controller, an engine sound enhancement (ESE) controller and a sound modification controller. When the vehicle is in a steady state driving condition, the sound modification controller activates the ANR controller and deactivates the ESE controller. In contrast, when the vehicle is in a non-steady state driving condition, the sound modification controller activates the ESE controller and deactivates the ANR controller. Preferably, when the vehicle switches between the steady state and non-steady state driving conditions, the sound modification controller activates and deactivates the ANR and ESE controllers at a rate that is not noticeable to a human. In summary, the system utilizes the ANR and ESE controllers to achieve an optimal passenger cabin experience for the driver during any driving condition.
Owner:VISTEON GLOBAL TECH INC
Process for producing aliphatic carboxylic acid
InactiveUS7319168B2Guaranteed uptimeShorten the timeOrganic compound preparationFractional distillationFractionating columnCarboxylic acid
It is an object of the present invention to provide a process for producing aliphatic carboxylic acid, which can stabilize operation of a distillation column upon production of aliphatic carboxylic acid by reducing a water content in an aqueous aliphatic carboxylic acid solution by a distillation column, and can shorten a time during the non-steady state such as at starting up of distillation column operation. The present invention is directed to a process for producing aliphatic carboxylic acid, which comprises an azeotropic distillation step of supplying an aqueous aliphatic carboxylic acid solution and an azeotropic solvent to an azeotropic distillation column to perform distillation, separating an azeotrope containing the azeotropic solvent and water as a distillate, and recovering aliphatic carboxylic acid with a reduced water content as bottom liquid, characterized in that a target value of an amount of the azeotropic solvent to be supplied is set depending on an amount of water in the aqueous aliphatic carboxylic acid solution supplied to the azeotropic distillation column, and the amount of the azeotropic solvent to be supplied is controlled at the target value.
Owner:NIPPON SHOKUBAI CO LTD
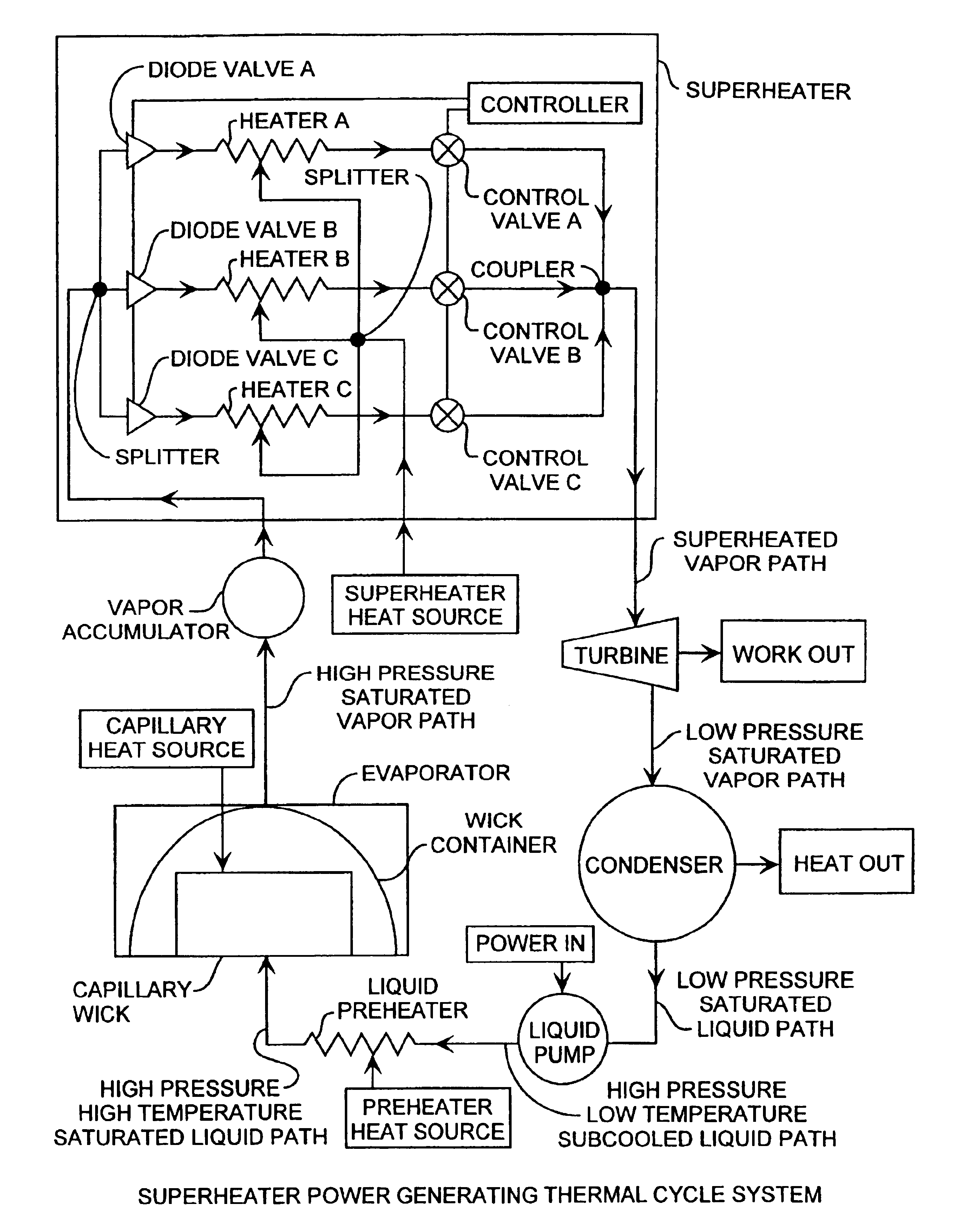
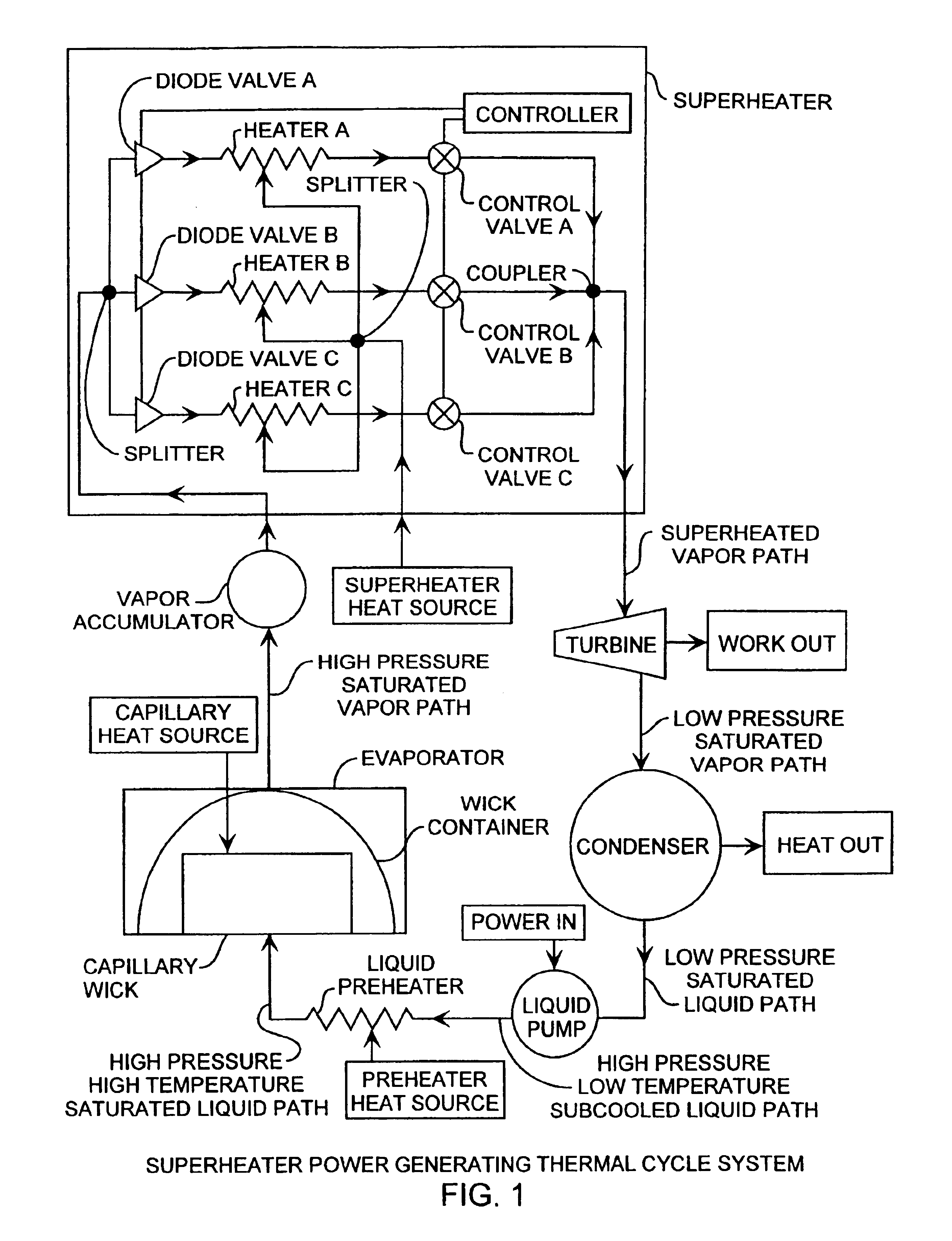
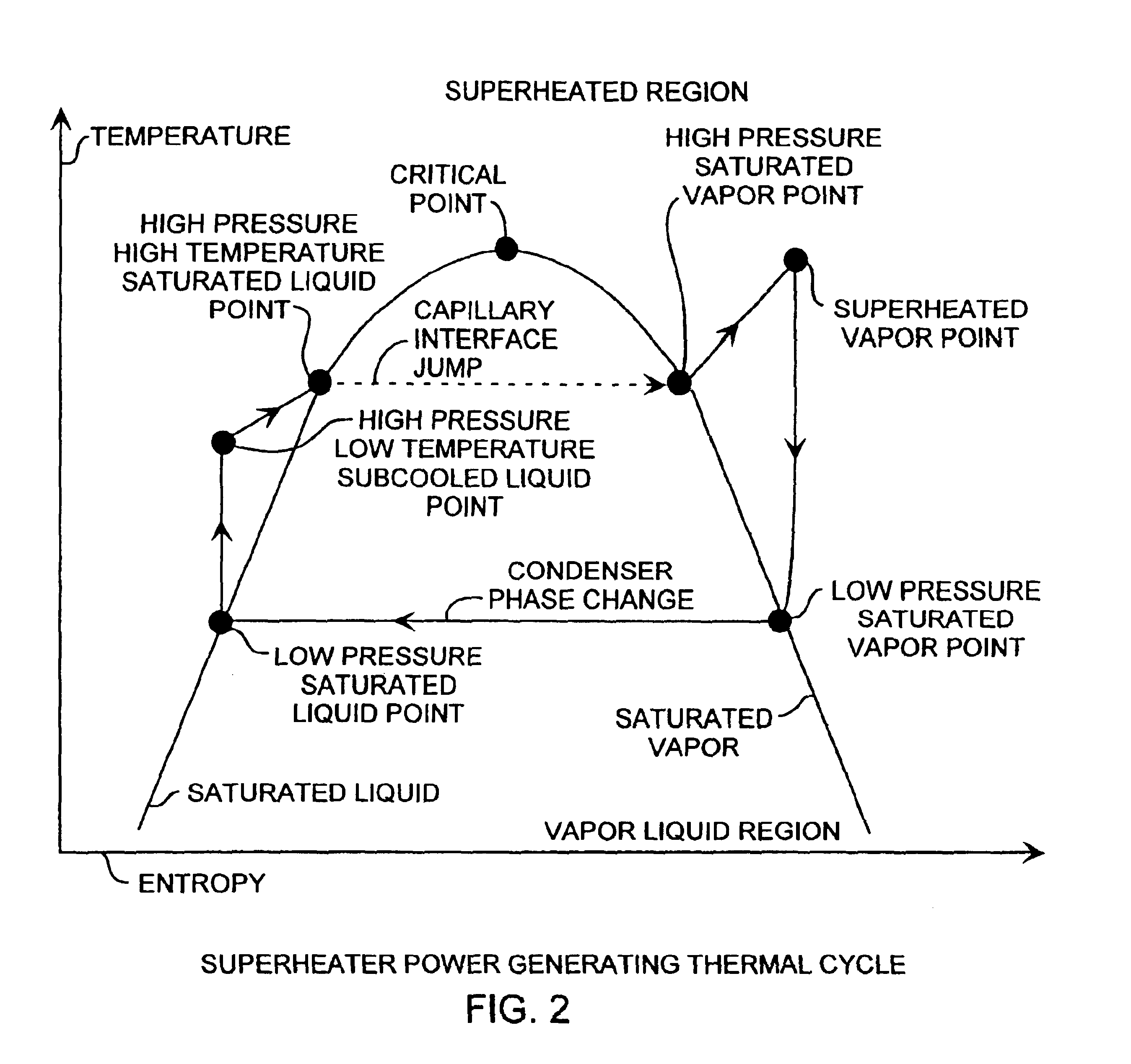
Superheater capillary two-phase thermodynamic power conversion cycle system
InactiveUS6918254B2Power generationImprove efficiencyIndirect heat exchangersSteam engine plantsThermal energyThermal energy storage
A two-phase thermodynamic power system includes a capillary device, vapor accumulator, superheater, an inline turbine, a condenser, a liquid pump and a liquid preheater for generating output power as a generator. The capillary device, such as a loop heat pipe or a capillary pumped loop, is coupled to a vapor accumulator, superheater, the inline turbine for generating output power for power generation, liquid pump and liquid preheater. The capillary device receives input heat that is used to change phase of liquid received from the liquid preheater, liquid pump and condenser into vapor for extra heating in the superheater used to then drive the turbine. The power system is well suited for space applications using a radioisotope, active nuclear or solar heat source. The system can use waste heat from various dynamic or static power systems as a heat source and waste heat from spacecraft components such as electronics as a heat source. These heat sources can be used separately or in any combination. The power system can be combined with thermal energy storage devices when operated with heat sources that are not steady state. Heat sources are useful for driving the capillary wick, superheater and liquid preheater for increased power efficiency and long lifetime operation. The power system is well suited for space receiving heat from a heat source to produce useful mechanical energy. A superheater in combination with a liquid pump and preheater are implemented for use with the evaporator for improved thermal efficiency while operating at maximum cycle temperatures well below other available power conversion cycles.
