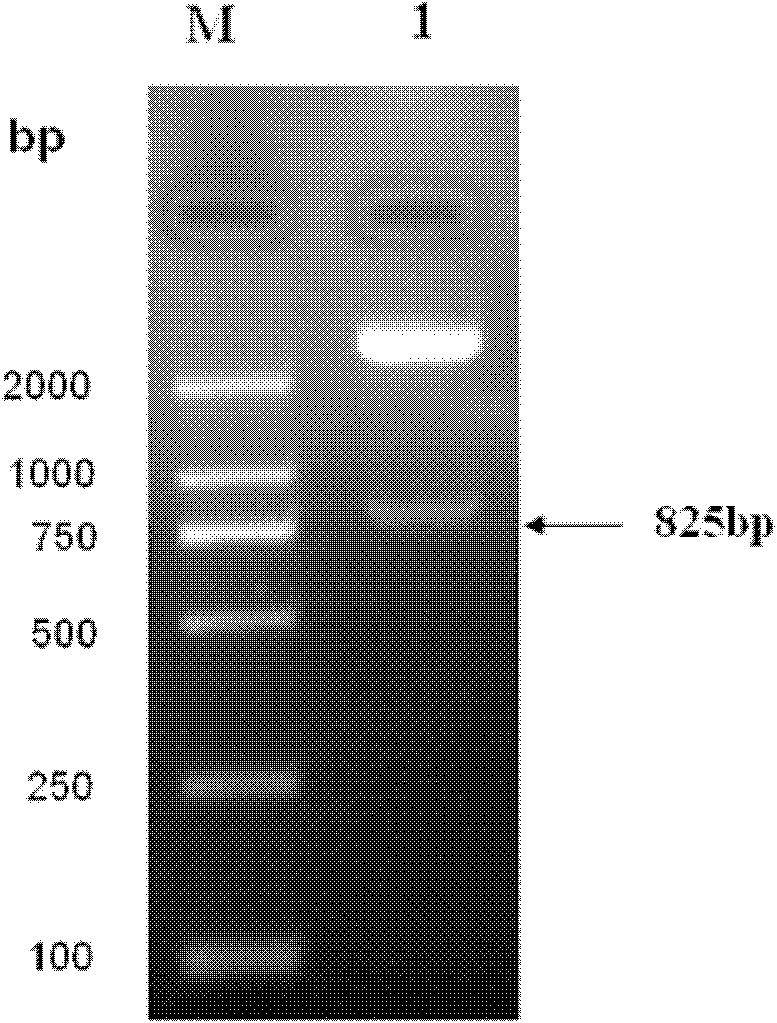
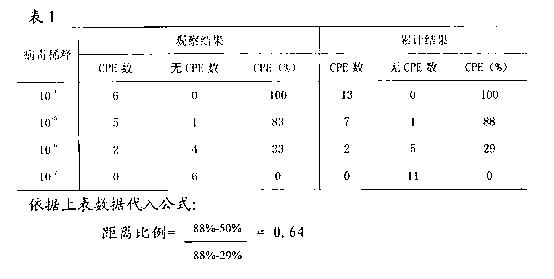
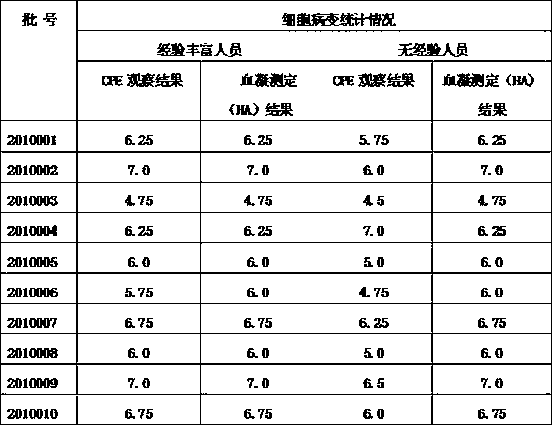
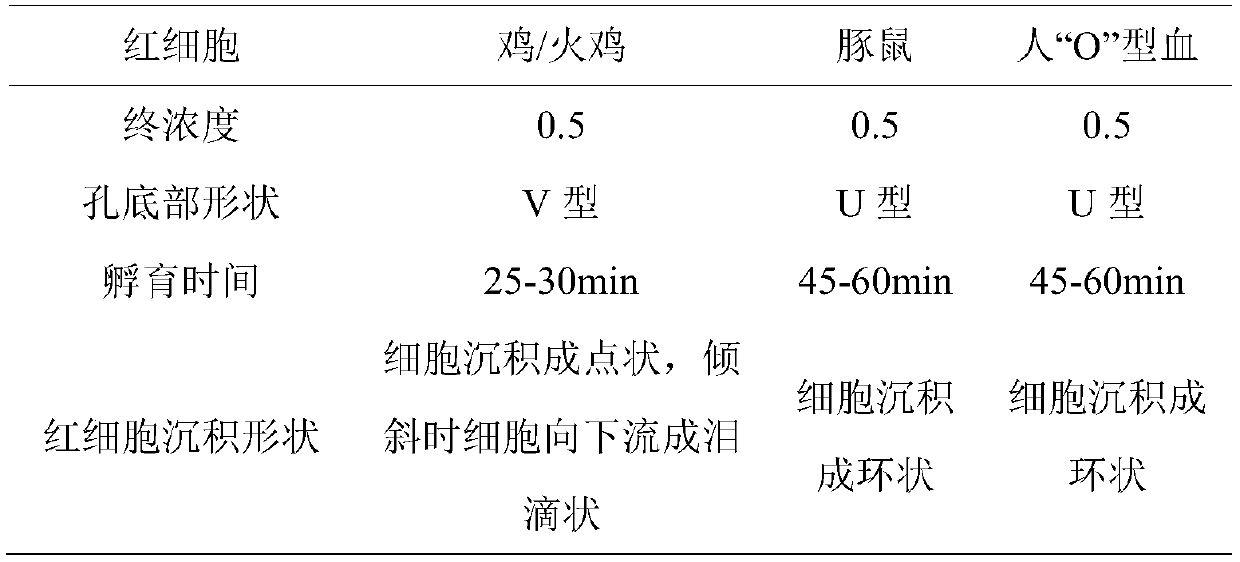
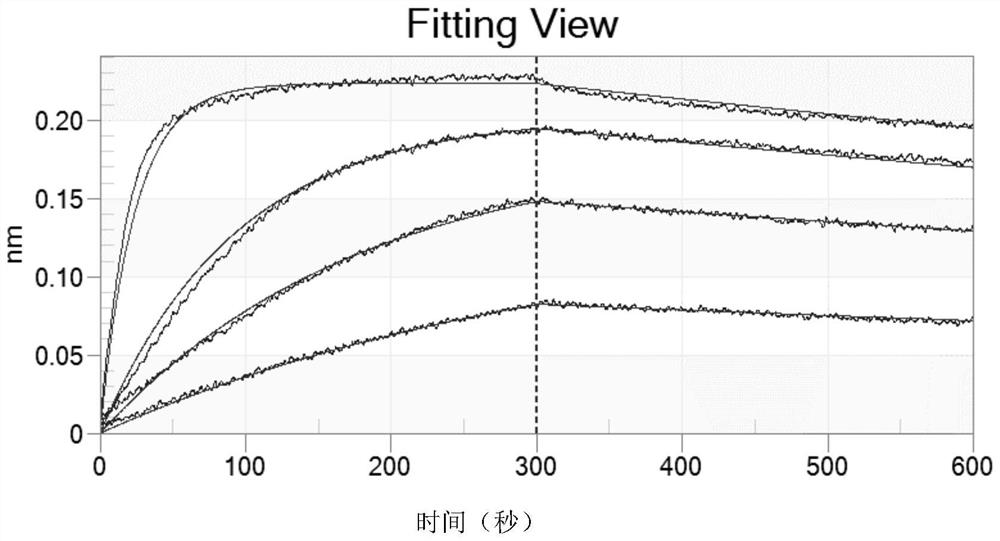
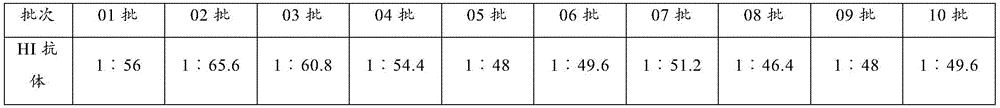
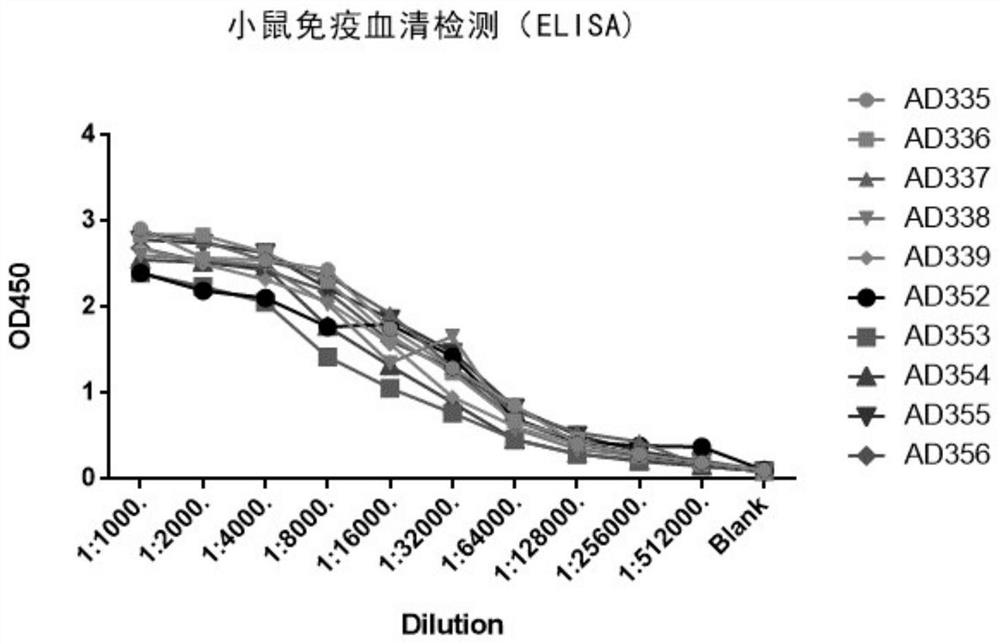
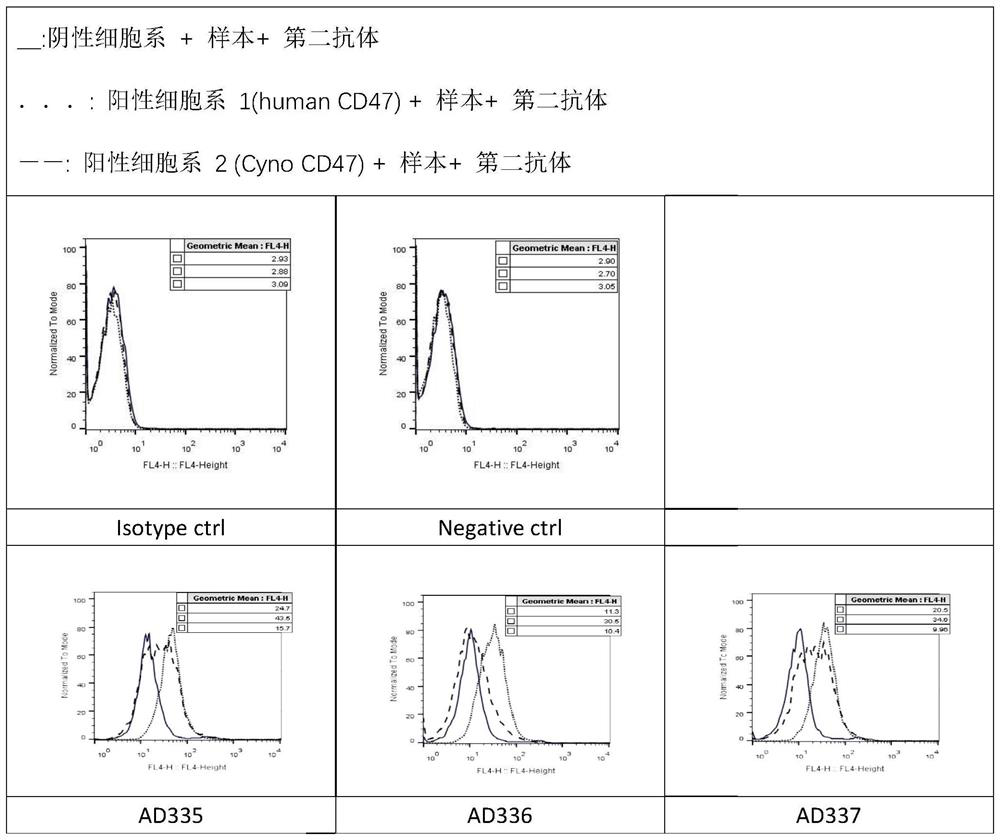
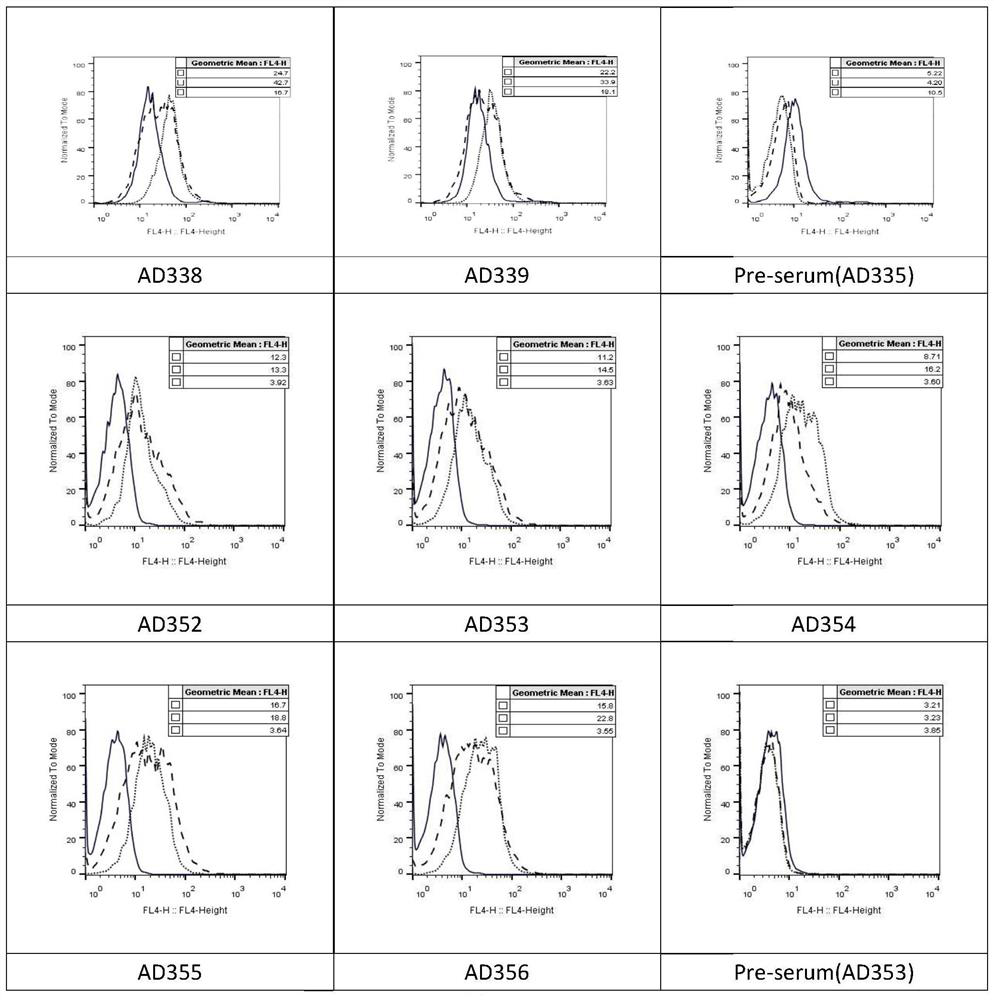
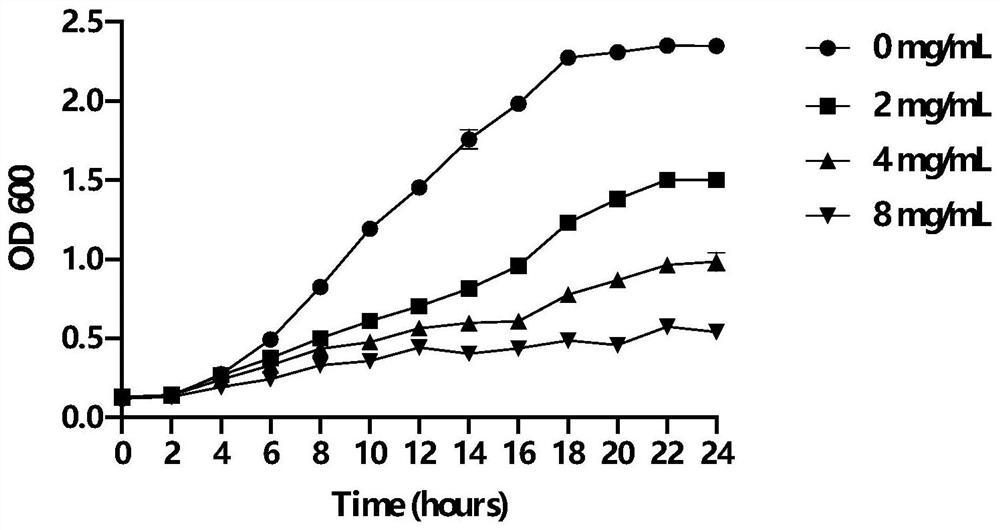
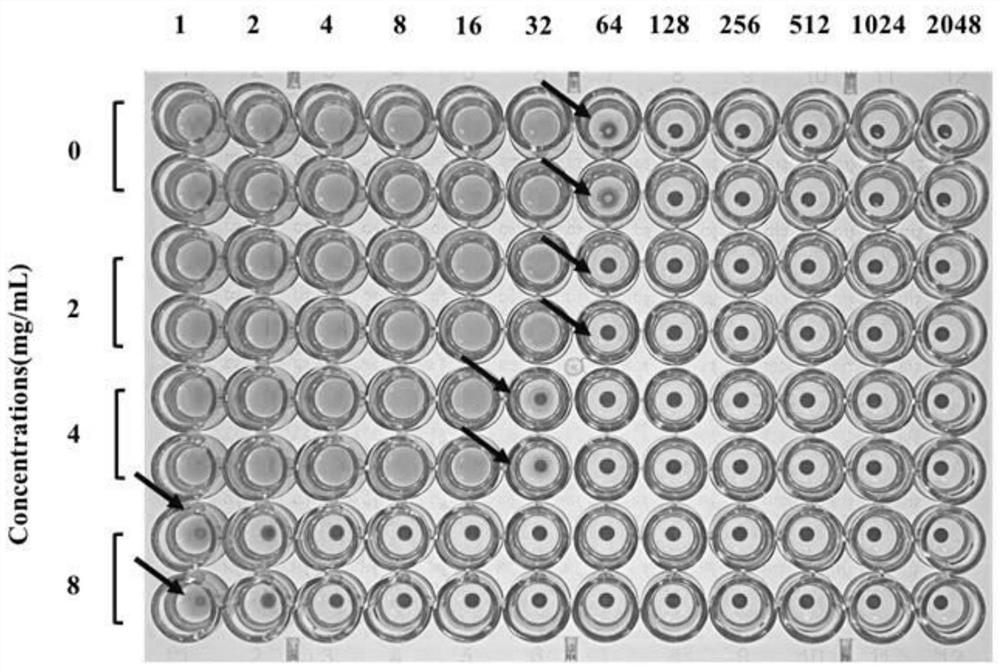
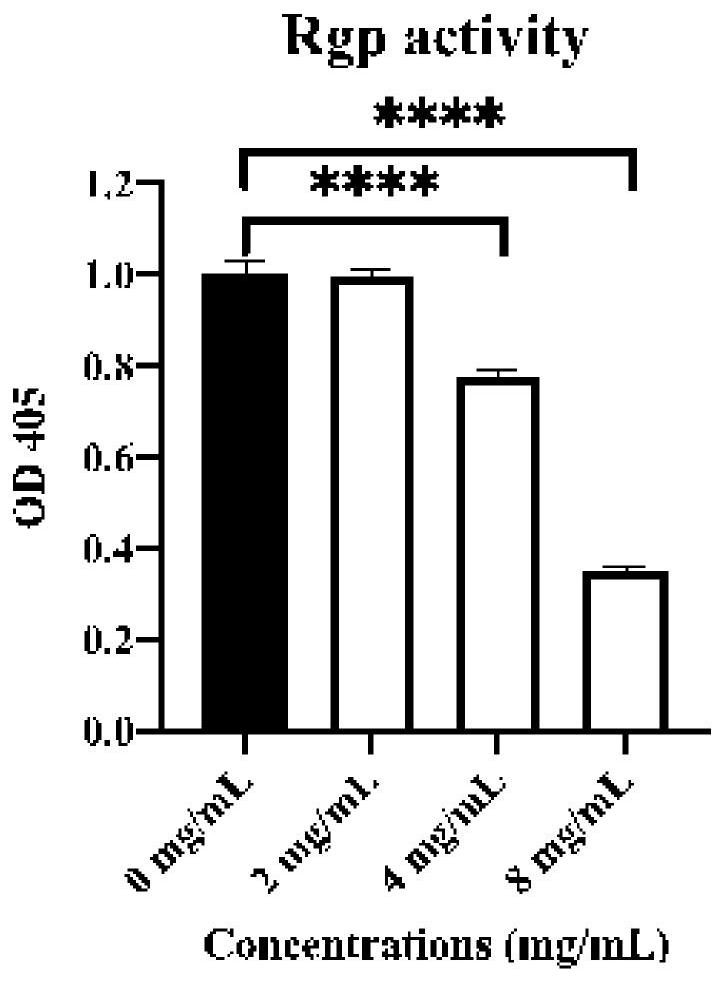
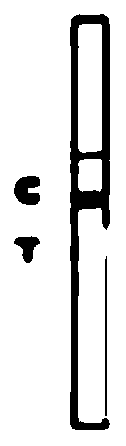Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "ERYTHROCYTES AGGLUTINATION" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
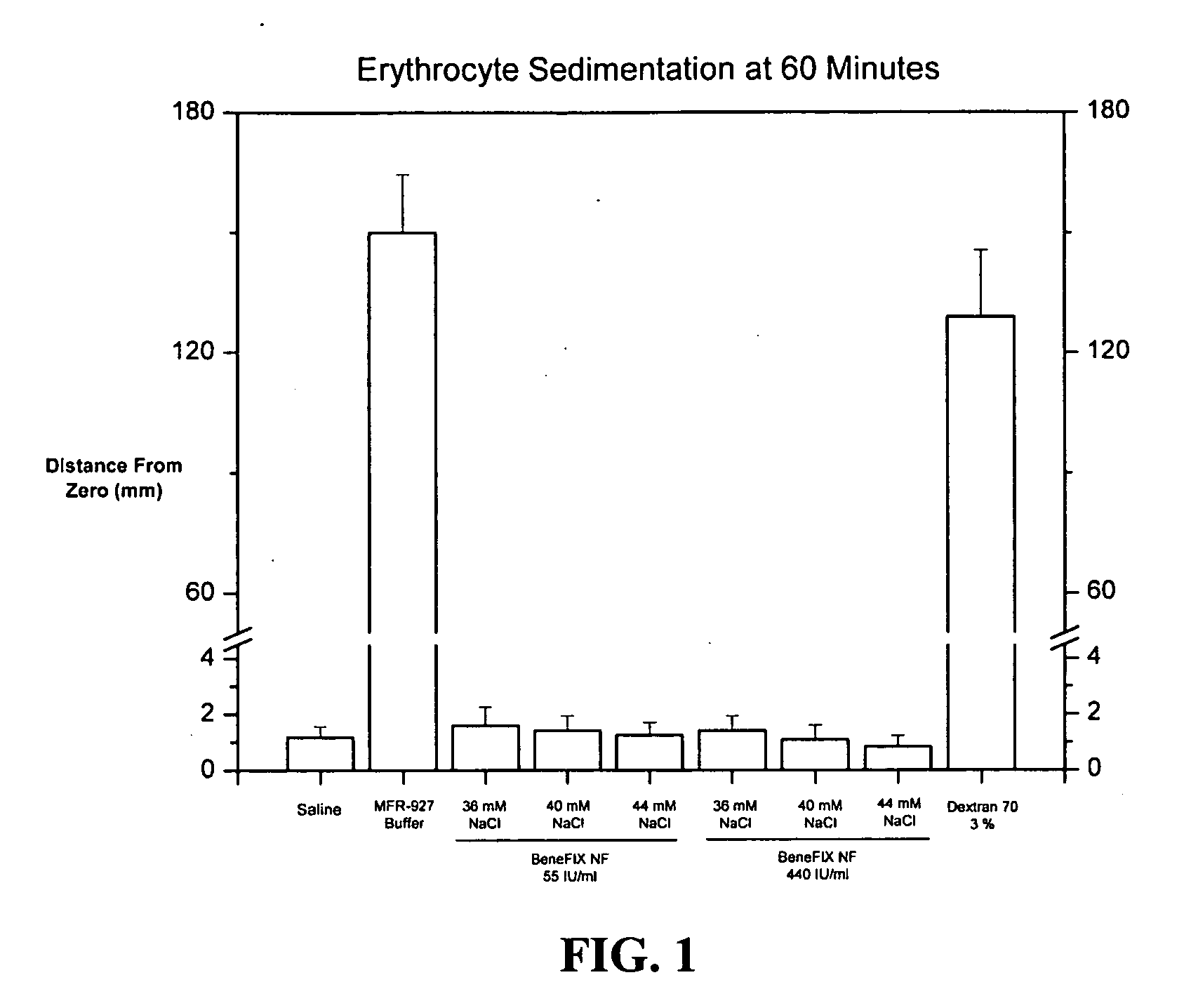
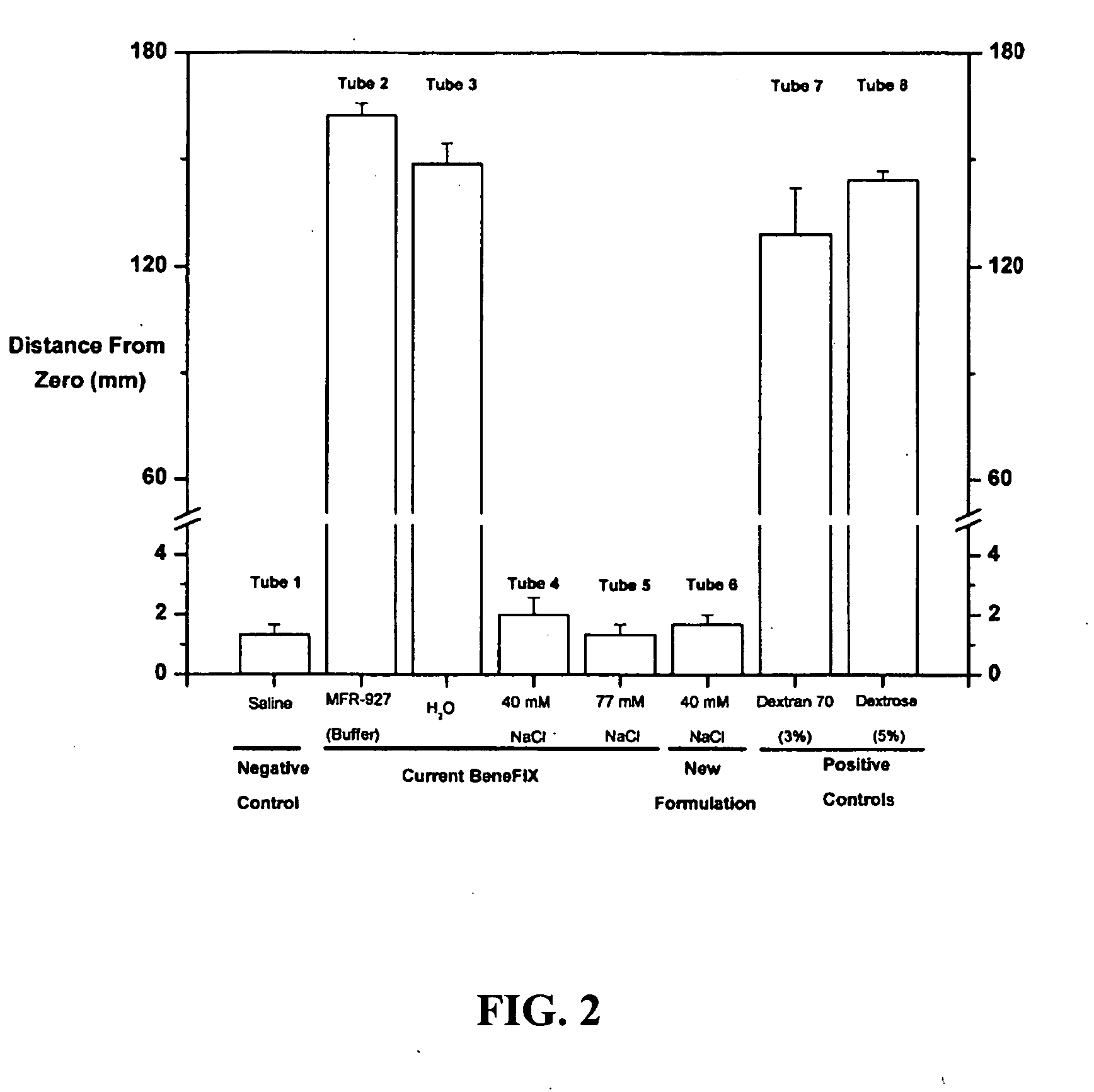
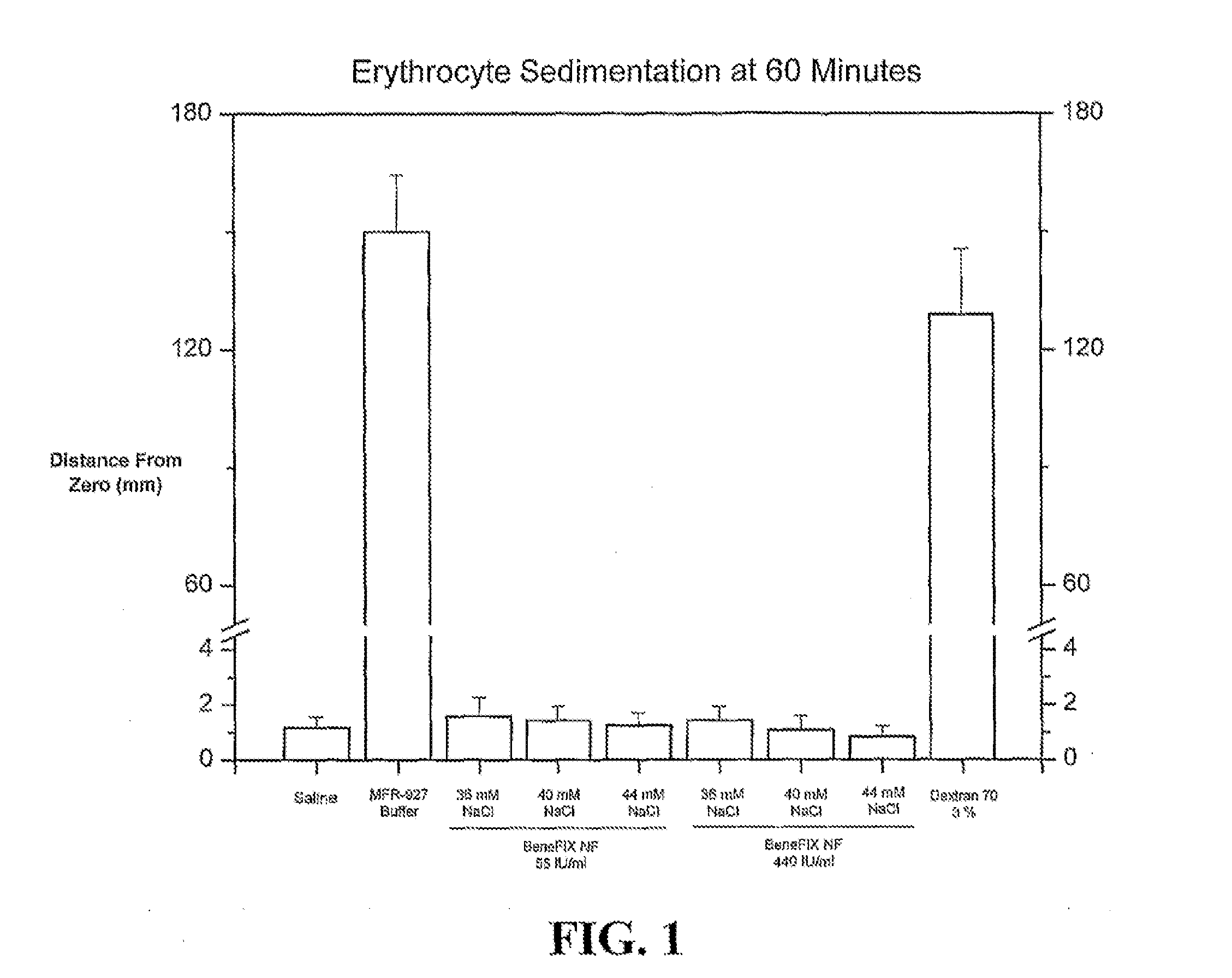
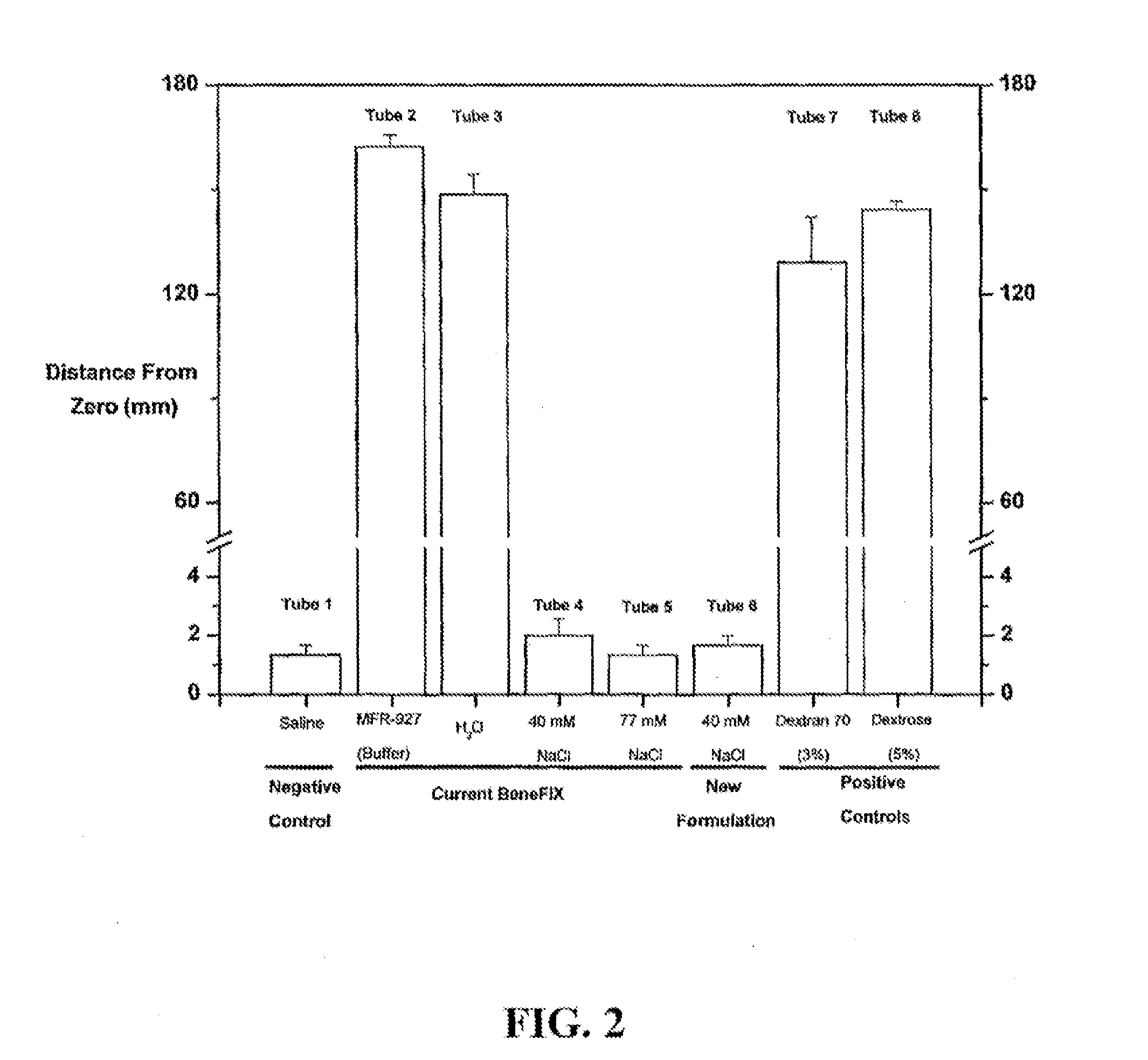
Sodium chloride solution for drug reconstitution or dilution
InactiveUS20070135343A1Prevent agglutinationIncrease ionic strengthBiocidePeptide/protein ingredientsHemolysisPresent method
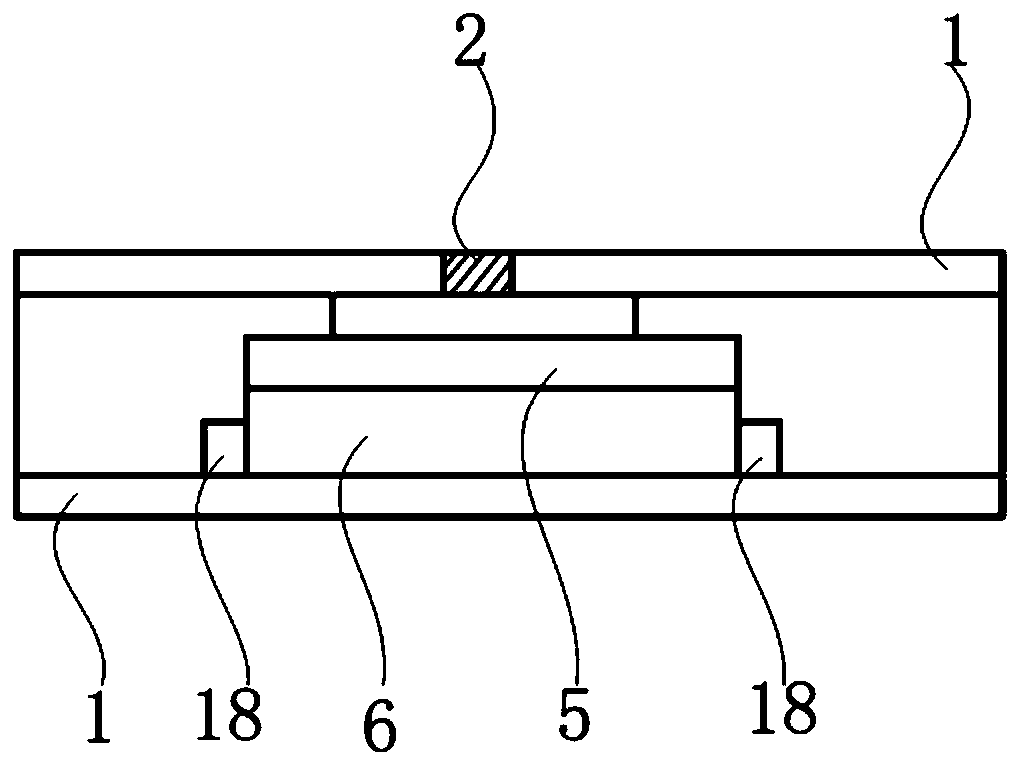
The invention provides methods for preparing pharmaceutical formulations for injection such that upon injection the formulation does not cause erythrocyte agglutination, hemolysis, and / or cell shrinkage. To prevent agglutination, a pharmaceutical formulation ready for injection needs to have a sufficient ionic strength. To prevent hemolysis or cell shrinkage, a pharmaceutical formulation ready for injection needs to be about isotonic with respect to plasma. The invention provides methods that prepare pharmaceutical formulations for injection that have both the sufficient ionic strength to prevent agglutination and the requisite tonicity to prevent significant hemolysis or cell dehydration or shrinkage. The present methods involve the use of sodium chloride solutions that are about 25 mM to about 150 mM for reconstituting lyophilized cakes (or other non-liquid pharmaceutical formulations) into solution or for diluting pharmaceutical formulation solutions.