Owner:THE AEROSPACE CORPORATION
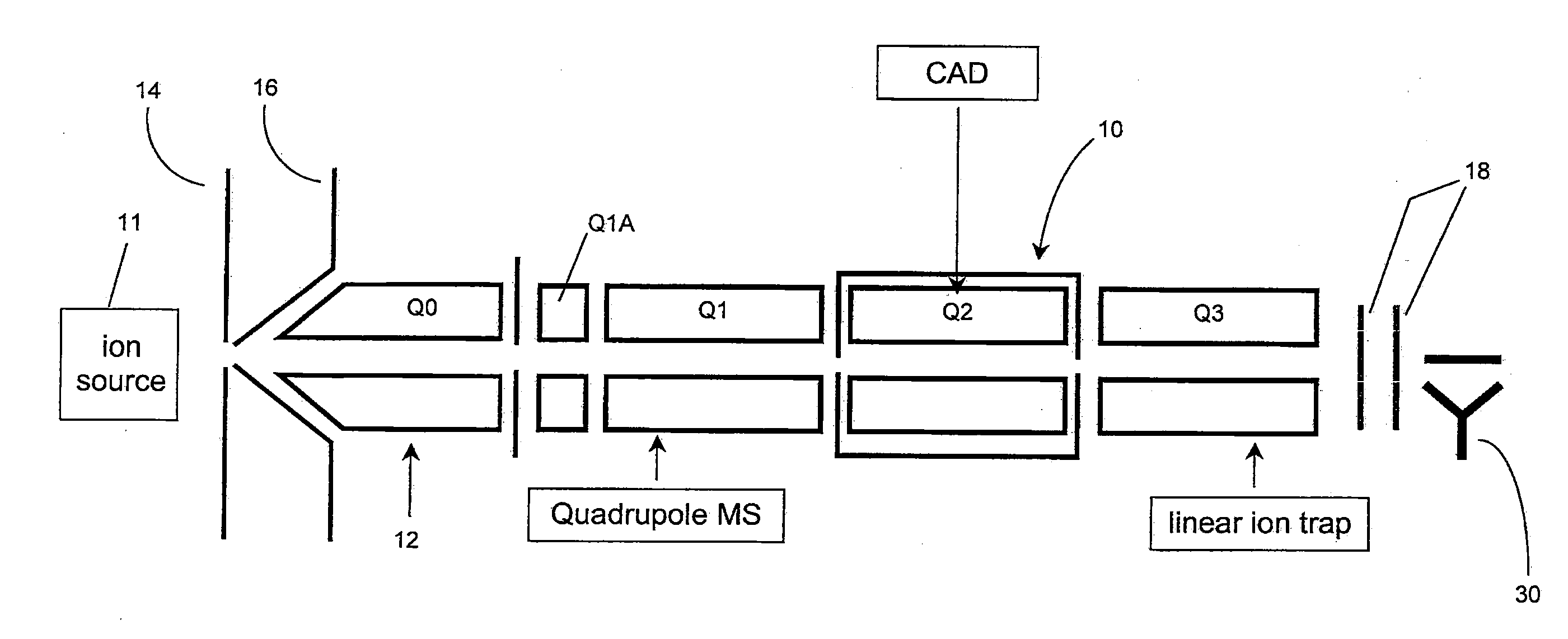
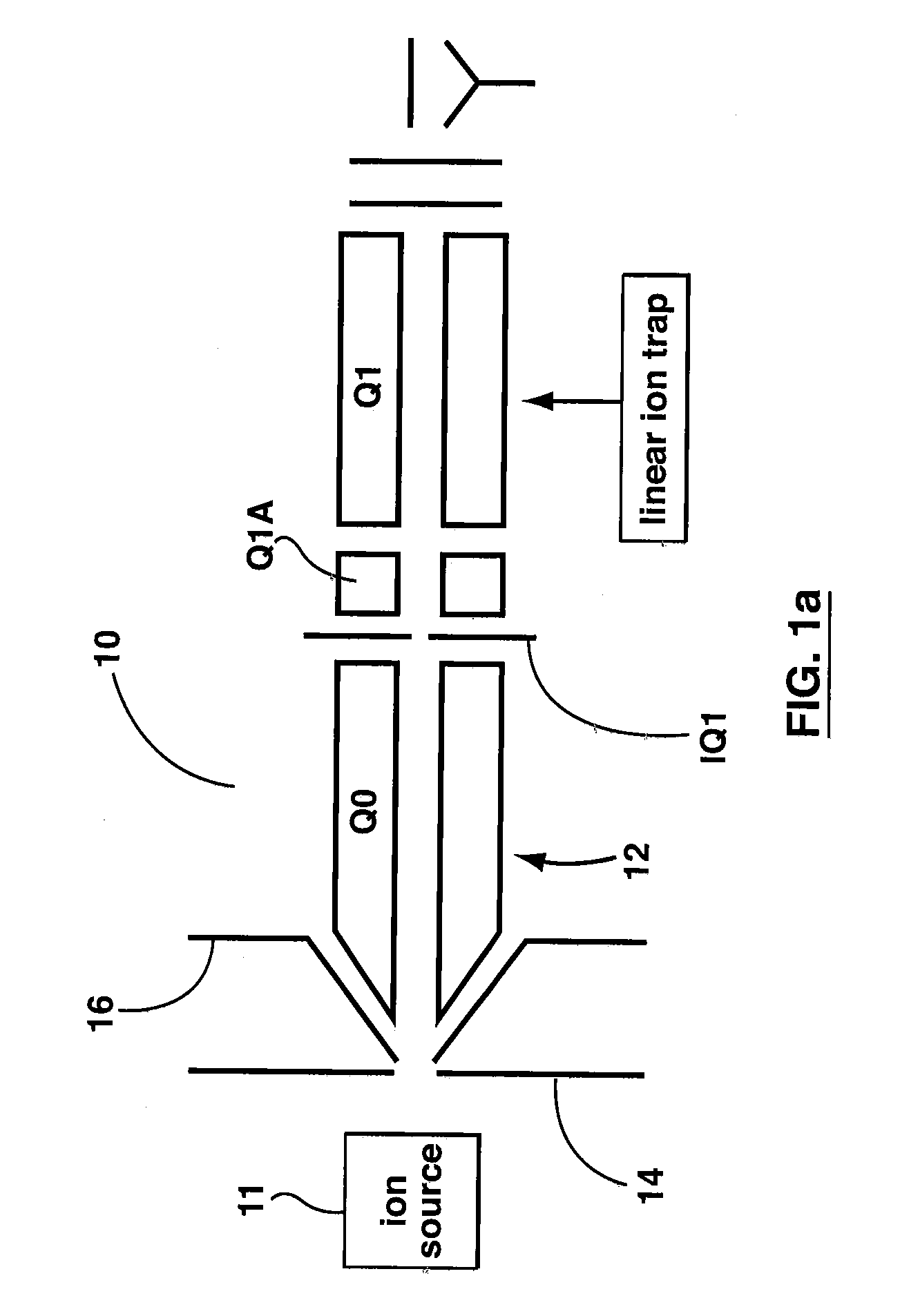
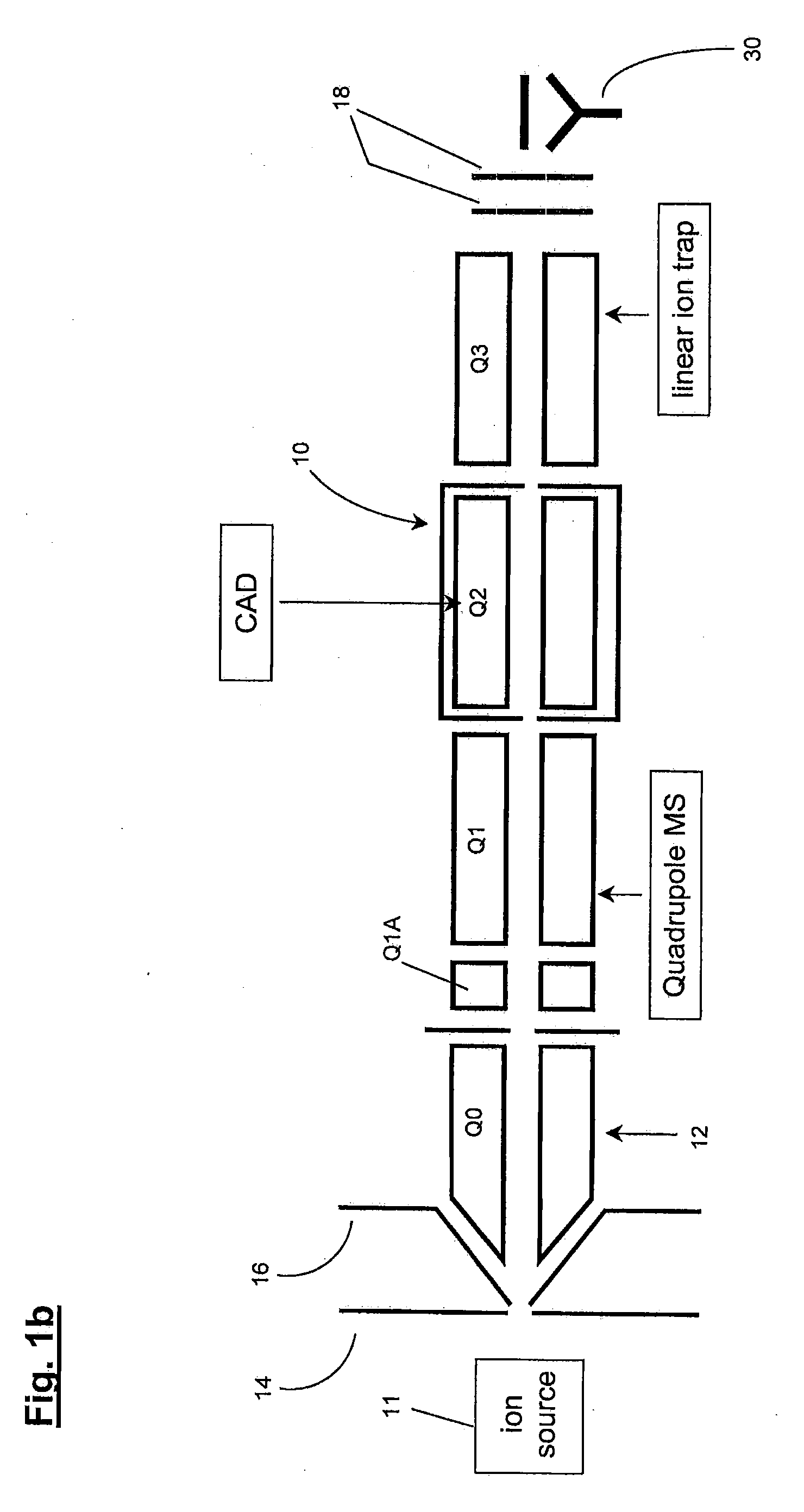
Method of operating a linear ion trap to provide low pressure short time high amplitude excitation with pulsed pressure
ActiveUS20090194684A1Reduce lossesHigh retention rateIsotope separationTube vacuum systemsStability parameterTrapping
Methods for fragmenting ions in an ion trap are described. These methods involve a) selecting parent ions for fragmentation; b) retaining the parent ions within the ion trap for a retention time interval, the ion trap having an operating pressure of less than about 1×10-4 Torr; c) providing a RF trapping voltage to the ion trap to provide a Mathieu stability parameter q at an excitement level during an excitement time interval within the retention time interval; d) providing a resonant excitation voltage to the ion trap during the excitement time interval to excite and fragment the parent ions; e) providing a non-steady-state pressure increase of at least 10% of the operating pressure within the ion trap by delivering a neutral gas into the ion trap for at least a portion of the retention time interval to raise the pressure in the ion trap to a varying first elevated-pressure in the range between about 6×10-5 Torr to about 5×10-4 Torr for a first elevated-pressure duration; and f within the retention time interval and after the excitement time interval, terminating the resonant excitation voltage and changing the RF trapping voltage applied to the ion trap to reduce the Mathieu stability parameter q to a hold level less than the excitement level to retain fragments of the parent ions within the ion trap. The excitation time interval and the first elevated-pressure duration substantially overlap in time.
Owner:MDS ANALYTICAL TECH A BUSINESS UNIT OF MDS +1
Advanced wafer refining
InactiveUS7008300B1Accessible costImprove refining methodSemiconductor/solid-state device testing/measurementSolid-state devicesManufacturing cost reductionElectrolysis
A refining apparatus having magnetically responsive refining elements that can be smaller than the workpiece being refined are disclosed. The refining apparatus can supply a parallel refining motion to the refining element(s) through magnetic coupling forces. The refining apparatus can supply multiple different parallel refining motions to multiple different refining elements solely through magnetic coupling forces to improve refining quality and versatility. New refining methods, refining apparatus, and refining elements disclosed. Methods of refining using frictional refining, chemical refining, tribochemical refining, and electrochemical refining and combinations thereof are disclosed. A refining chamber can be used. New methods of control are refining disclosed. The new magnetic refining methods, apparatus, and magnetically responsive refining elements can help improve yield and lower the cost of manufacture for refining of workpieces having extremely close tolerances such as semiconductors wafers. Refining fluids are preferred. Reactive refining aids are preferred. Electro-refining for adding and removing material is disclosed. A method to use business calculations combined with physical measurements to improve control is discussed. Use of business calculations to change the cost of finishing semiconductor wafers is discussed. The method can help cost of manufacture forecasting for pre-ramp-up, ramp-up, and commercial manufacture. Actively based accounting can be preferred for some applications. New methods and new apparatus for non-steady state refining control are disclosed.
Owner:SEMCON TECH
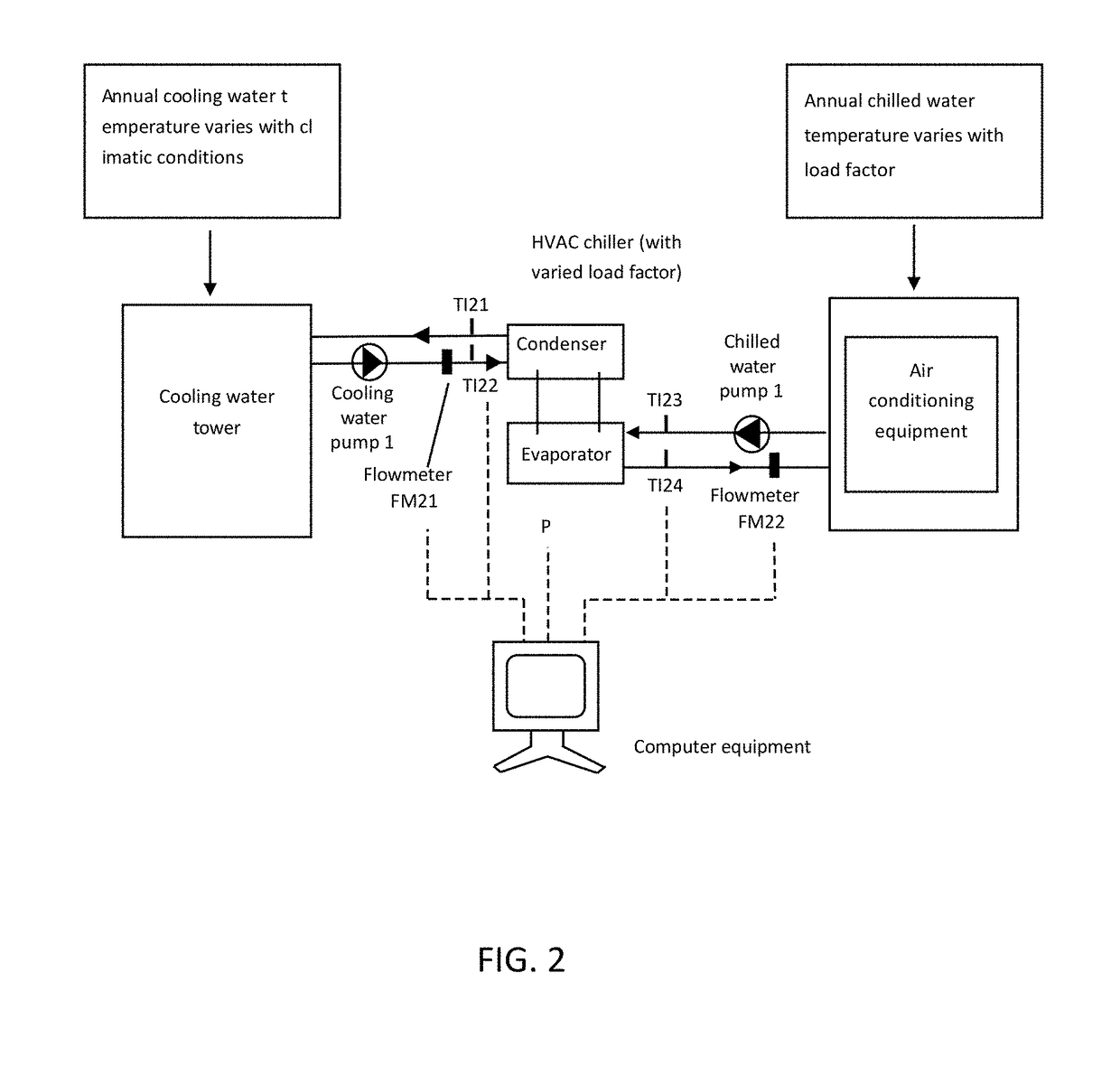
Method of verifying and analyzing energy efficiency ratio EER of a heating ventilation and air conditioning HVAC chiller unit
ActiveUS20170277816A1Avoid wasting electricityLower standardsMechanical apparatusData processing applicationsBusiness efficiencyManagement indicators
A method of verifying and analyzing energy efficiency ratio (EER) of an HVAC chiller unit in accordance with the present invention provides verification and analysis of HVAC chiller units to build daily steady-state data and non-steady state data out of field dynamic EER values and provides analysis of the steady-state data, based on selected integer temperatures and tenfold load factors in the annual scale that are subject to the dynamic changes in temperatures and load factors along with chiller seasonal operation to build monthly or seasonal running EER trend, and to determine-management index values for a period of time and to determine whether energy consumption meets specified criteria, as a basis of comparison of calculations, and resulting amplitude ratios between before and after the energy-saving improvement and of totally saved energy.
Owner:CHEN CHU FU
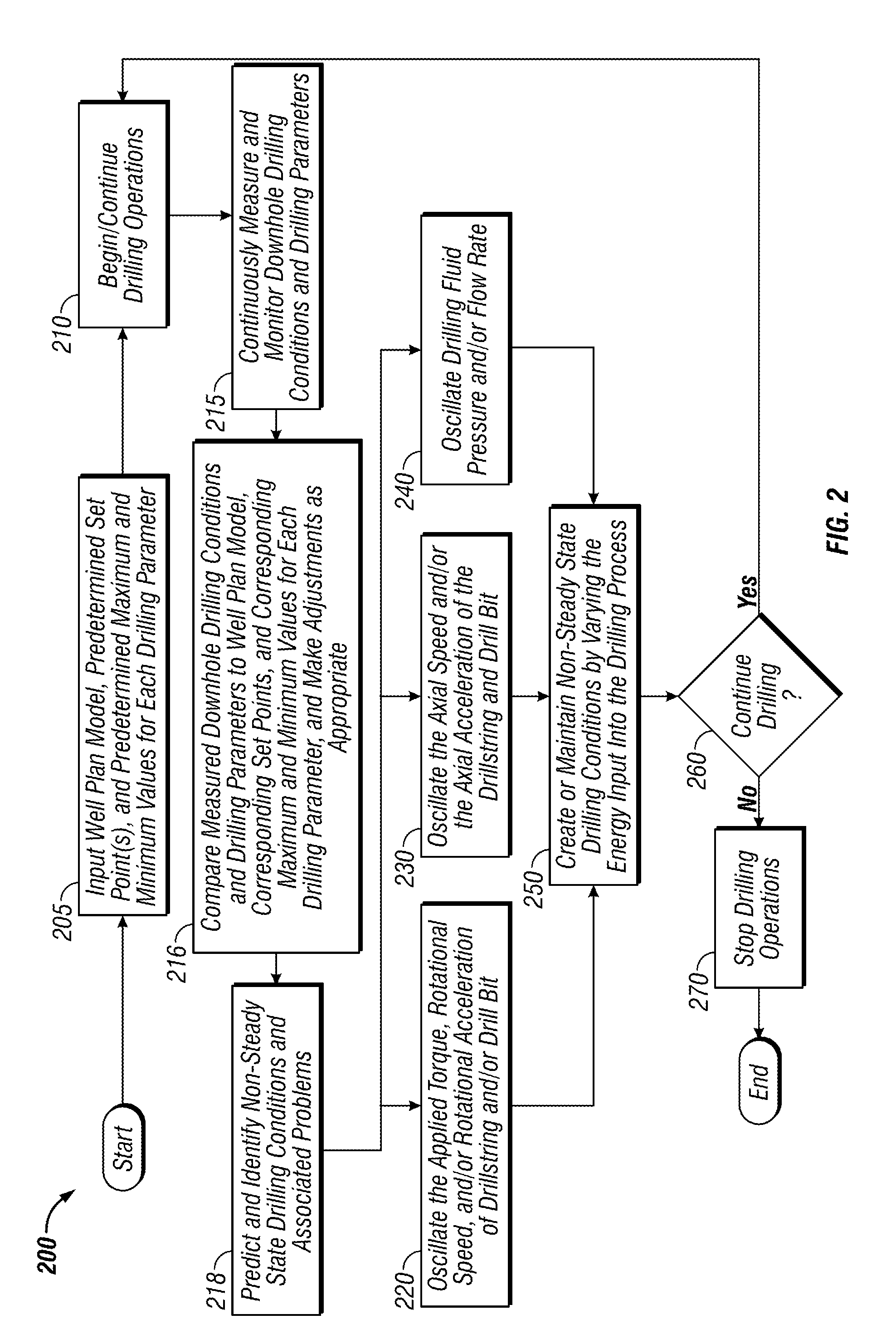
Systems and methods for improving drilling efficiency
ActiveUS20120217067A1Readily apparentDrilling machines and methodsAutomatic control for drillingWell drillingDrilling system
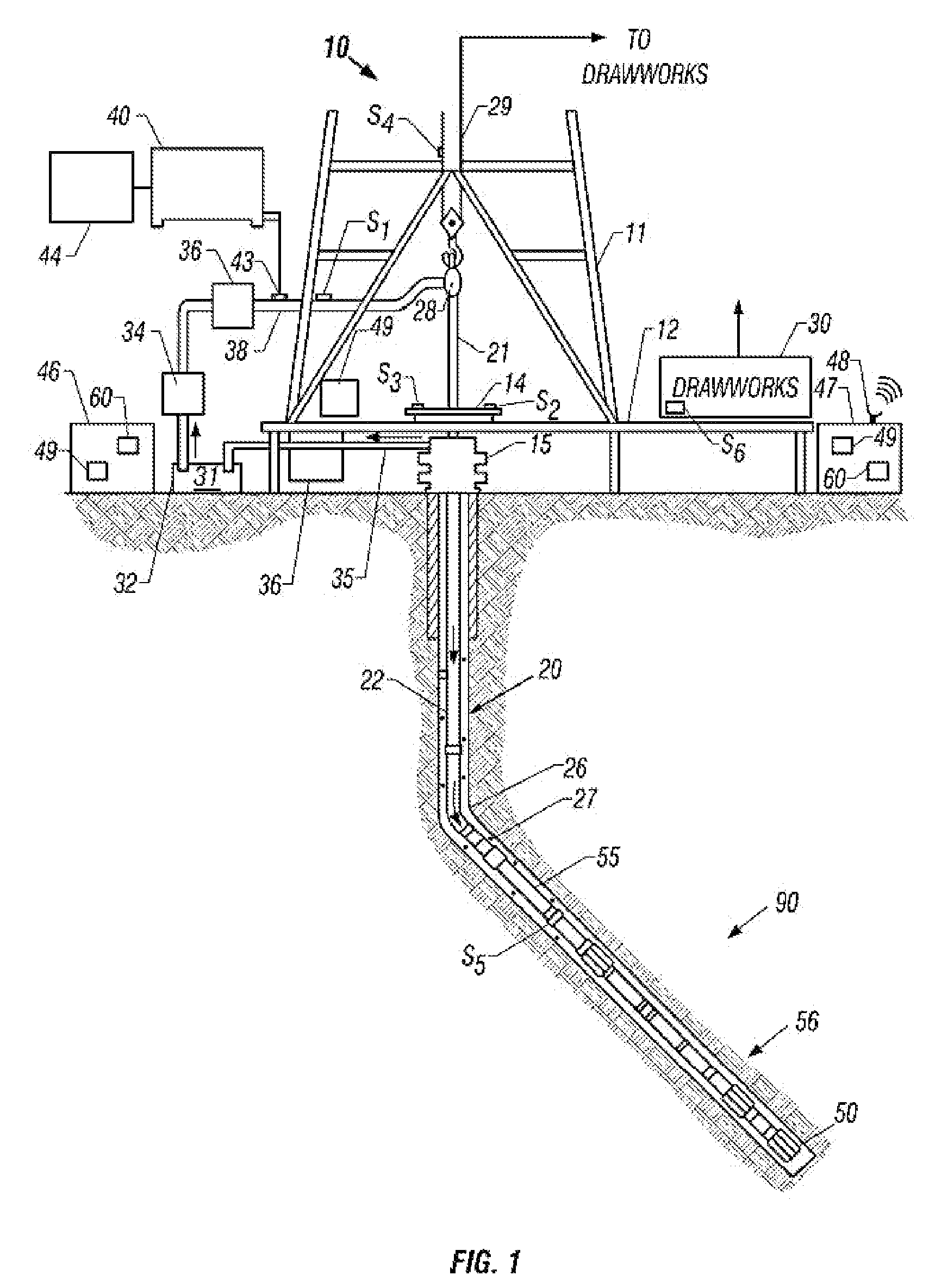
A method for drilling a borehole in an earthen formation comprises (a) providing a drilling system including a drillstring having a longitudinal axis, a bottom-hole assembly coupled to a lower end of the drillstring, and a drill bit coupled to a lower end of the bottom-hole assembly. In addition, the method comprises (b) rotating the drill bit at a rotational speed. Further, the method comprises (c) applying weight-on-bit to the drill bit and advancing the drill bit through the formation to form the borehole. Still further, the method comprises (d) pumping a drilling fluid down the drillstring to the drill bit. The drilling fluid has a flow rate down the drillstring. Moreover, the method comprises (e) oscillating the rotational speed of the drill bit during (c). The method also comprises (f) generating non-steady state conditions in the borehole during (e).
Owner:NAT OILWELL VARCO LP
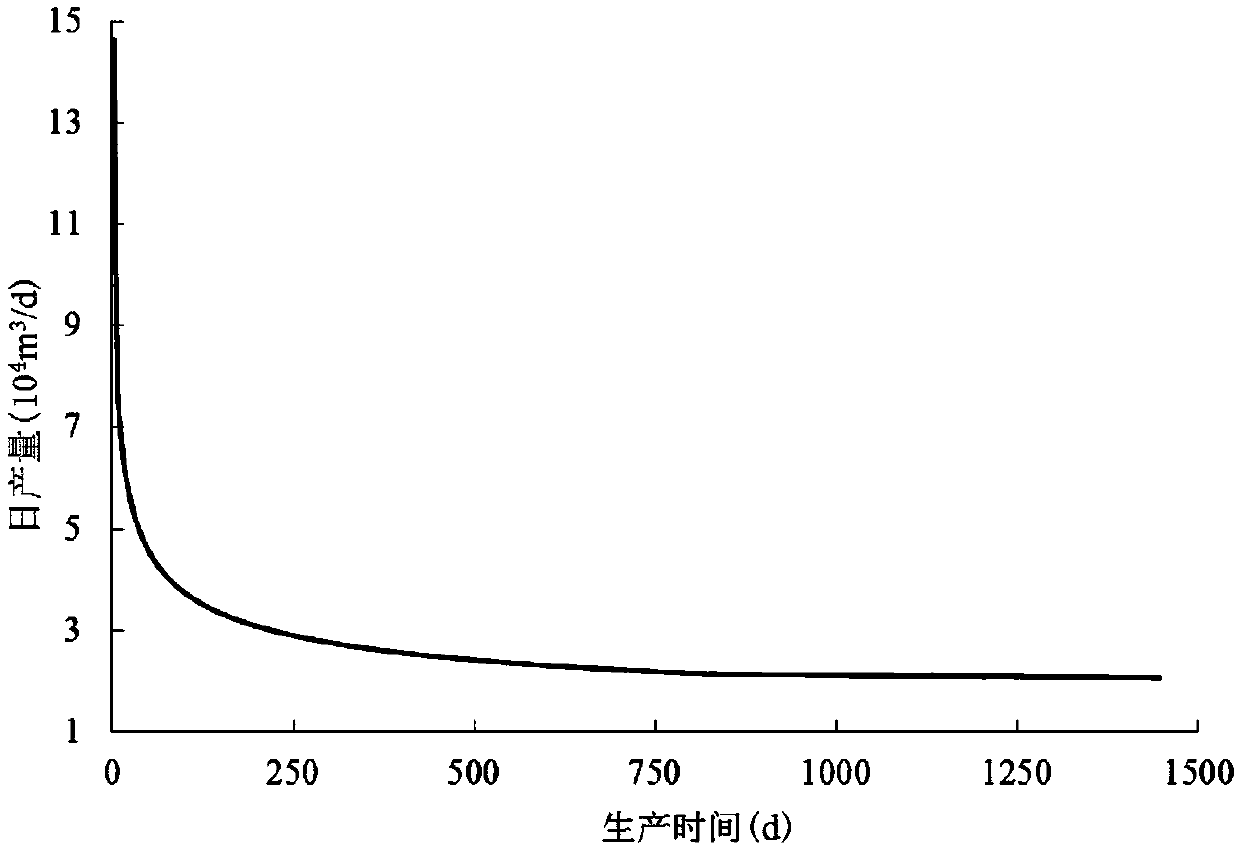
Method for calculating non-steady state yield of volume fractured horizontal well of shale gas reservoir
The invention discloses a method for calculating a non-steady state yield of a volume fractured horizontal well of a shale gas reservoir. The method comprises the following steps of dividing the reservoir into a volume fractured region and a non-transformed matrix region, and collecting basic parameters of the volume fractured region, the non-transformed matrix region and the horizontal well, wherein the volume fractured region is Darcy flow, and the non-transformed matrix region is Knudson flow; according to a mass conservation law, establishing a steady-state yield formula of the volume fractured horizontal well of the shale gas reservoir; based on a pressure propagation formula, the steady-state yield formula and a matter balance equation, calculating the non-steady state yield of the volume fractured horizontal well of the shale gas reservoir by applying a continuous quasi-steady method; and repeating the previous step, and performing calculation to obtain the non-steady state yield of the volume fractured horizontal well of the shale gas reservoir. The method fully considers a process that a pressure is propagated to a boundary gradually in a production process of the volume fractured horizontal well of the shale gas reservoir, different seepage laws of shale gas in a volume transformation region and the non-transformed matrix region, and adsorption and desorption effects of the shale gas, so that actual conditions are better met.