Owner:WYETH LLC
Heavy chain and light chain variable region for resisting to canine parvovirus antibody and gene engineering antibody
ActiveCN109627331AHigh activityPrevent agglutinationImmunoglobulins against virusesGenetic engineeringMonoclonal antibodyGene engineering
The invention discloses a heavy chain and light chain variable region for resisting to a canine parvovirus antibody and a gene engineering antibody, and belongs to the technical field of antibody engineering. An amino acid sequence and a nucleotide sequence of the heavy chain and light chain variable region for resisting to the canine parvovirus antibody provide supports for construction of the high-affinity and low-immunity canine parvovirus antibody. A variable region sequence of a monoclonal antibody for canine parvovirus and a murine constant region are further assembled, so that the geneengineering antibody for resisting to canine parvovirus is obtained, good activity for neutralizing CPV viruses is shown, and the gene engineering antibody has a restraining effect on erythrocyte agglutination of canine parvovirus, can be applied to research of a canine source of the monoclonal antibody for canine parvovirus and other fields, and has important meaning for pushing of development ofcanine source monoclonal antibody medicine.
Owner:CHANGCHUN SR BIOLOGICAL TECH
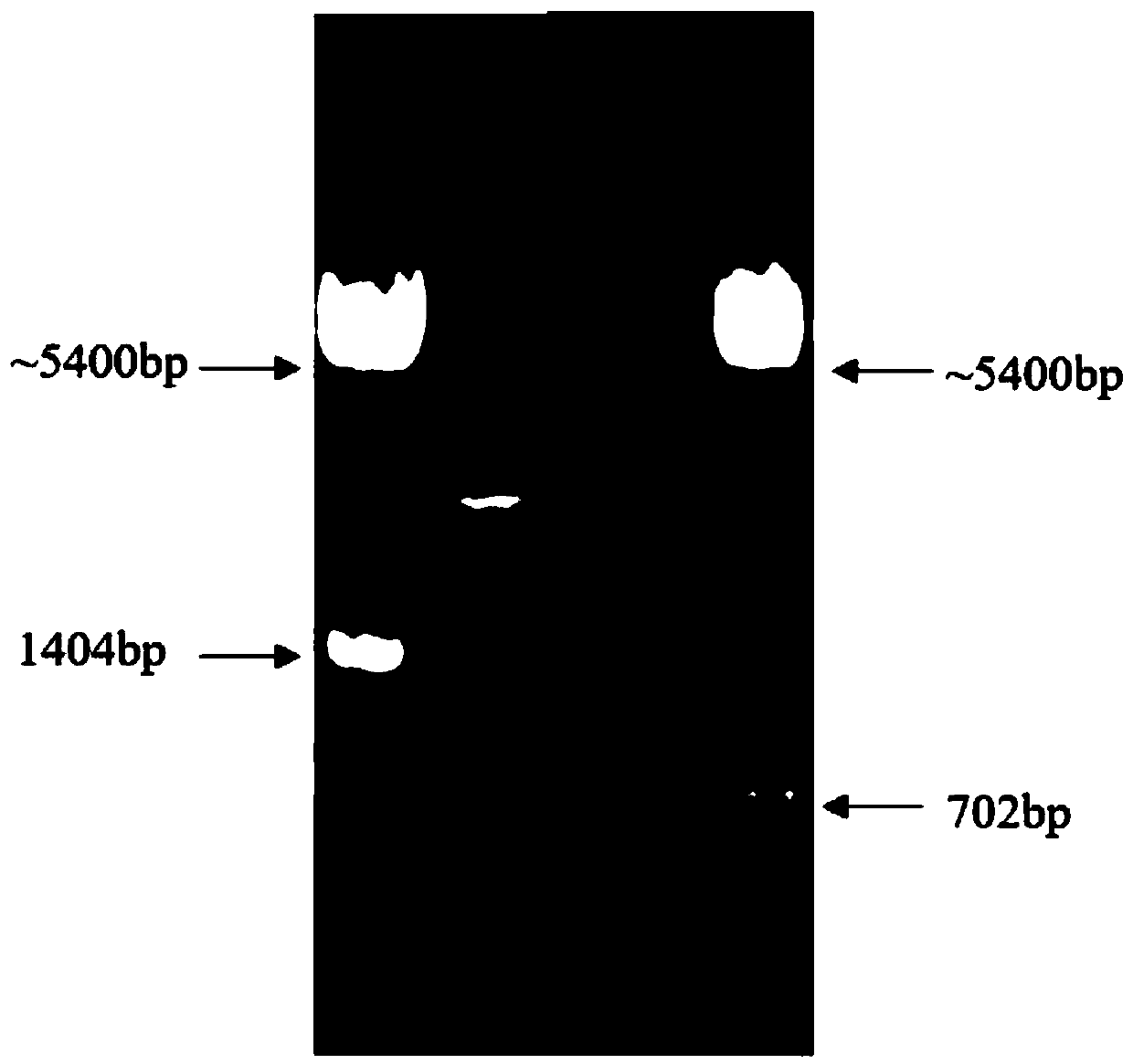
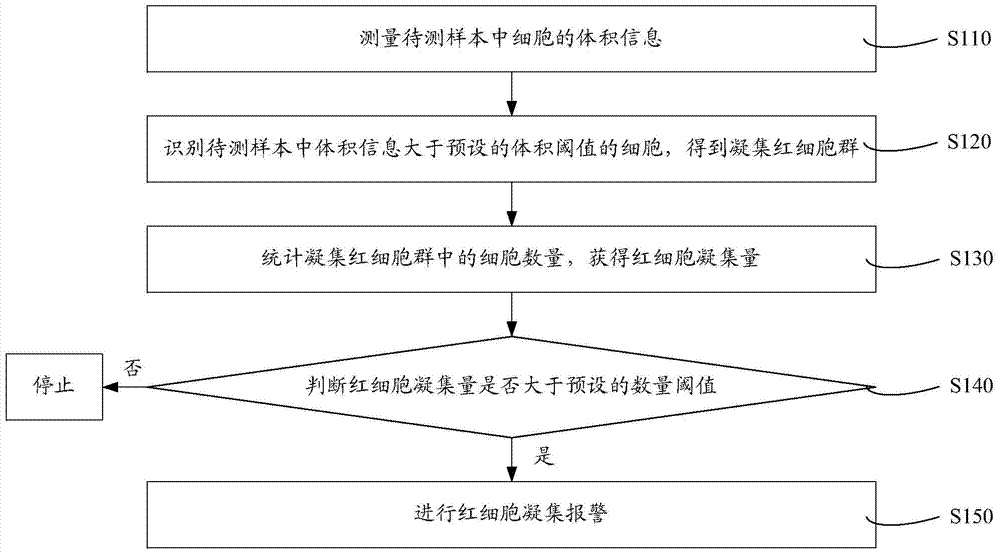
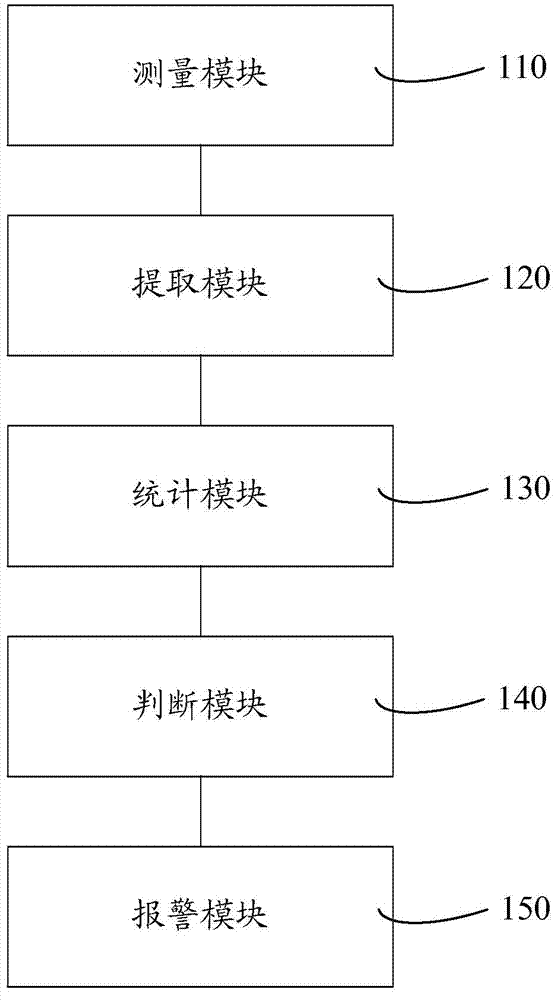
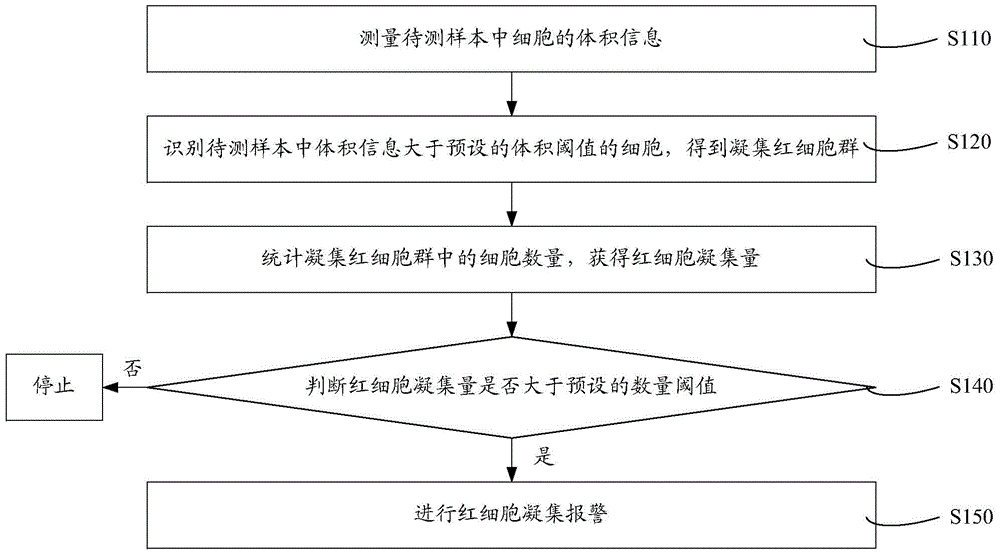
Cell analyzer, erythrocyte agglutination warning method and erythrocyte agglutination warning system of the cell analyzer
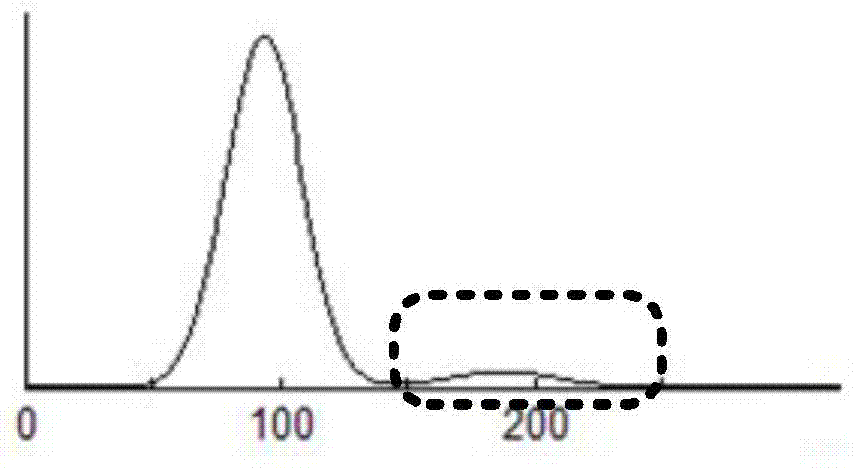
A cell analyzer, an erythrocyte agglutination warning method and an erythrocyte agglutination warning system of the cell analyzer. The erythrocyte agglutination warning method includes following steps: (1) measuring volume information of cells in a sample; (2) recognizing the cells of which the volume information is larger than a preset