Owner:SOUTHWEST PETROLEUM UNIV
Experimental method and experimental device for determinating volcanic gas-water relative permeability
InactiveCN104316449AAccurate measurementEasy to usePermeability/surface area analysisVolcanic GasesExperimental methods
The invention relates to an experimental method and an experimental device for determinating volcanic gas-water relative permeability. Through experimental steps of preparing a volcanic core, measuring permeability and porosity of a rock sample gas, exerting a confining pressure and performing rock sample gas water displacement, a relative permeability curve when gas saturation increases and water saturation decreases in the volcanic core is determinated by a non-steady-state method.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
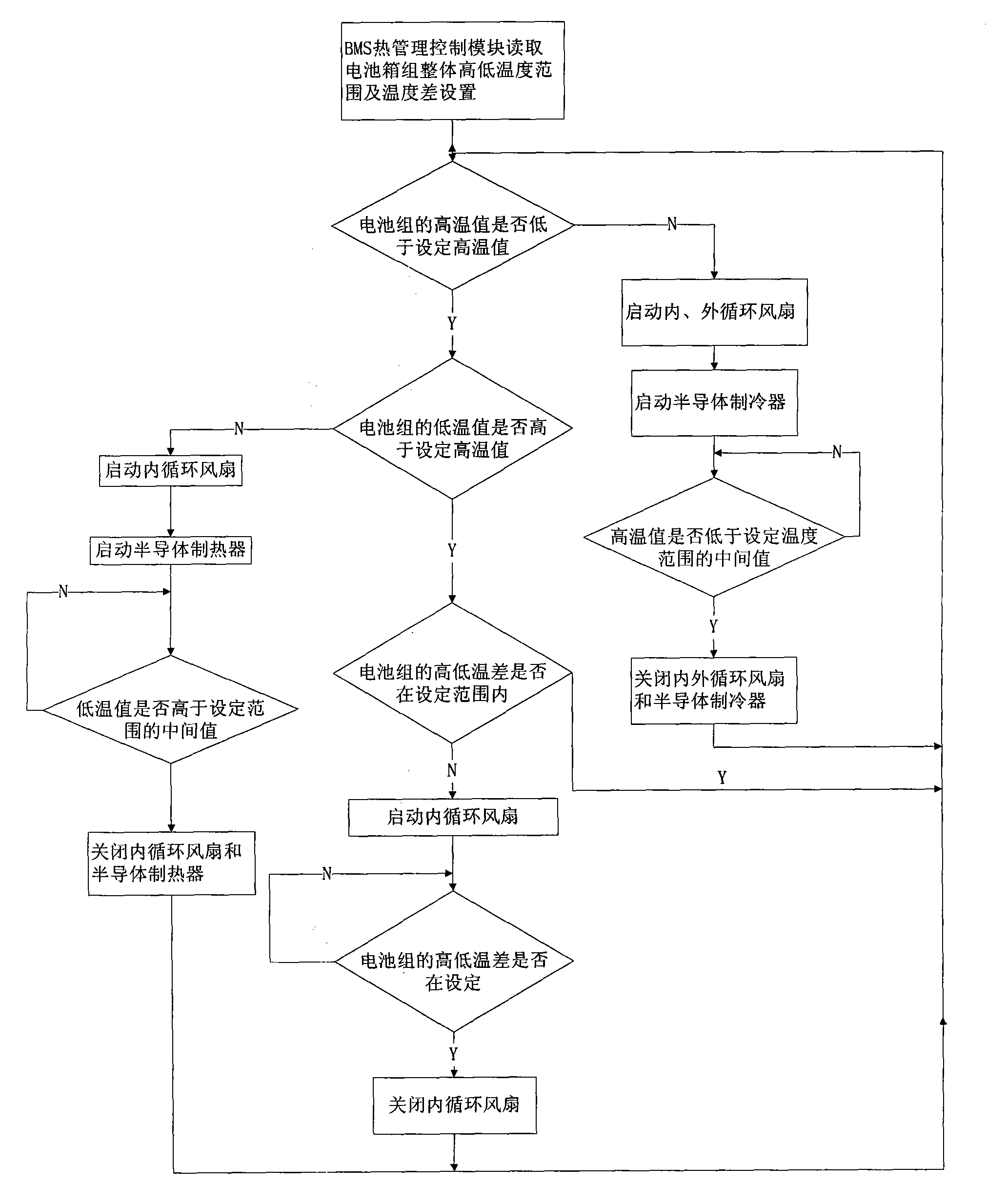
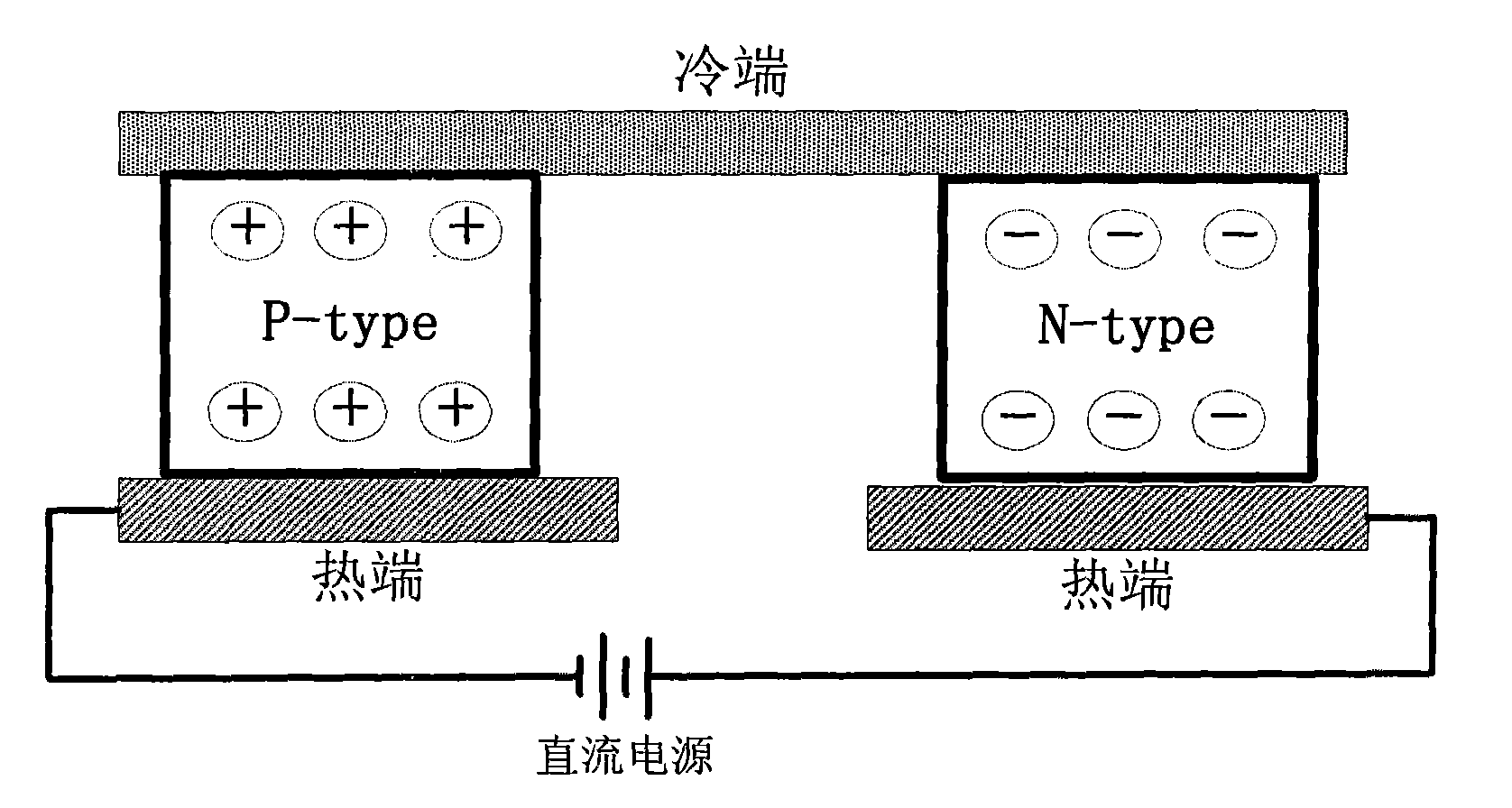
Thermal management method and device for power battery pack with function of automatically controlling non-steady-state temperature field
InactiveCN102403543AAchieve sealingContinuously workingSecondary cells servicing/maintenancePower batteryAutomatic control
The invention relates to a thermal management device for a power battery pack with a function of automatically controlling a non-steady-state temperature field, which comprises a battery pack, a temperature collection unit, a semiconductor cooler / heater, an external circulating fan, an internal circulating fan and a controller. A power battery monomer is arranged in the battery pack; the temperature collection unit is arranged on the pole of the power battery monomer; the semiconductor cooler / heater comprises a cold end plate and a hot end plate, automatic switching can be achieved between the two end plates, the two end plates are arranged on the shell of the battery pack, the cold end plate is arranged inside, the hot end plate is arranged outside, the cold end plate is a cooling plate and the hot end plate is a heat dissipating plate in forward powering-up, and the cold end plate is a heating plate and the hot end plate is a heat absorbing plate in reverse powering-up; the external circulating fan is arranged on the hot end plate of the semiconductor cooler / heater and is used for heat exchange between the hot end plate and external air; the internal circulating fan is arranged on the cold end plate of the semiconductor cooler / heater and is used for air circulation in the battery pack and heat exchange between the cold end plate and air in the batter pack; and the controller is used for controlling the thermal management device. Meanwhile, the invention discloses a method for implementing thermal management of the thermal management device.
Owner:SHANGHAI ZHONGKE SHENJIANG ELECTRIC VEHICLE
Advanced wafer planarizing
InactiveUS7037172B1Cost changeImprove controlProgramme controlNuclear monitoringElectricityManufacturing cost reduction
An apparatus for planarizing is disclosed. A method of planarizing is disclosed. Methods of planarizing using frictional planarizing, chemical planarizing, tribochemical planarizing, CVD planarizing, and electrochemical planarizing and combinations thereof are disclosed. A planarizing chamber can be used. New methods of control are planarizing disclosed. The new planarizing methods and apparatus, can help improve yield and lower the cost of manufacture for planarizing of workpieces having extremely close tolerances such as semiconductor wafers. Cost of manufacture parameters are used for control. Methods to determine preferred changes to process control parameters are disclosed. Cost of manufacture models can be used and are disclosed. Process models can be used and are disclosed. A method to use business calculations combined with physical measurements to improve control is discussed. Use of business calculations to change the cost of finishing semiconductor wafers is discussed. The method can help cost of manufacture forecasting for pre-ramp-up, ramp-up, and commercial manufacture. Activity based accounting can be preferred for some applications. Planarizing fluids are preferred. Reactive planarizing aids are preferred. Electro-planarizing for adding and removing material is disclosed. New methods and new apparatus for non-steady state planarizing control are disclosed.
Owner:SEMCON TECH
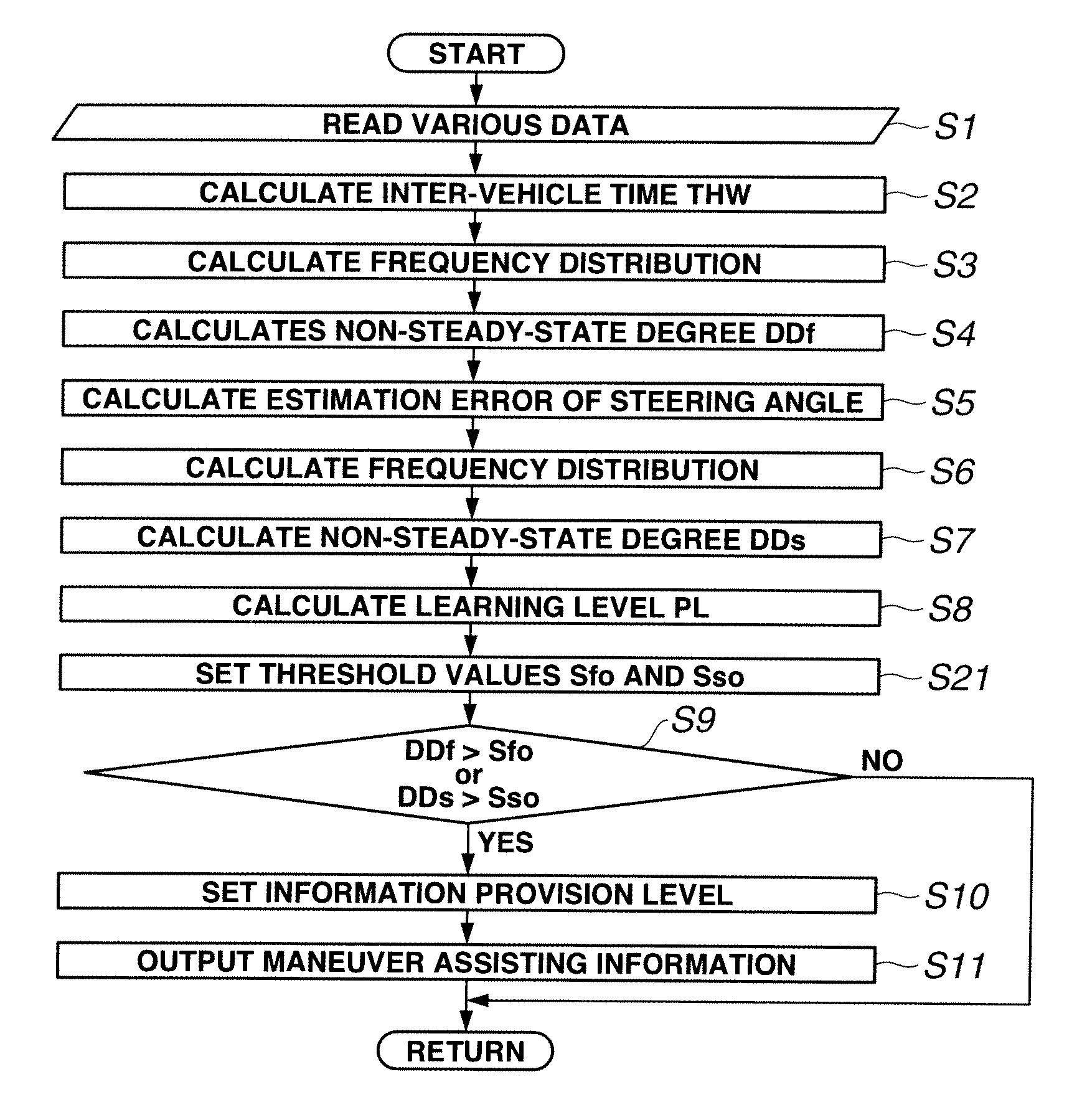
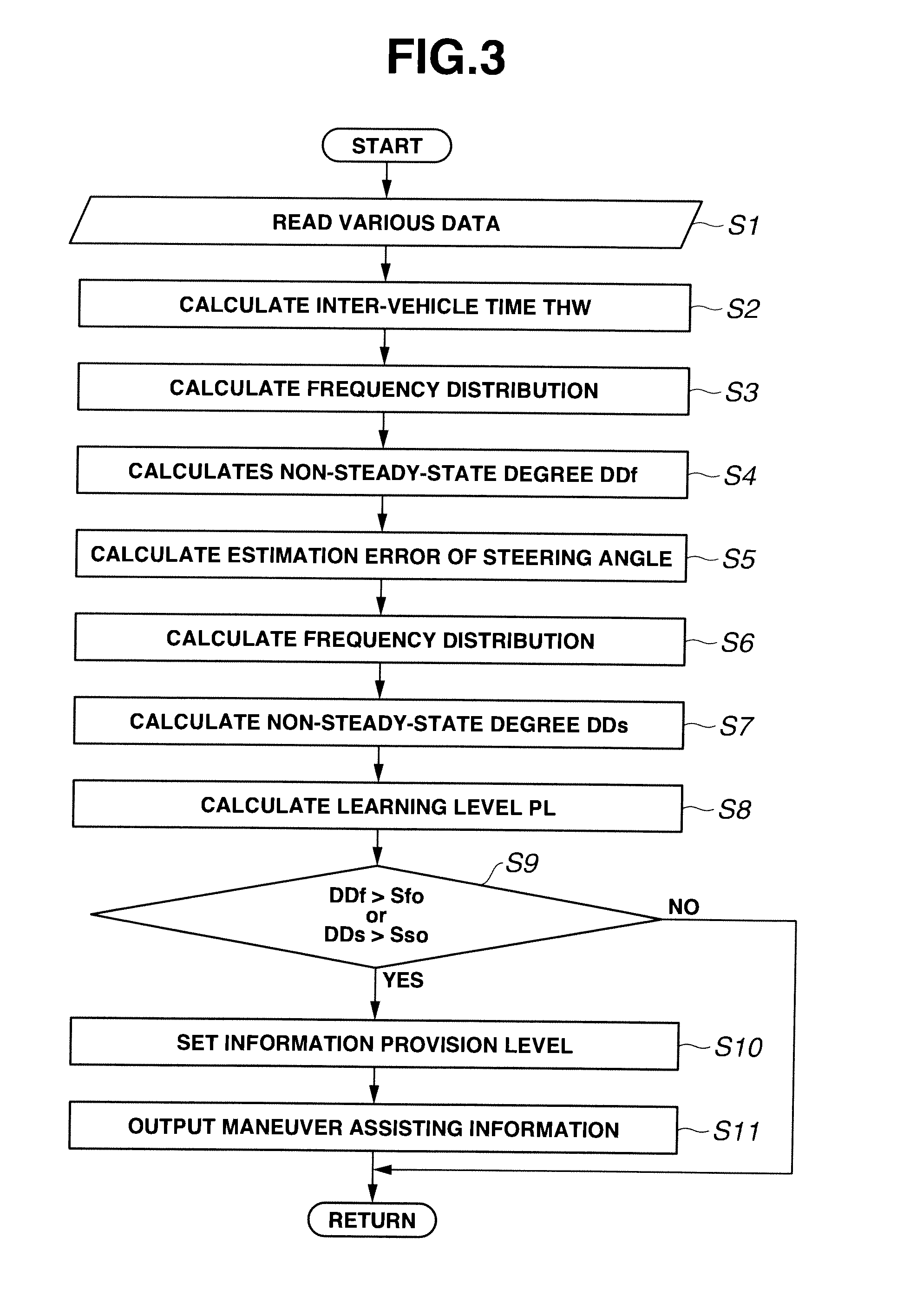
Driving maneuver assisting apparatus and method for assisting driving maneuver
InactiveUS20110153532A1Digital computer detailsExternal condition input parametersDriver/operatorEngineering
Owner:NISSAN MOTOR CO LTD
Non-steady state detection device and non-steady state detection method for permeability of low-permeability rock
ActiveCN103616322AAccurate measurementTest accuratePermeability/surface area analysisPorosityRock core
The invention discloses a non-steady state detection device and a non-steady state detection method for permeability of low-permeability rock. The detection device comprises a rock core holder for holding a rock core sample, wherein the side wall of the holder is connected to an annular pressure pump; the holder is provided with an inlet pipeline and an outlet pipeline; an inlet buffering container is arranged in the inlet pipeline; an outlet buffering container is arranged in the outlet pipeline; a check loop is connected to the holder in parallel; a check cylinder is arranged in the check loop. Due to the adoption of the non-steady state detection device for the permeability of the low-permeability rock, the permeability of the low-permeability rock core can be detected quickly and accurately; the permeability of the low-permeability rock core sample can be detected through two non-steady state detection methods; the device is simple to operate, accurate to test and capable of adjusting the volume of the inlet and outlet buffering containers according to a porosity of the rock core so as to ensure the detection precision of the permeability.
Owner:PETROCHINA CO LTD
Versatile wafer refining
InactiveUS7377836B1Easy to organizeReduce manufacturing costElectrolysis componentsSemiconductor/solid-state device testing/measurementManufacturing cost reductionElectrolysis
Methods of refining using a plurality of refining elements are discussed. A refining apparatus having refining elements that can be smaller than the workpiece being refined are disclosed. New refining methods, refining apparatus, and refining elements disclosed. Methods of refining using frictional refining, chemical refining, tribochemical refining, and electrochemical refining and combinations thereof are disclosed. A refining apparatus having magnetically responsive refining elements that can be smaller than the workpiece being refined are disclosed. The refining apparatus can supply a parallel refining motion to the refining element(s) for example through magnetic coupling forces. The refining apparatus can supply multiple different parallel refining motions to multiple different refining elements for example solely through magnetic coupling forces to improve refining quality and versatility. A refining chamber can be used. New methods of control are refining disclosed. The new refining methods, including magnetic refining methods, apparatus, and refining elements, including magnetically responsive refining elements, can help improve yield and lower the cost of manufacture for refining of workpieces having extremely close tolerances such as semiconductor wafers. New methods of control are also discussed. Methods and apparatus which use processor readable memory devices are discussed. Refining fluids are preferred. Reactive refining aids are preferred. Electro-refining for adding and removing material is disclosed. New methods and new apparatus for non-steady state refining control are disclosed.
Owner:SEMCON TECH
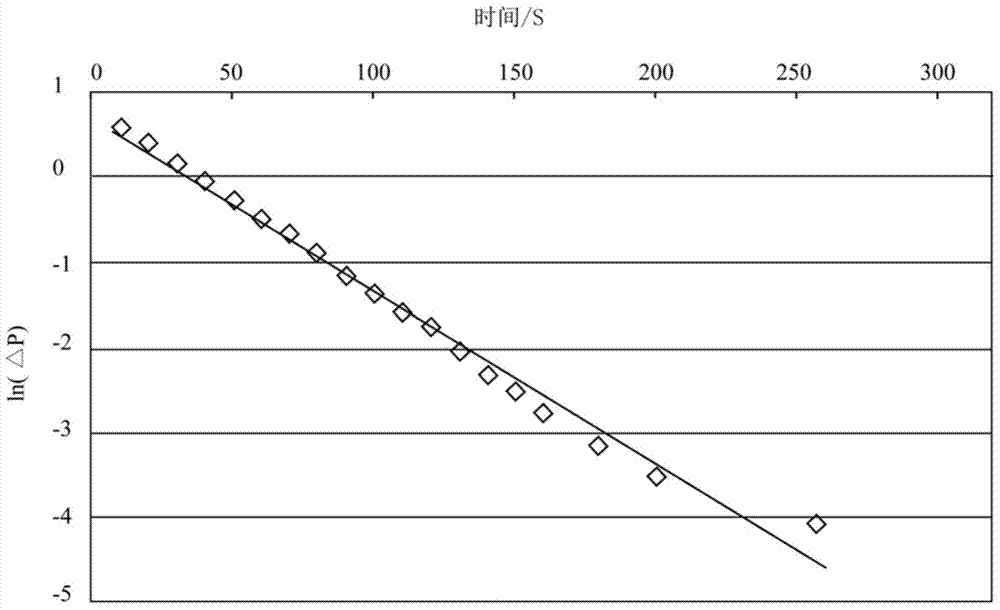
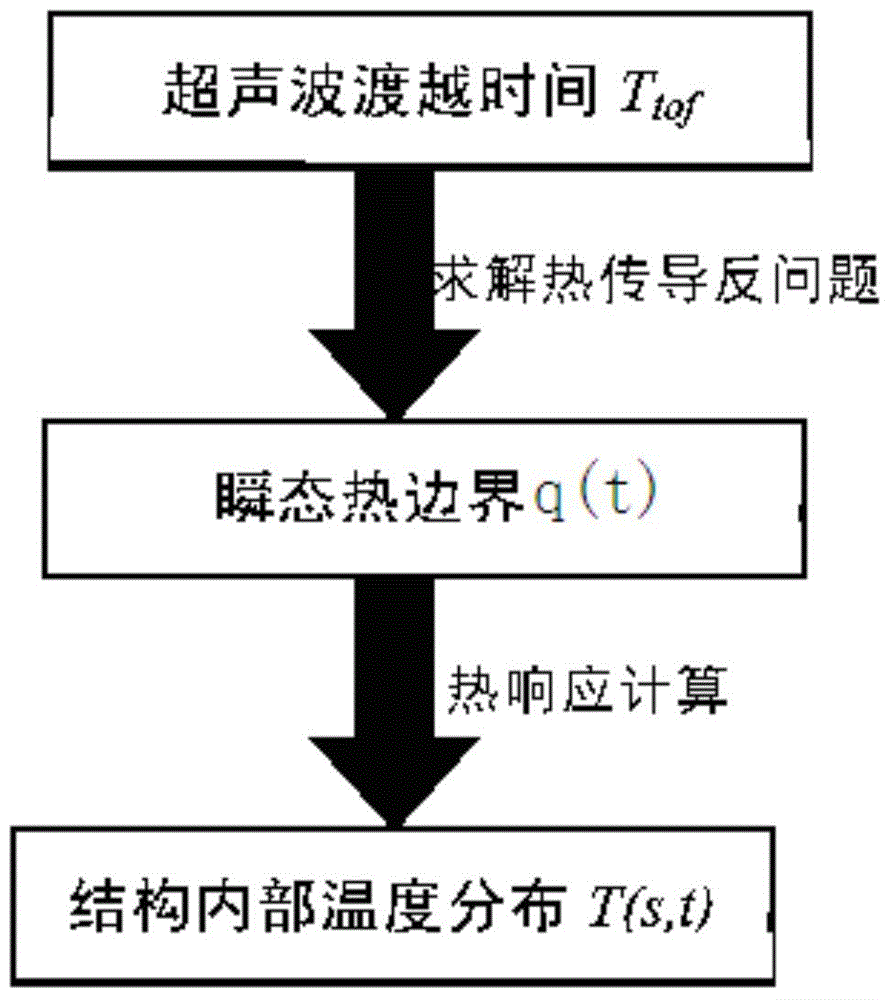
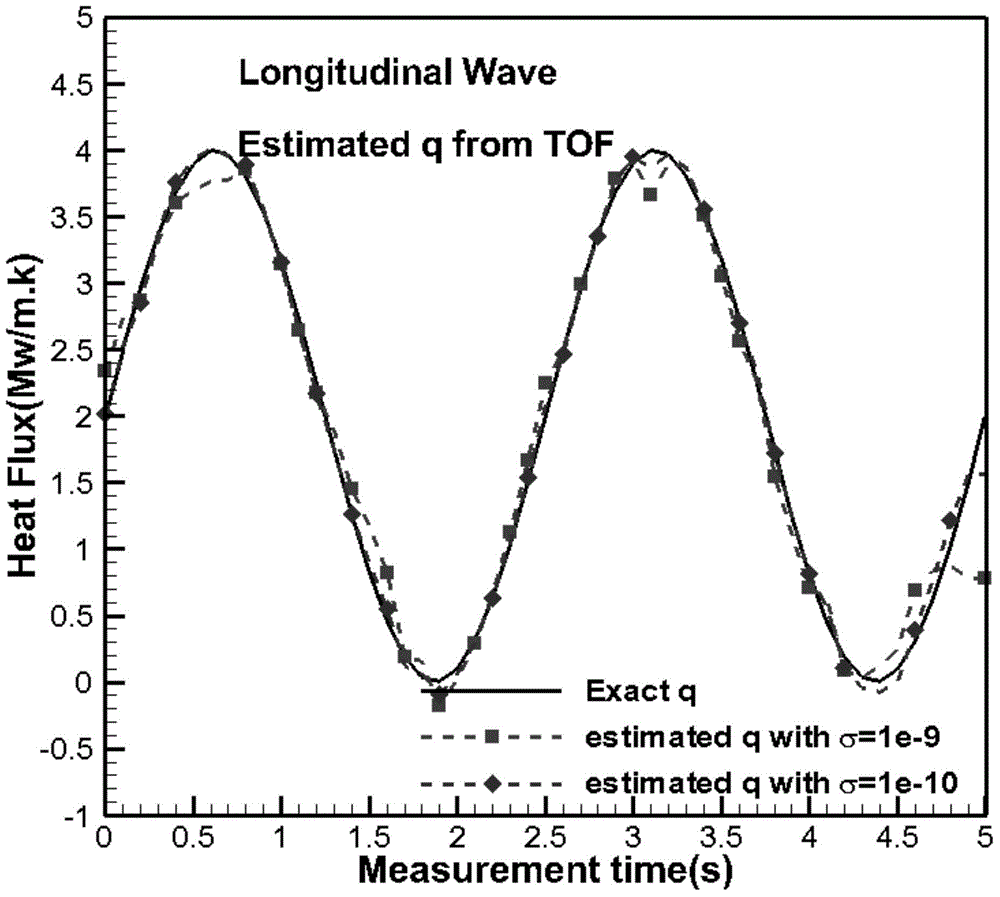
Method for reconstructing nonuniform temperature field inside structure and based on transient-state thermal boundary inversion
ActiveCN104792435AHigh precisionQuick responseThermometers using physical/chemical changesTransient stateSolid structure
The invention discloses a method for reconstructing a nonuniform temperature field inside structure and based on transient-state thermal boundary inversion. The method includes that on the basis of transition time of ultrasonic pulse echo, transient-state thermal boundary conditions causing temperature change of the structure is inverted; on this basis, non-steady-state temperature distribution inside the structure is reconstructed by solving a thermal conduction equation. Compared with existing ultrasonic temperature measuring methods, the method has the advantages that ultrasonically-detected inside temperature is not acquired directly through transition time and is acquired through inverted transient-state thermal boundary conditions, so that temperature in the method is not a single average value on a transmission path any more but specific temperature distribution, and the method is higher in temperature resolution and higher in stability and can realize realtime and high-accuracy reconstruction of temperature distribution inside solid structure at different moments.
Owner:CALCULATION AERODYNAMICS INST CHINA AERODYNAMICS RES & DEV CENT
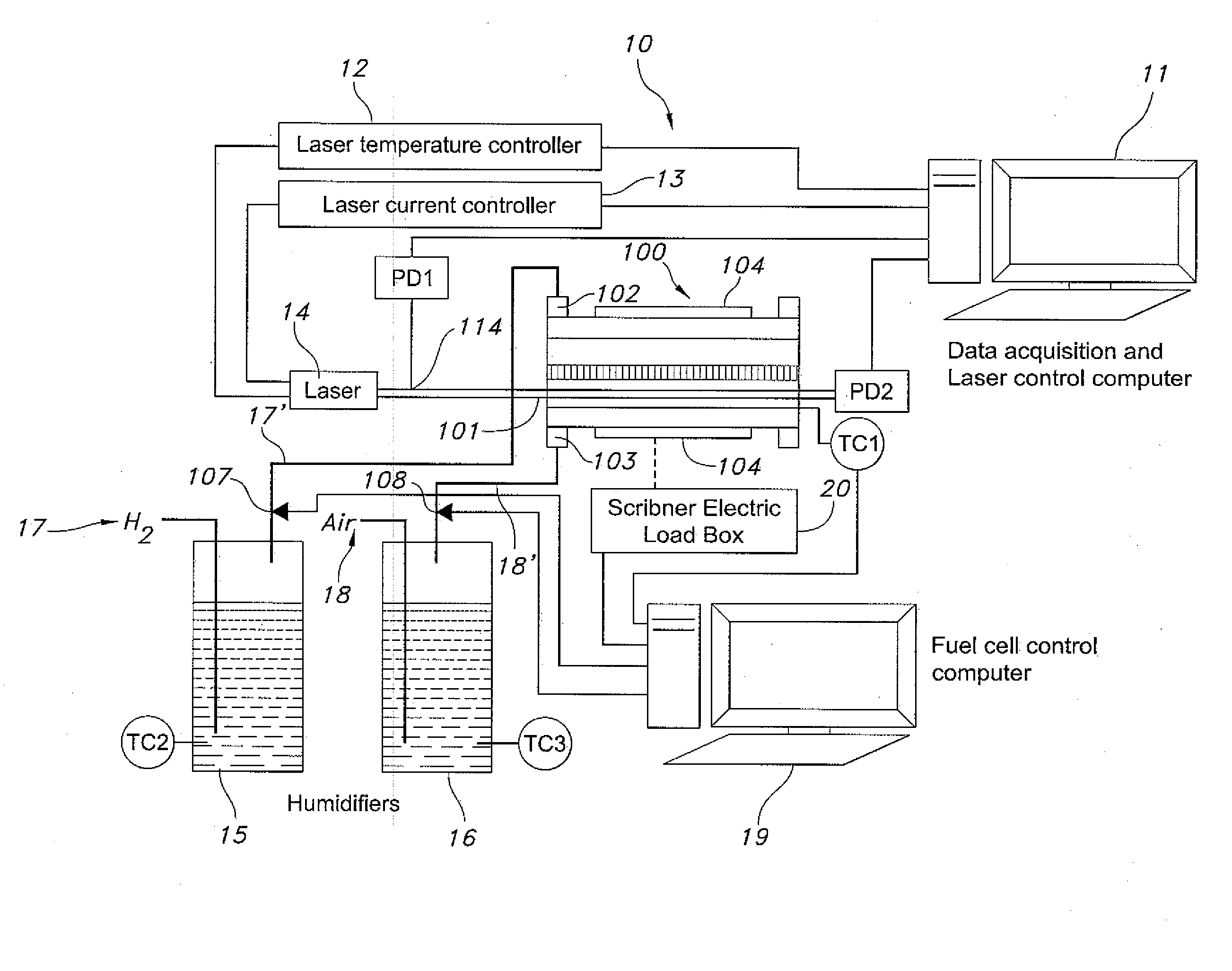
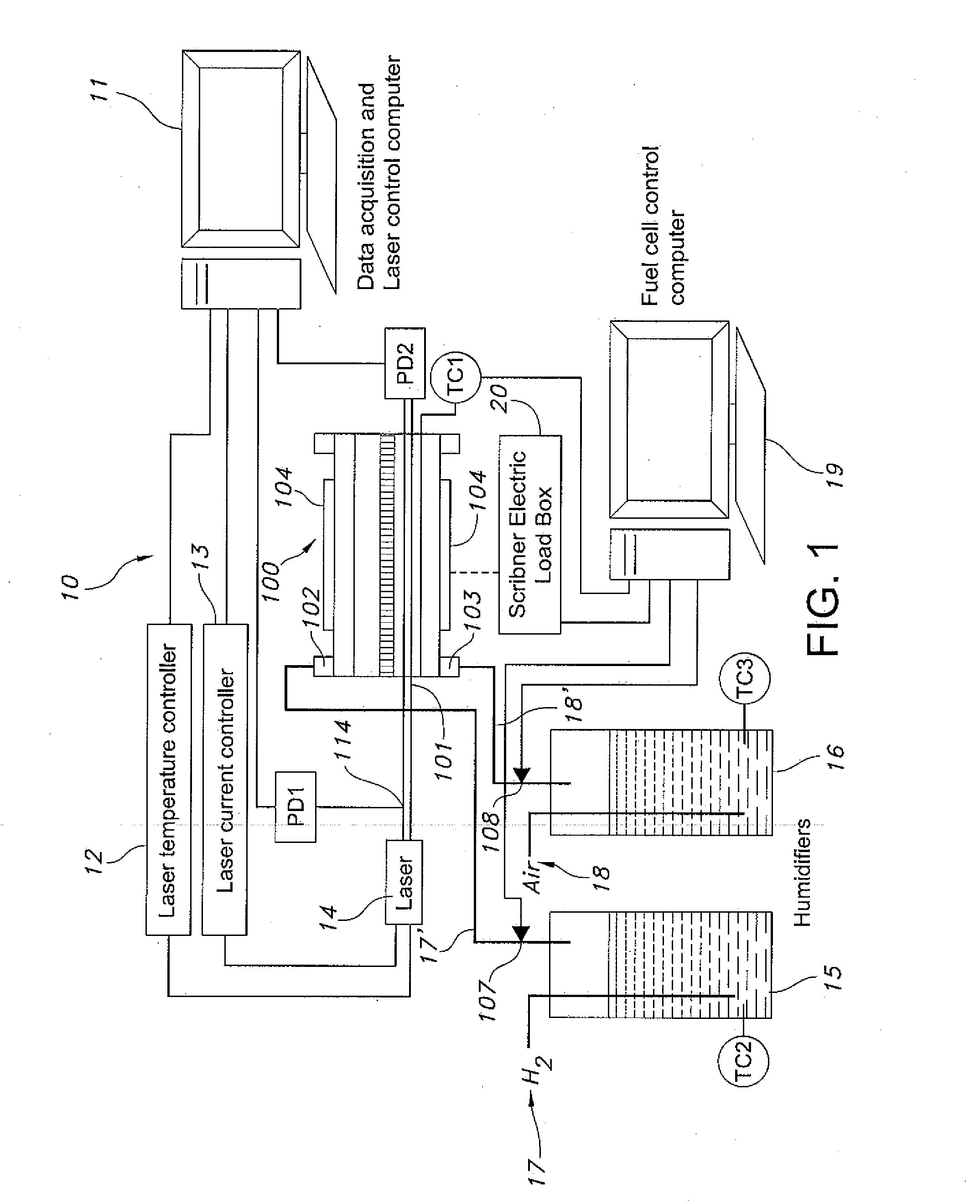
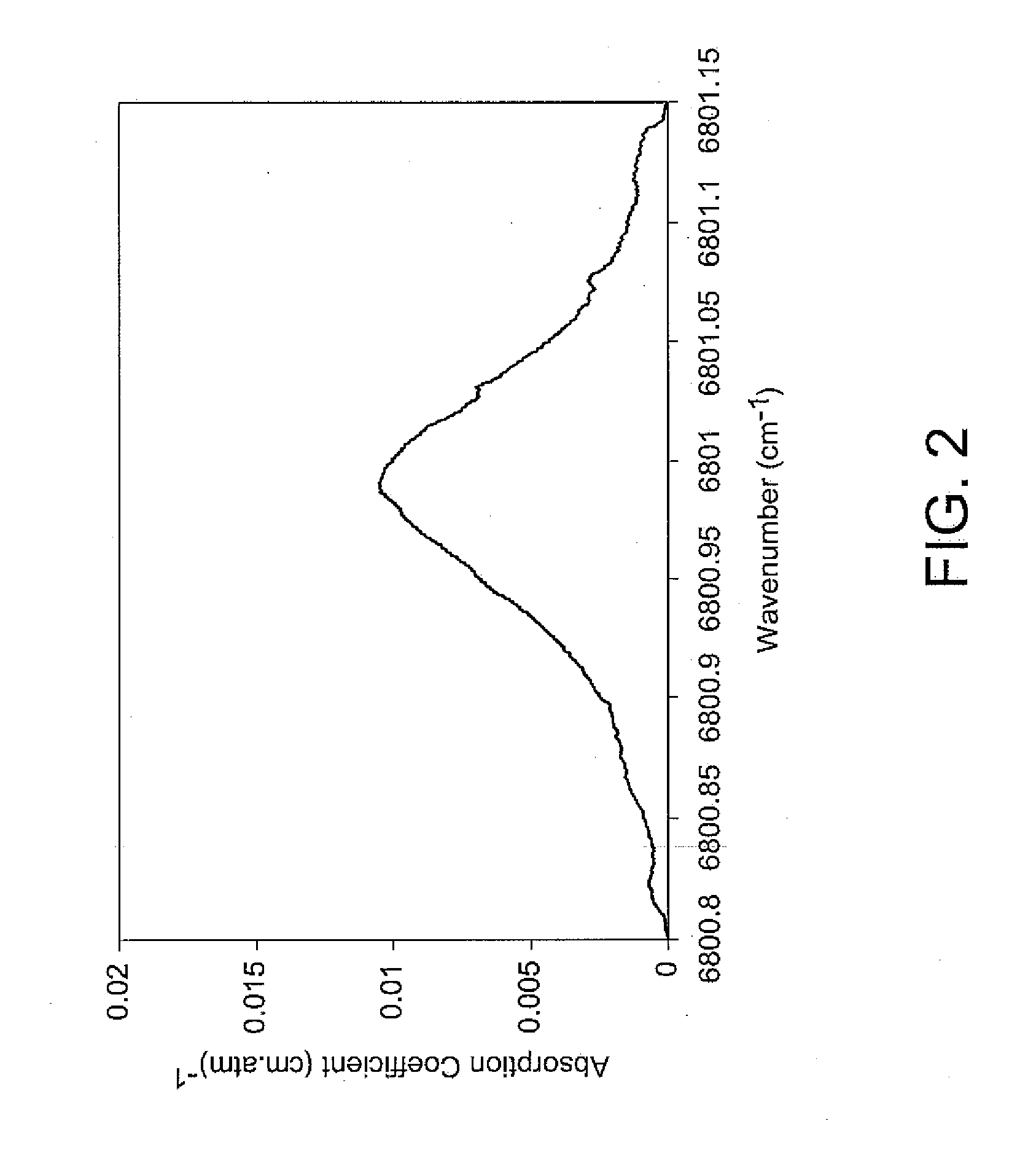
Fiber optic based in-situ diagnostics for PEM fuel cells
InactiveUS20080118783A1Fuel cell heat exchangeFinal product manufactureElectrochemical responseFiber