volume threshold value to obtain an agglutinated erythrocyte group; (3) counting the number of the cells in the agglutinated erythrocyte group to obtain an agglutinated erythrocyte amount; (4) determining whether the agglutinated erythrocyte amount is large than a preset number threshold value, and if the agglutinated erythrocyte amount is large than the preset number threshold value, carrying out erythrocyte agglutination warning. Determination on the content of certain components in the cells is unnecessary since the agglutinated erythrocyte is recognized directly according to the volume of the cells, so that compared with a conventional erythrocyte agglutination warning method, the method can improve a warning accuracy and is simple, convenient and quick.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Avian influenza H9N2 subtype virus strain and application thereof
ActiveCN103789273AStrong specificityImprove featuresSerum immunoglobulinsMicroorganism based processesSerum igeAvian influenza virus
The invention provides an avian influenza H9N2 subtype virus strain with the preservation number of CGMCC No.6757. The avian influenza H9N2 subtype virus strain provided by the invention has favorable specificity and immunogenicity, the erythrocyte agglutination effect of allantoic fluid can not be inhibited by anti-NDV (Newcastle Disease Virus) positive serum, anti-EDS76 positive serum, anti-M41 positive serum, anti-H5 subtype avian influenza virus positive serum and anti-H7 subtype avian influenza virus positive serum, but can be inhibited by H9 subtype avian influenza virus positive serum, and immunized chicken can generate an HI (Hemagglutination Inbition) antibody which is specific for H9. The avian influenza H9N2 subtype virus strain provided by the invention can be used as a vaccine strain for preventing H9 subtype avian influenza of birds, and can be used for authenticating avian influenza viruses and researching epidemiology so as to have favorable market application prospects.