The present disclosure relates to in-situ, line-of-sight measurements of partial pressure and temperature associated with at least one flow channel of a fuel cell. Tunable diode laser absorption spectroscopy (TDLAS) is employed for measurements for which water transition states sensitive to temperature and partial pressure are utilized. Measurements are achievable for a fuel cell operating under both steady-state and time-varying load conditions. For steady-state operation, the water partial pressure increases with increasing current density on a cathode side of the fuel cell due to production of water by electrochemical reaction. Temperature in a gas phase remains relatively constant since the fuel cell housing temperature is controlled externally. For non-steady-state operation of the fuel cell through a time-varying current profile, the water partial pressure responds to the load changes rapidly and follows a current profile. The gas temperature varies in response to the dynamic loading and departures from steady-state conditions become more apparent at higher fuel cell operating temperatures.
Owner:UNIV OF CONNECTICUT
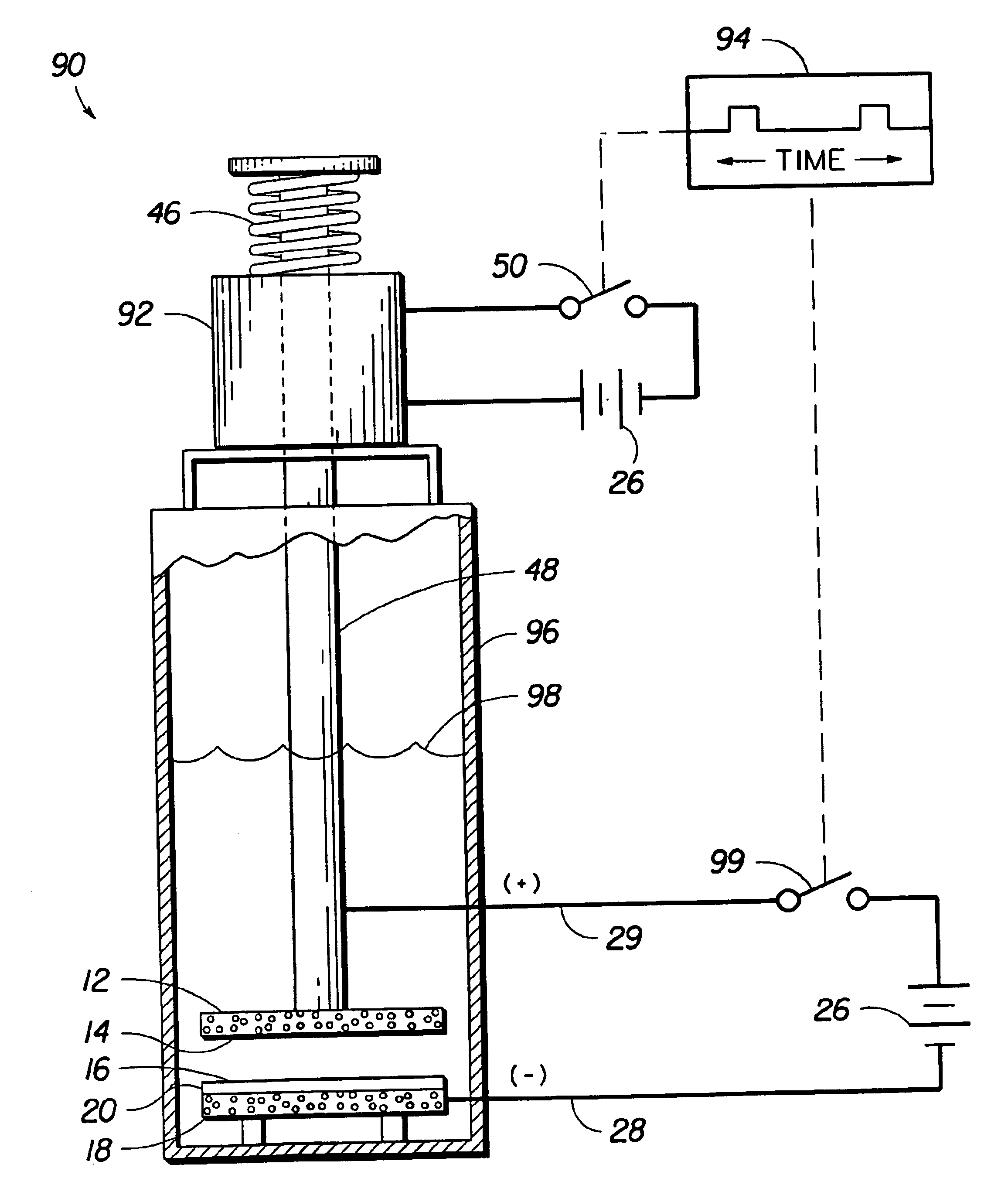
Electrochemical apparatus with retractable electrode
InactiveUS6860976B2Water/sewage treatment using germicide/oligodynamic-processWater/sewage treatment apparatusContact pressureElectrochemistry
Electrochemical apparatus and methods that support periodic, non-steady state, or discontinuous operation without suffering degradation of materials or loss of efficiency. The invention provides a means for positioning one or more electrodes into contact with electrolyte and means for retracting the one or more electrodes out of contact with the electrolyte. The means for positioning and means for retracting may be the same device or different devices. The means for positioning and means for retracting may be designed to provide automatic, passive, or fail-safe retraction of the electrode upon a given shutdown condition, such as a voltage of less than one Volt being applied between the first and second electrodes, expiration of a time period, an ozone concentration greater than a setpoint ozone concentration, contact pressure of less than 5 psig, and combinations thereof.
Owner:LYNNTECH INT +1
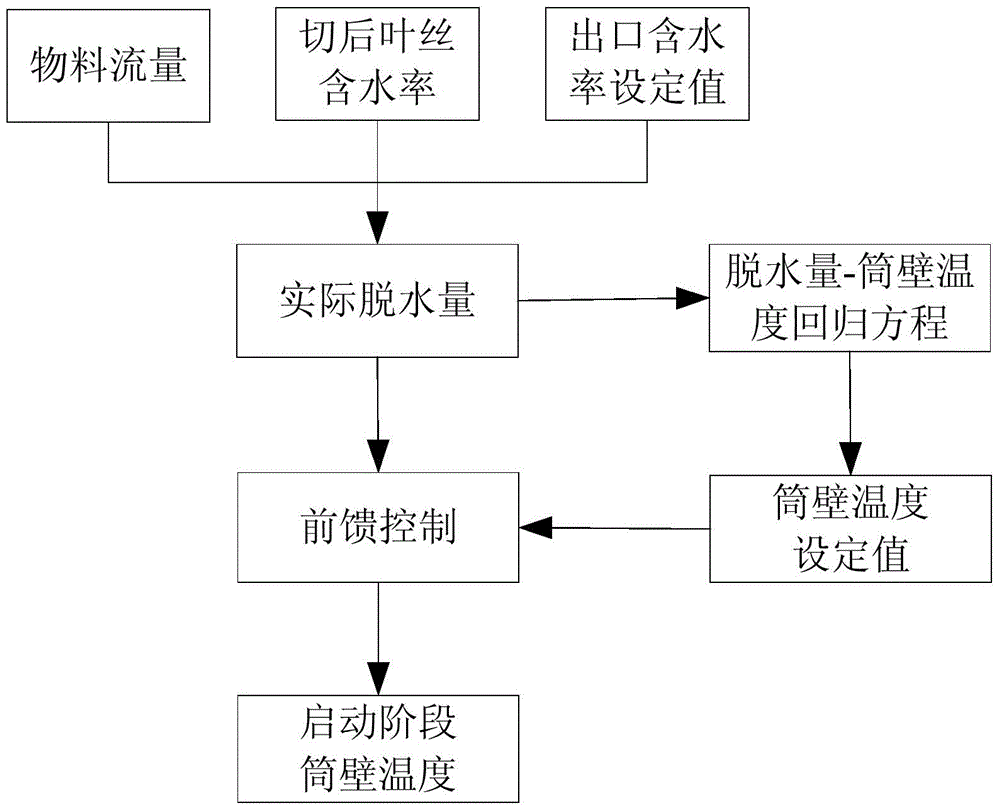
Shred drier cylinder wall temperature prediction model based on cut tobacco shred moisture content
InactiveCN104886751AImprove accuracyIncrease the steady state timeTobacco preparationPulp and paper industryMoisture
The invention discloses a shred drier cylinder wall temperature prediction model based on the cut tobacco shred moisture content. The shred drier cylinder wall temperature prediction model includes the following steps: (1) the after-cut tobacco shred moisture content (W1) is collected through a moisture instrument at the position of a belt at an inlet of a tobacco shred temporary storage cabinet; (2) the actual dehydration amount deltaFW is calculated according to the tobacco shred drying outlet moisture content setting value (SPW4); (3) according to the dehydration amount-cylinder wall temperature stepwise regressive equation, the cylinder wall temperature setting valve TSP is calculated; and (4) an operating person inputs the TSP into a formula list, a drier gets into the starting stage, feedforward control is carried out according to the formula, and calculation is carried out to obtain Tpv. By means of the shred drier cylinder wall temperature prediction model based on the cut tobacco shred moisture content, through decreasing of the difference value between the cylinder wall temperature setting value at the starting stage and the cylinder wall temperature actual value in the production steady state, the non-steady-state time of the starting stage is shortened, and decreasing of the number of dry head materials in the thin plate drying process and improving of the moisture content process capability index of discharged materials are achieved.
Owner:HONGYUN HONGHE TOBACCO (GRP) CO LTD
Development of Protein-Based Biotherapeutics That Penetrates Cell-Membrane and Induces Anti-Angiogenic Effect - Improved Cell-Permeable Suppressor of Cytokine Signaling (iCP-SOCS3) Proteins, Polynucleotides Encoding the Same, and Anti-Angiogenic Compositions Comprising the Same
InactiveUS20160060313A1Good effectImprove efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsSolubilityApoptosis
In principle, protein-based biotherapeutics offers a way to control biochemical processes in living cells under non-steady state conditions and with fewer off-target effects than conventional small molecule therapeutics. However, systemic protein delivery in vivo has been proven difficult due to poor tissue penetration and rapid clearance. Protein transduction exploits the ability of some cell-penetrating peptide (CPP) sequences to enhance the uptake of proteins and other macromolecules by mammalian cells. Previously developed hydrophobic CPPs—named membrane translocating sequence (MTS), membrane translocating motif (MTM) and macromolecule transduction domain (MTD)—are able to deliver biologically active proteins into a variety of cells and tissues. Various cargo proteins fused to these CPPs have been used to test the functional and / or therapeutic efficacy of protein transduction. Previously, recombinant proteins consisting of suppressor of cytokine signaling 3 (SOSC3) fused to the fibroblast growth factor (FGF) 4-derived MTM were developed to inhibit inflammation and apoptosis. However, this SOCS3 fusion proteins expressed in bacteria cells were hard to be purified in soluble form. To address these critical limitations, CPP sequences called advanced MTDs (aMTDs) have been developed in this art. The development of this art has been accomplished by (i) analyzing previous developed hydrophobic CPP sequences to identify specific critical factors (CFs) that affect intracellular delivery potential and (ii) constructing artificial aMTD sequences that satisfy each critical factor. Furthermore, solubilization domains (SDs) have been incorporated into the aMTD-fused SOCS3 recombinant proteins to enhance solubility with corresponding increases in protein yield and cell- / tissue-permeability. These recombinant SOCS3 proteins fused to aMTD / SD having much higher solubility / yield and cell- / tissue-permeability have been named as improved cell-permeable SOCS3 (iCP-SOCS3) proteins. Previously developed SOCS3 recombinant proteins fused to MTM were only tested or used as anti-inflammatory agents to treat acute liver injury. In the present art, iCP-SOCS3 proteins have been tested for use as anti-angiogenic agents. Since SOCS3 is known to be an endogenous inhibitor of pathological angiogenesis, we reasoned that iCP-SOCS3 could be used as a protein-based intracellular replacement therapy for inhibiting angiogenesis in tumor cells. The results demonstrated in this art support this following reasoning: Cancer treatment with iCP-SOCS3 results in reduced endothelial cell viability, loss of cell migration potential and suppressed vascular sprouting potentials. In the present invention with iCP-SOCS3, where SOCS3 is fused to an empirically determined combination of newly developed aMTD and customized SD, macromolecule intracellular transduction technology (MITT) enabled by the advanced MTDs may provide novel protein therapy against cancer cell-mediated angiogenesis.
Owner:CELLIVERY THERAPEUTICS
Sound modification system and method
InactiveUS8542844B2Minimize NVH and other noiseOptimal passenger compartment experienceEar treatmentGain controlDriver/operatorNoise reduction
A sound modification system and a method of managing sound in a vehicle. The system includes an active noise reduction (ANR) controller, an engine sound enhancement (ESE) controller and a sound modification controller. When the vehicle is in a steady state driving condition, the sound modification controller activates the ANR controller and deactivates the ESE controller. In contrast, when the vehicle is in a non-steady state driving condition, the sound modification controller activates the ESE controller and deactivates the ANR controller. Preferably, when the vehicle switches between the steady state and non-steady state driving conditions, the sound modification controller activates and deactivates the ANR and ESE controllers at a rate that is not noticeable to a human. In summary, the system utilizes the ANR and ESE controllers to achieve an optimal passenger cabin experience for the driver during any driving condition.
Owner:VISTEON GLOBAL TECH INC
Diffusion based metal oxide gas sensor
ActiveUS20140212979A1Material analysis by electric/magnetic meansMaterial analysis by optical meansDiffusionAnalyte
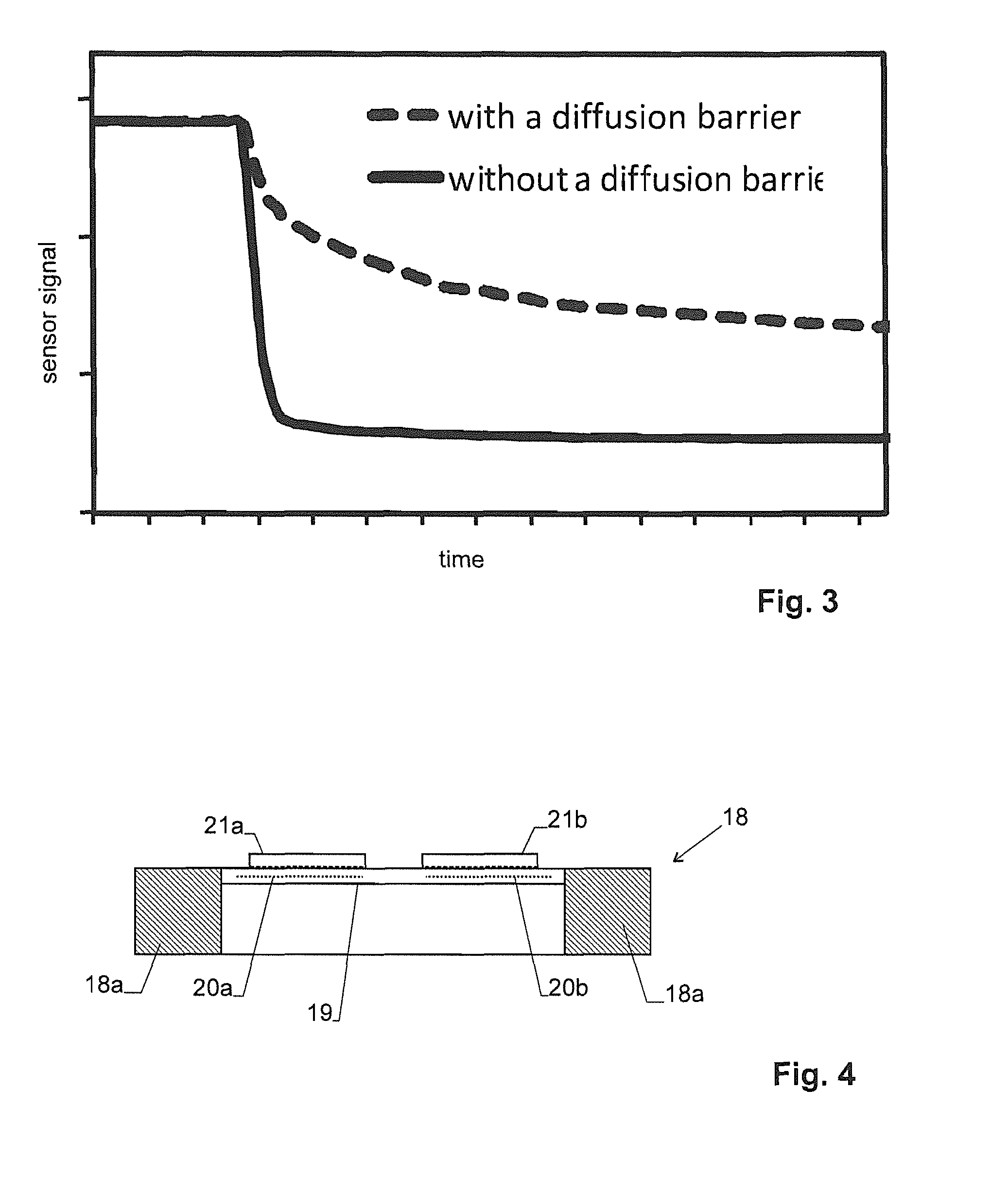
A measuring device is provided for determining the type and / or concentration a gaseous analyte from a set of analytes in a gaseous carrier. It comprises a housing having a passage to a cavity. A gas sensor with a heated metal-oxide sensing layer is arranged in the cavity. In order to gain a better understanding of the type of the analyte, diffusion effects are exploited by taking into account that the diffusion process through the passage as well as the catalytic reaction rate at the metal-oxide sensing layer depend on the type of the analyte. These material parameters can be determined by taking several measurements in a non-steady state of the concentration of the analyte within the cavity or while varying the reaction rate.
Owner:SENSIRION AG
Systems and methods for improving drilling efficiency
Owner:NAT OILWELL VARCO LP
System and method for single-scan rest-stress cardiac pet
ActiveUS20150230762A1High first-pass extraction rateFew and slow biochemical reactionIn-vivo radioactive preparationsComputerised tomographsRadioactive tracerCardiac muscle
The present invention provides a system and method for performing a single-scan rest-stress cardiac measurement. In one aspect, the system includes a positron emission tomography (PET) imaging system, a source of a first PET radiotracer for administration to a subject, a source of a second PET radiotracer for administration to a subject, and a processor. The processor has non-transient computer readable media programmed with instructions to obtain PET images of the subject administered with the radiotracer. Furthermore, the computer readable media is programmed with instructions to process the PET images with a non-steady-state, multi-compartment parametric model. An output of the non-steady-state, multi-compartment parametric model is a measure of myocardial blood flow for both a rest state and a stress state of the subject.
Owner:THE GENERAL HOSPITAL CORP
High-temperature step combination-performance testing device for phase-change heat storage device and testing method
ActiveCN105606645ABroaden the scope of testingEnsure consistencyMaterial heat developmentData acquisitionEngineering
The invention discloses a high-temperature step combination-performance testing device for a phase-change heat storage device and a testing method. The testing device comprises a filter, a draught fan, an air discharging valve, a flow meter, an air feeding valve, an air heater, an experiment connecting pipe, the phase-change heat storage device, a cooler, six high-temperature electrically operated valves, a plurality of temperature sensors, a plurality of pressure sensors and a data collection and control system. The highest temperature of heat transferring fluid of the testing device can be 800 DEG C, and the testable range is expanded; meanwhile, the step heat-storage technology of the phase-change heat storage device can be researched, the structure is compact, testing of the real and non-steady-state condition of a solar high-temperature air collector can be achieved, and testing is more comprehensive. According to the testing device, the used heat transferring working medium is air, and the testing device is environmentally friendly and free of pollution.
Owner:XI AN JIAOTONG UNIV
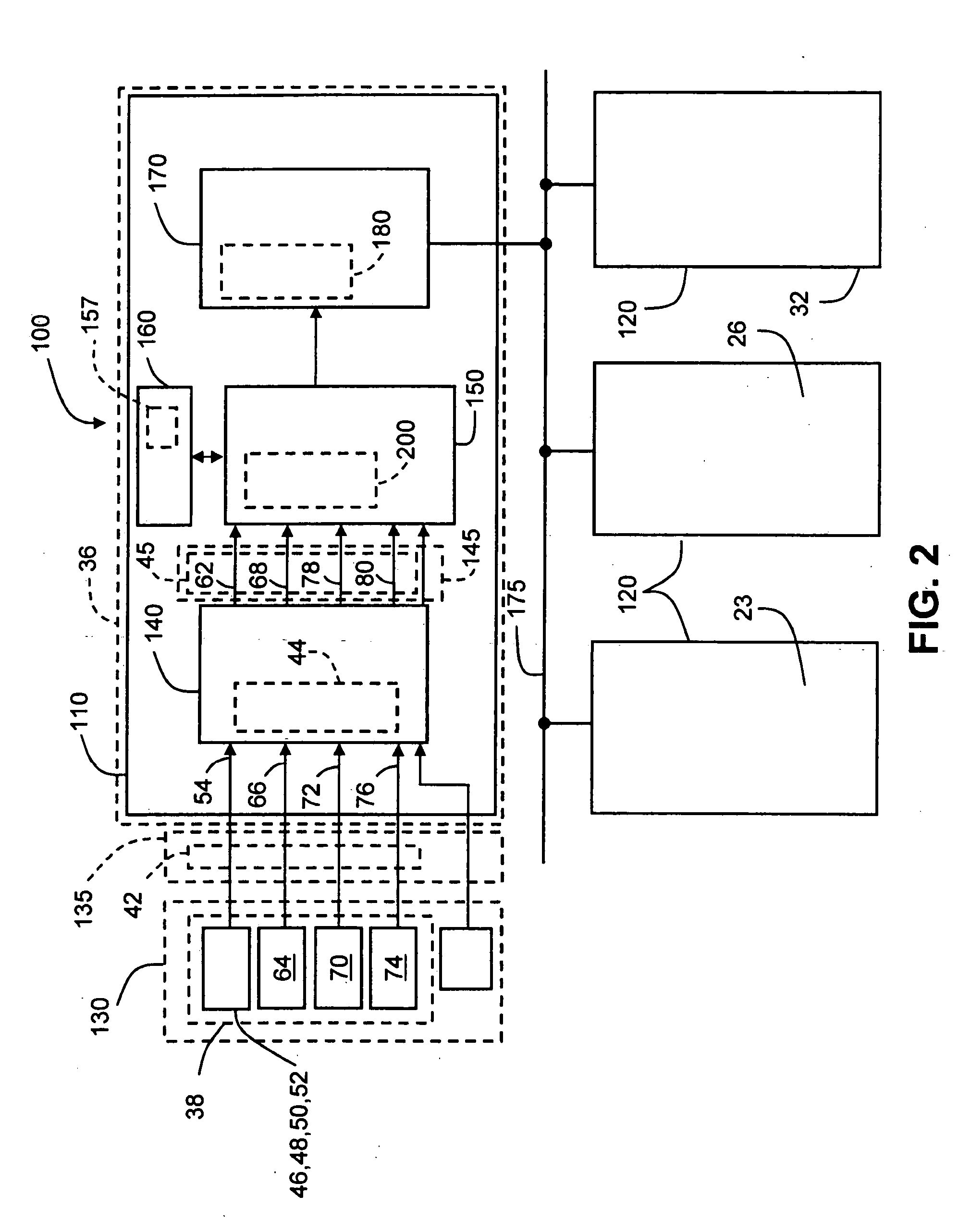
Method and apparatus for estimating steering behavior for integrated chassis control
InactiveUS20050222731A1Digital data processing detailsAutomatic steering controlVehicle dynamicsUndersteer and oversteer
A method and apparatus for providing integrated chassis control of a vehicle over the entire range of the vehicle dynamic state, including steady state and non-steady state steering conditions and linear and non-linear tire behavior, based on the general steer equation by using an estimated understeer and oversteer steering behavior indicator. The method and apparatus are particularly adapted to provide a yaw control apparatus and method. The steering behavior indicator may be calculated as a function of certain vehicle dynamic state inputs. A weighting factor for the calculation of the steering behavior indicator is determined as a function of certain vehicle dynamic state indication parameters.
Owner:GM GLOBAL TECH OPERATIONS LLC
Method and system for determining sustainable throughput over wireless networks
Sustainable average data throughput rates for data transfer between a sender and a receiver are determined for a network in a steady-state condition. Delivery performance for data transferred in non-steady-state conditions is disregarded in determining sustainable average data throughput rates. The rates may be used to adapt file delivery to network conditions.