Owner:北京华都诗华生物制品有限公司
Pharmaceutical- or gene-carrier compositions with reduced hemagglutinating activity
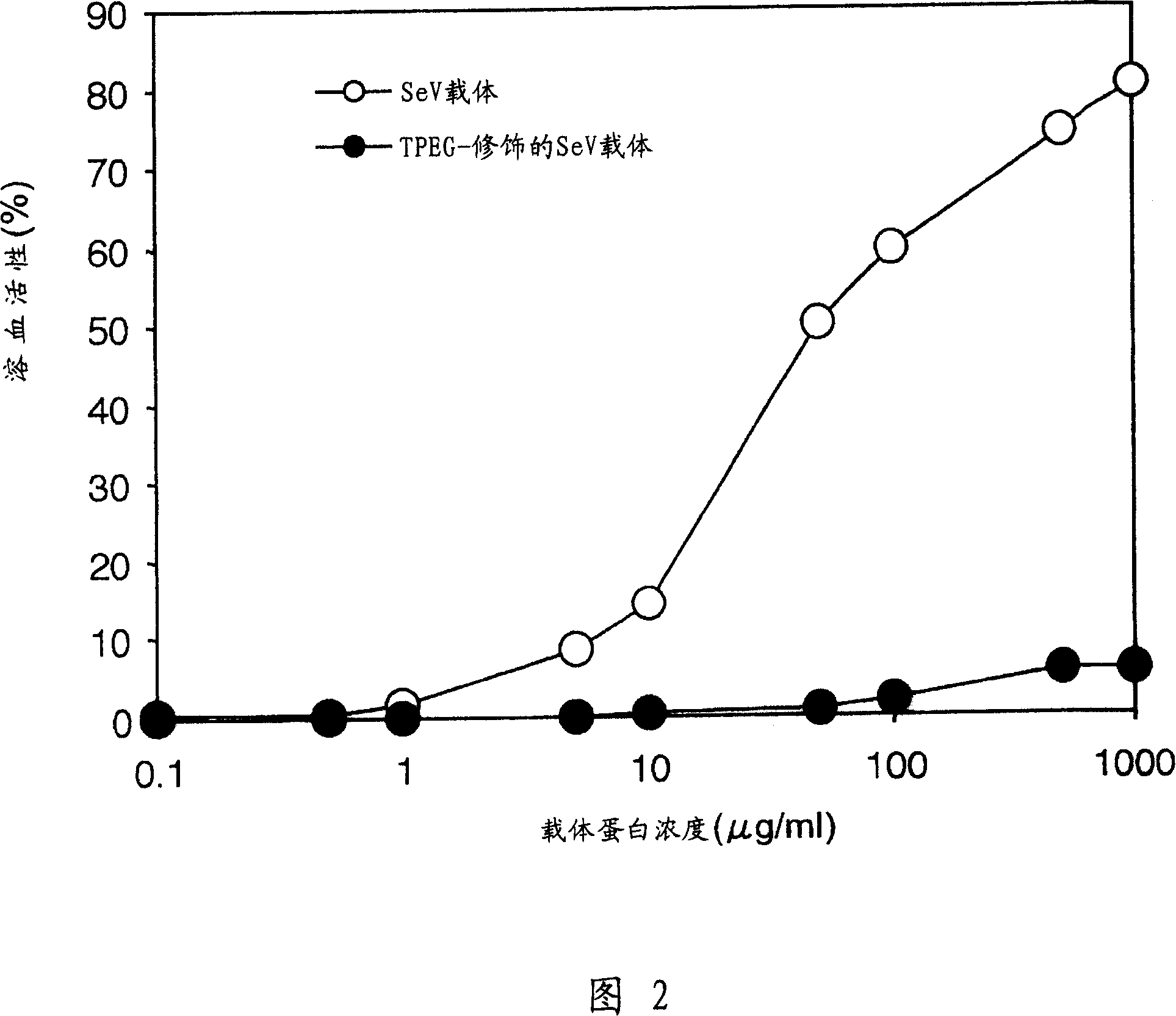
InactiveUS20050250718A1Reduced activityRetain abilitySsRNA viruses negative-senseBiocideRed blood cellIn vivo
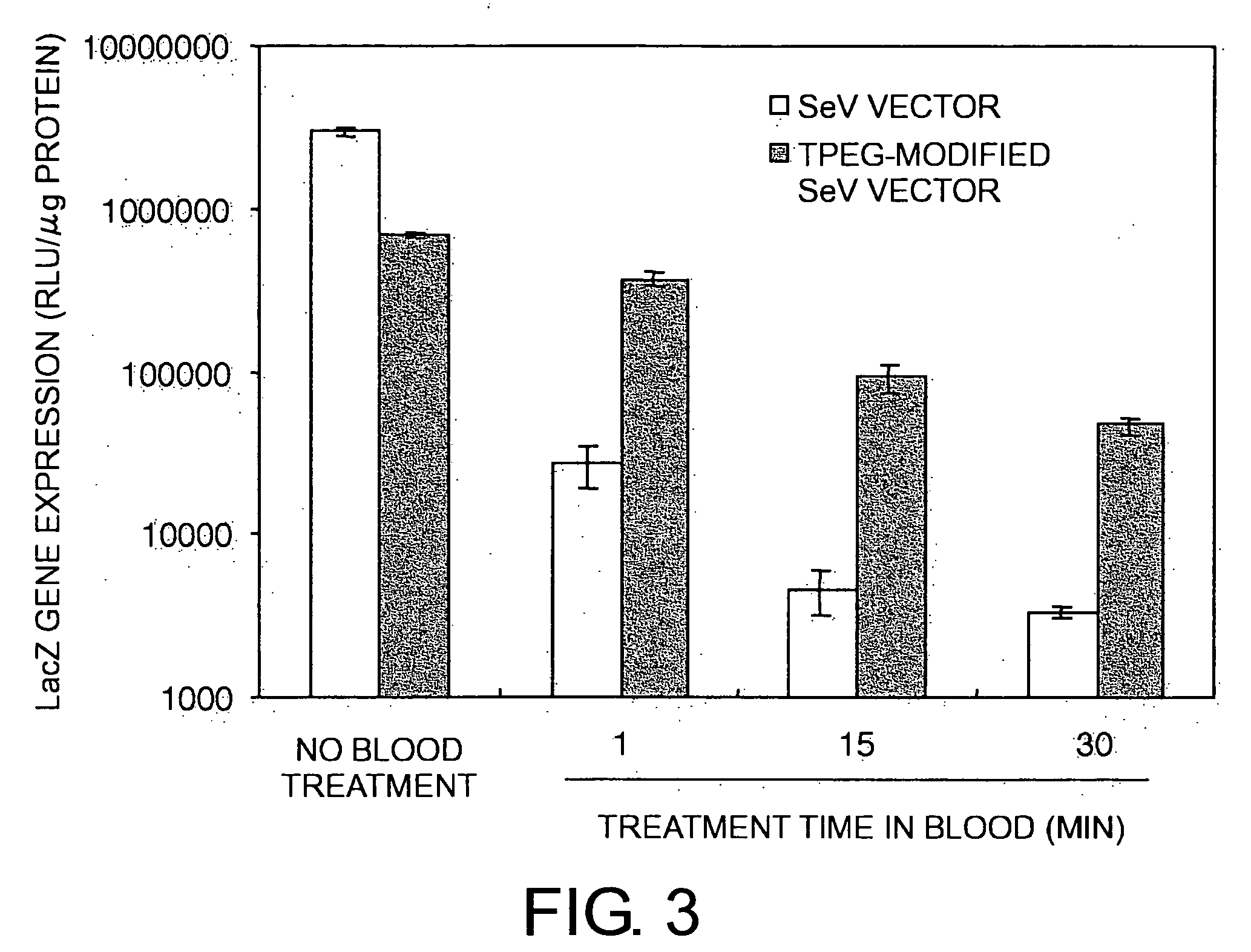
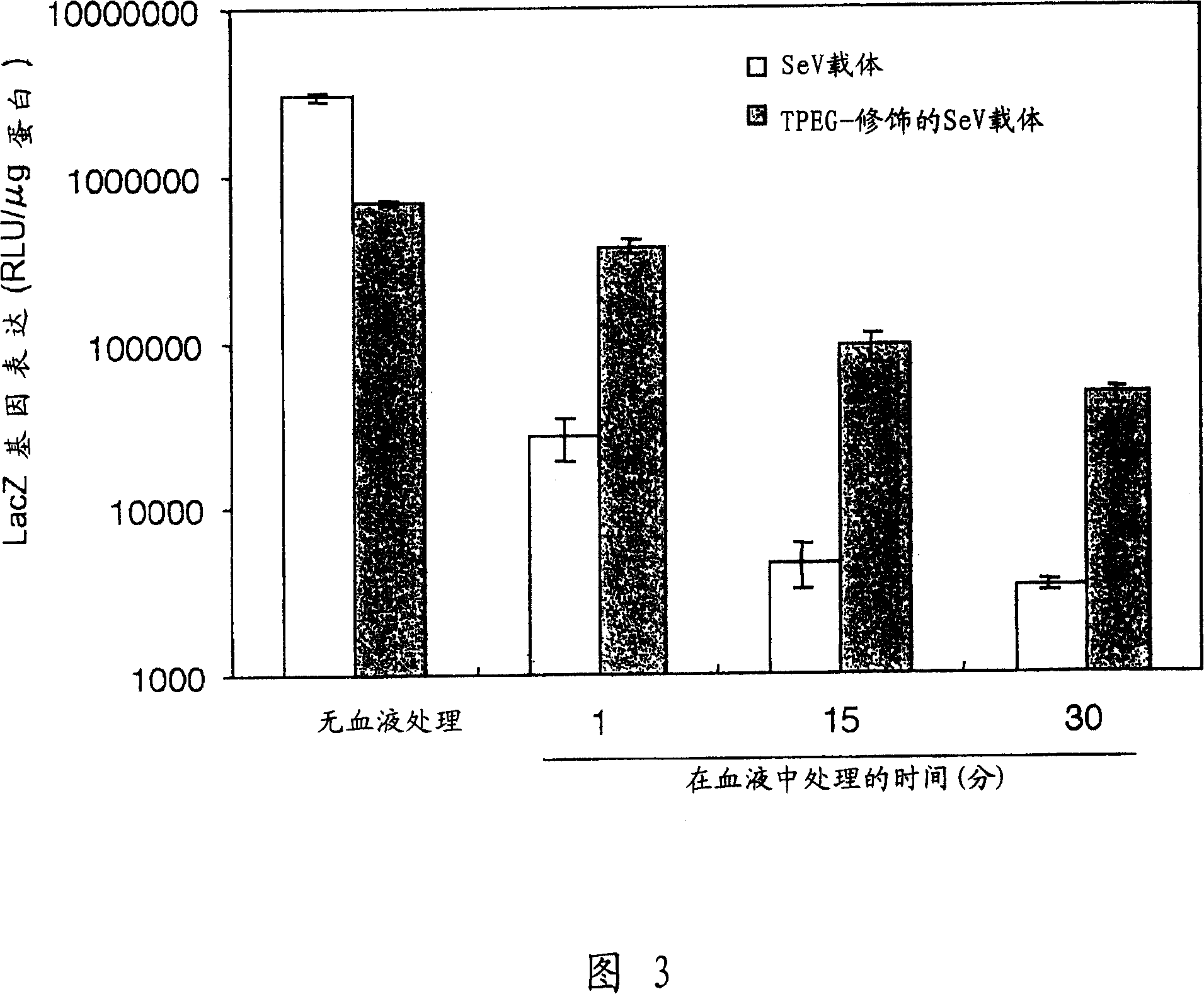
The present invention provides pharmaceutical- or gene-carrier compositions with reduced hemagglutinating activity. By attaching a compound to a minus-strand RNA virus envelope protein having hemagglutinating activity, a pharmaceutical- or gene-carrier composition with lower hemagglutinating activity than a composition to which the compound has not been attached can be successfully constructed. For example, an embodiment of the present invention provides a viral vector whose erythrocyte agglutination activity and hemolytic activity are significantly lowered, and whose stability in blood is remarkably elevated. The pharmaceutical- or gene-carrier compositions provided in this invention can be preferably used for transferring pharmaceuticals or genes in vivo.
Owner:DNAVEC RES
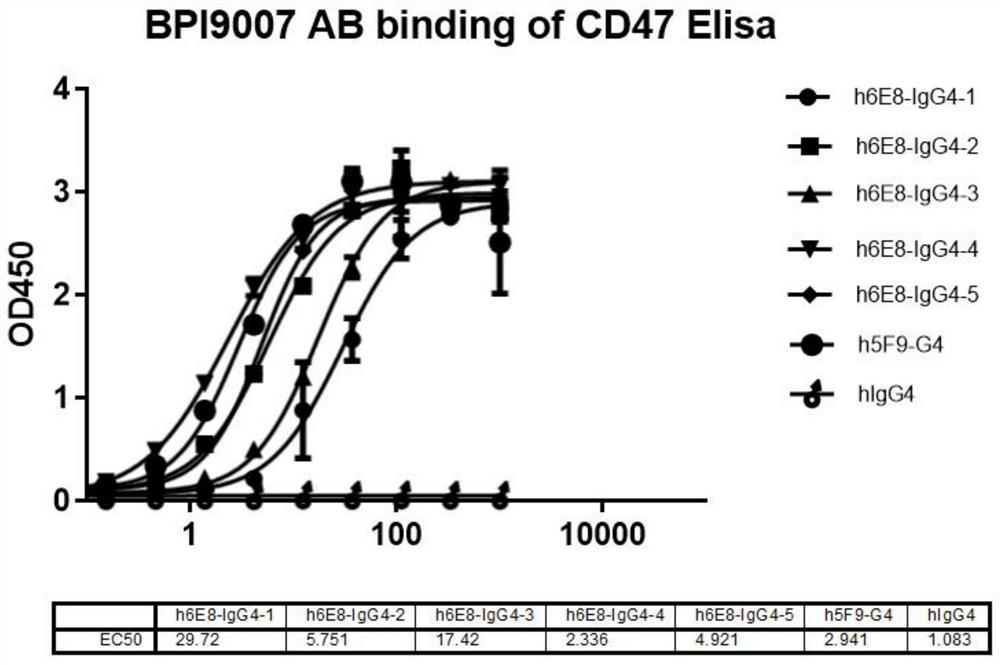
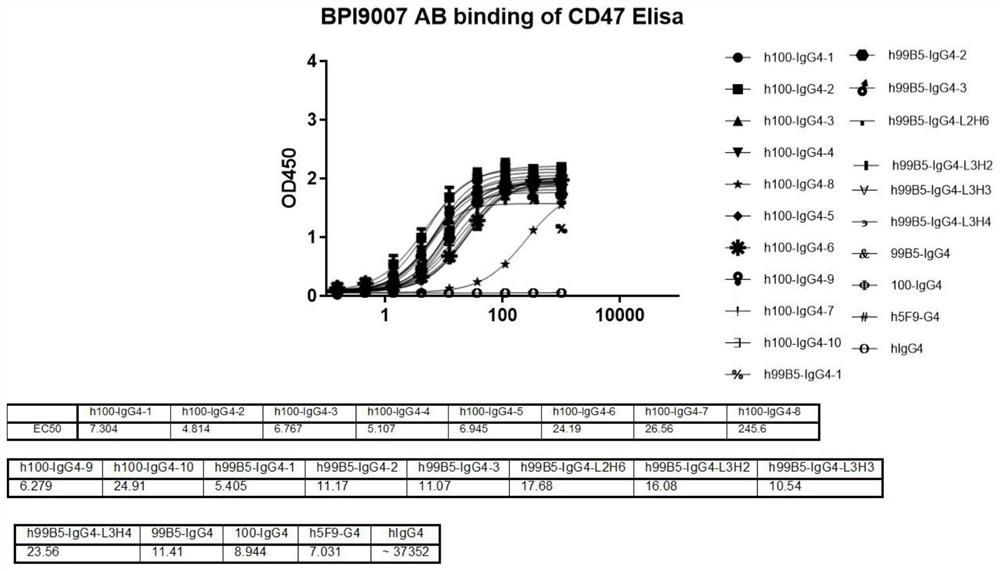
Anti-CD47 antibody and application thereof
ActiveCN110872350AImprove anti-tumor effectLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesAntigen Binding Fragment