Owner:OPANGA NETWORKS
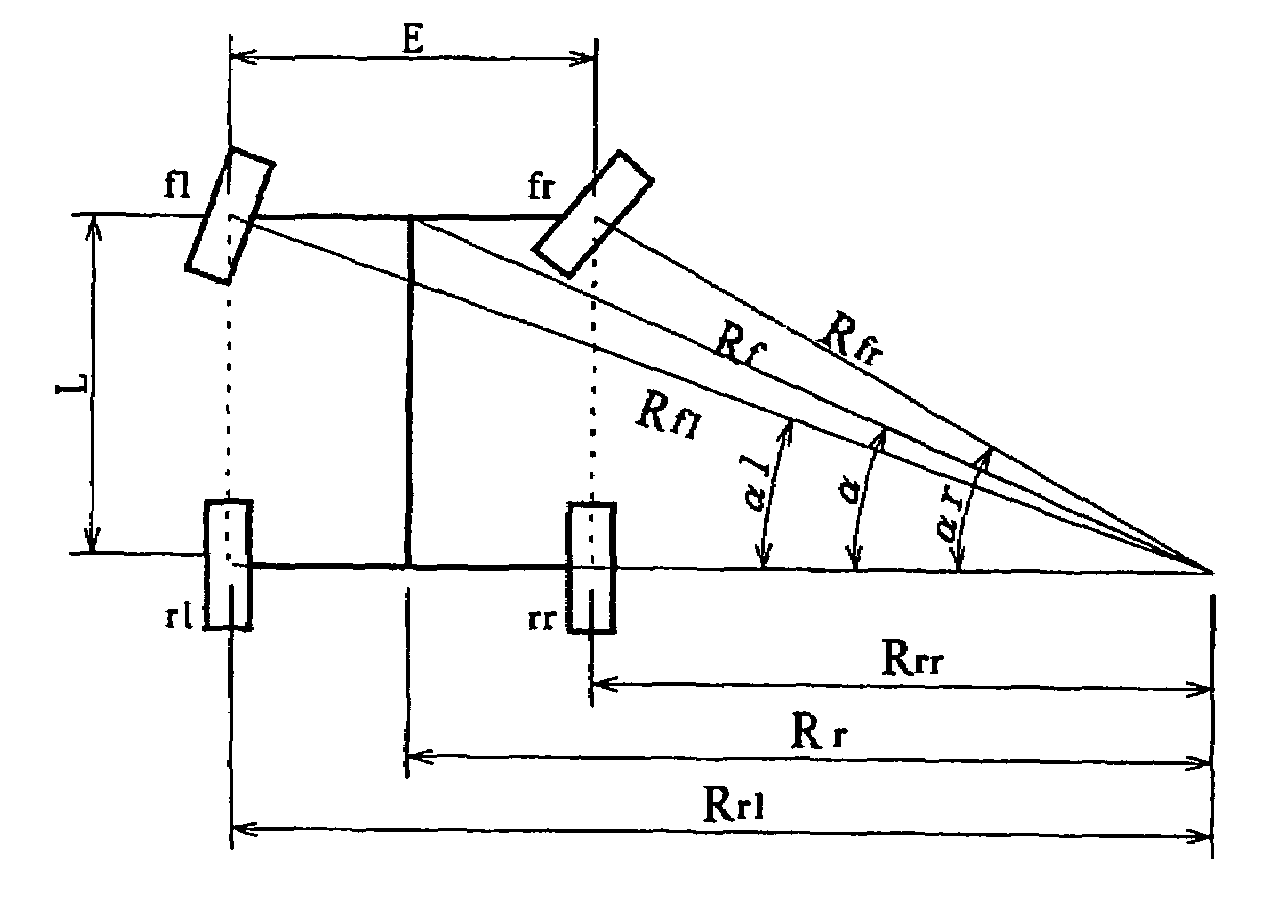
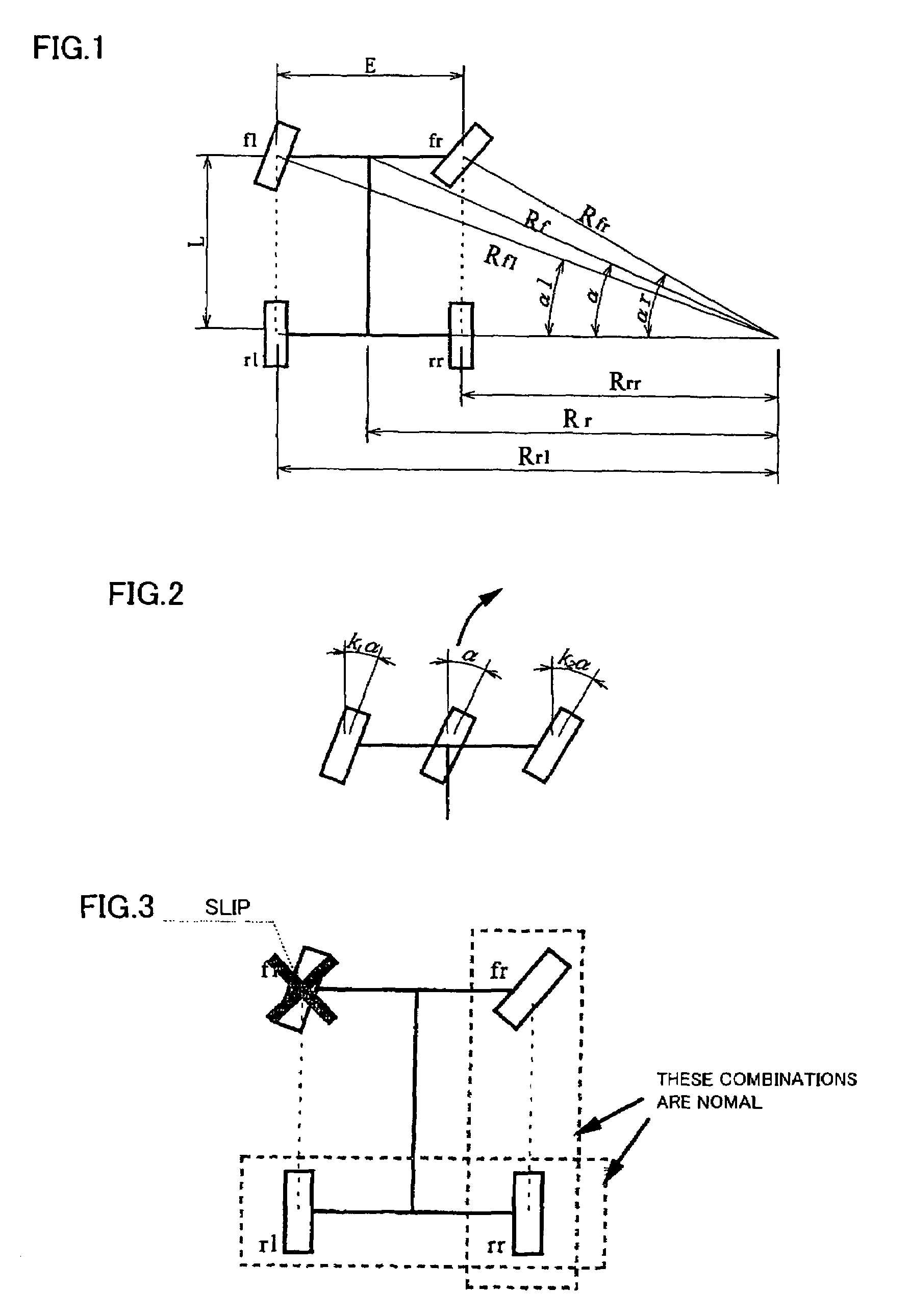
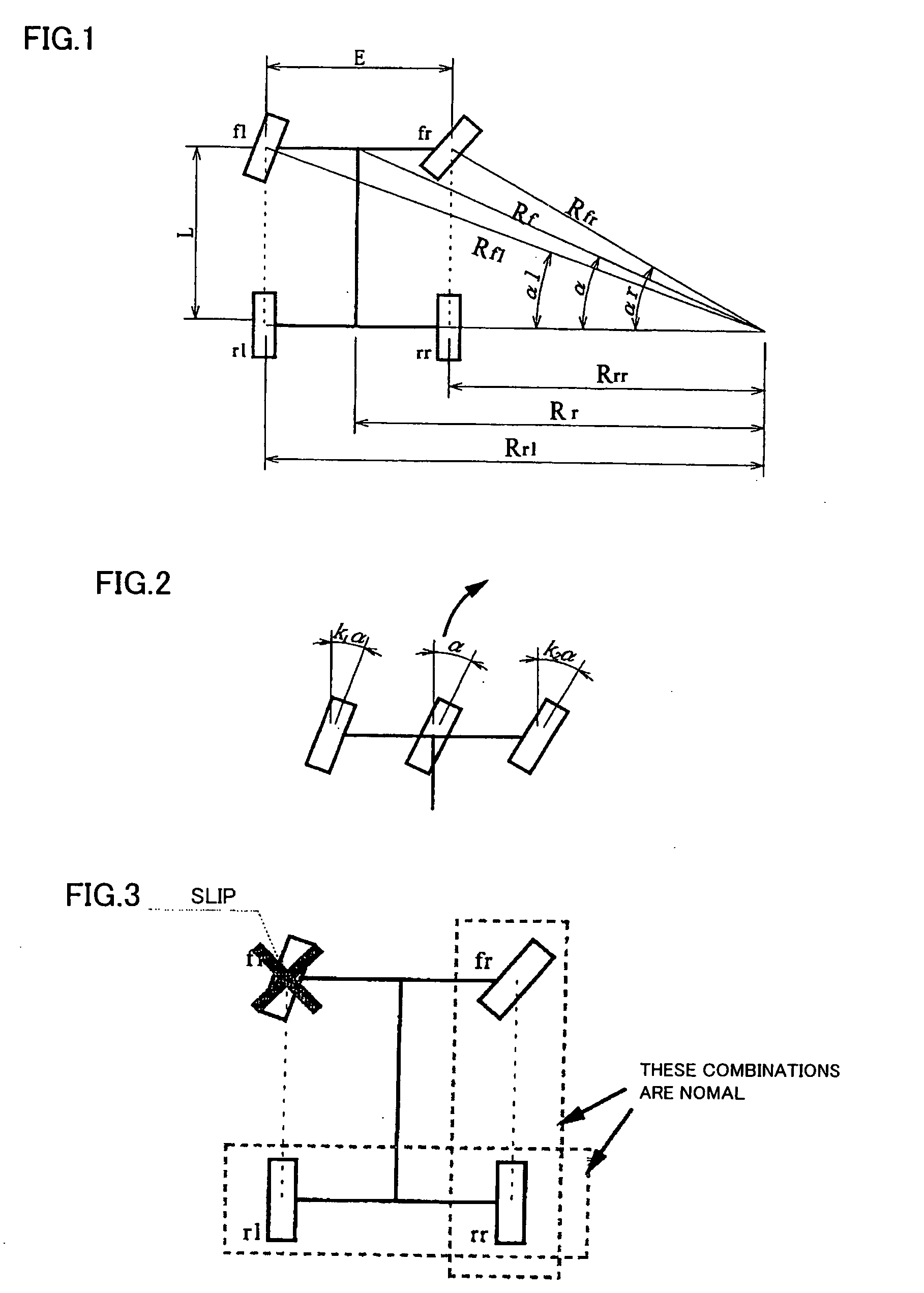
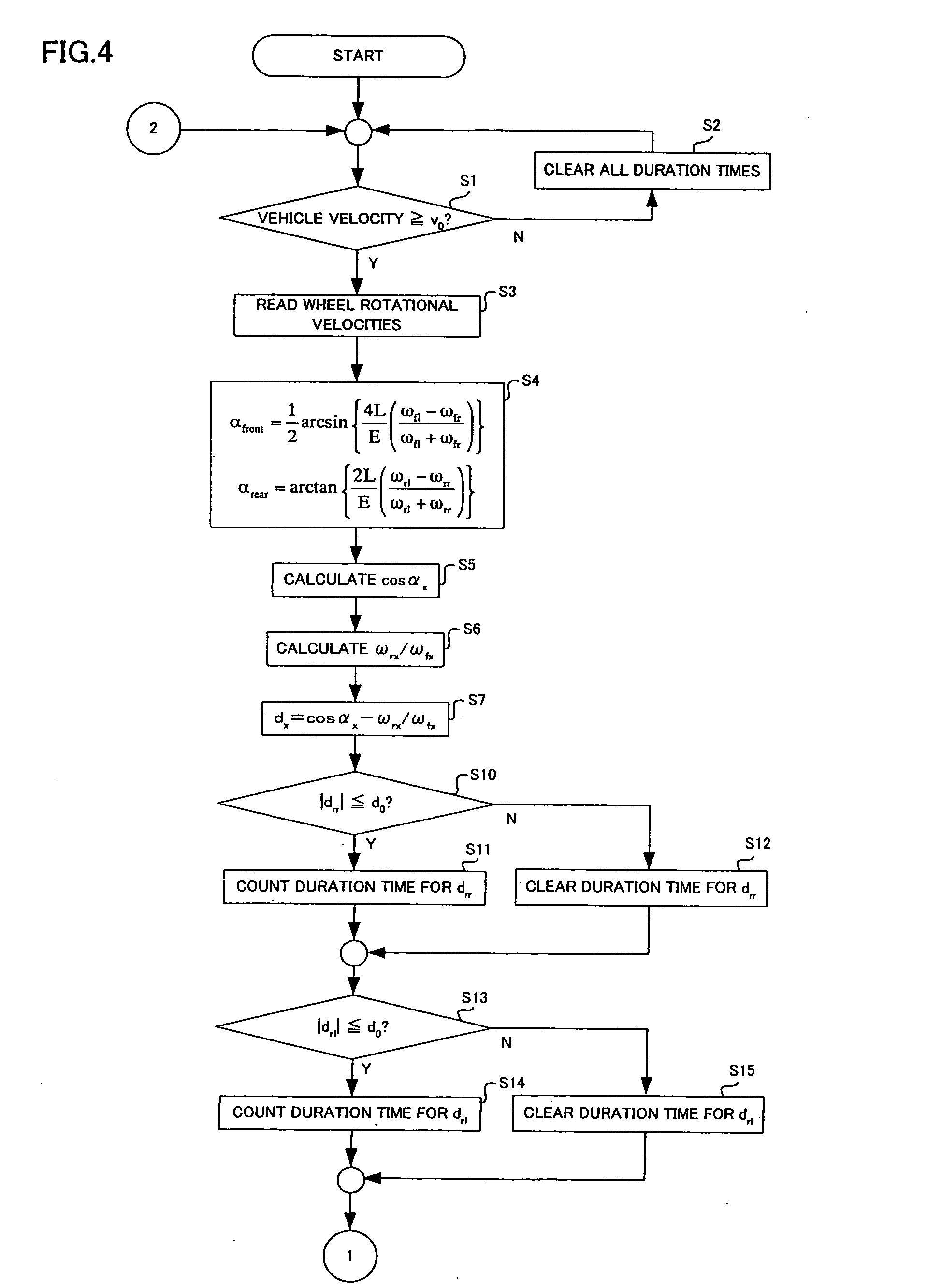
Steering angle estimating apparatus for vehicle
ActiveUS7440829B2Correct steering angle can be estimatedEfficient implementationSteering initiationsDigital data processing detailsSteering angleEngineering
The present invention provides a steering angle estimating apparatus for vehicle that can accurately determine a steady state at all the steering angles, and can estimate the steering angle as much as possible even in a non-steady state. In the steering angle estimating apparatus for vehicle that has wheel rotational velocity sensors for four wheels, respectively, and estimates the steering angle of the vehicle based on wheel rotational velocities from the wheel rotational velocity sensors, relationships in the wheel rotational velocities between respective combinations of two wheels of the front and rear wheels on right and left sides are compared so that slip of the four wheels is detected.
Owner:NSK LTD +1
Production of toughened and reinforced tungsten carbide composite material with non-stablized yttrium jargonia
The method is to prepare the tungsten carbide compound material of the non-steady state yttrium zircite. First to match the powdeZrO21-8wt%, powderCo10-20wt% and the residue is powderWC; then to mix the mixture in the globe mill for 35-50h; last to get the compound metal ceramic material by the chill-pressing, vacuum sinter and hot isostatic press. The material has the good wearing and bending resistances.
Owner:UNIV OF SCI & TECH BEIJING
Electrochemical apparatus with retractable electrode
InactiveUS20050058886A1Non-aqueous electrolyte accumulator electrodesWater/sewage treatment using germicide/oligodynamic-processContact pressureEngineering
Owner:LYNNTECH INT
Steering angle estimating apparatus for vehicle
ActiveUS20070083308A1Efficient implementationCorrect steering angle can be estimatedSteering initiationsDigital data processing detailsSteering angleEngineering
The present invention provides a steering angle estimating apparatus for vehicle that can accurately determine a steady state at all the steering angles, and can estimate the steering angle as much as possible even in a non-steady state. In the steering angle estimating apparatus for vehicle that has wheel rotational velocity sensors for four wheels, respectively, and estimates the steering angle of the vehicle based on wheel rotational velocities from the wheel rotational velocity sensors, relationships in the wheel rotational velocities between respective combinations of two wheels of the front and rear wheels on right and left sides are compared so that slip of the four wheels is detected.
Owner:NSK LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com