The invention relates to the technical field of antibody drugs, in particular to an anti-CD47 antibody or an antigen binding fragment thereof, a pharmaceutical composition containing the anti-CD47 antibody or the antigen binding fragment thereof and their application. The anti-CD47 antibody or the antigen binding fragment thereof provided by the invention has significant antitumor activity, has high affinity with the human CD47 protein, can block the ability of SIRPa binding to CD47 on the cell surface, does not have significant erythrocyte agglutination activity, and can be applied to preparation of antitumor drugs.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Sodium chloride solution for drug reconstitution or dilution
InactiveUS20170021022A1Prevent agglutinationIncrease ionic strengthPeptide/protein ingredientsInorganic non-active ingredientsPresent methodHemolysis
The invention provides methods for preparing pharmaceutical formulations for injection such that upon injection the formulation does not cause erythrocyte agglutination, hemolysis, and / or cell shrinkage. To prevent agglutination, a pharmaceutical formulation ready for injection needs to have a sufficient ionic strength. To prevent hemolysis or cell shrinkage, a pharmaceutical formulation ready for injection needs to be about isotonic with respect to plasma. The invention provides methods that prepare pharmaceutical formulations for injection that have both the sufficient ionic strength to prevent agglutination and the requisite tonicity to prevent significant hemolysis or cell dehydration or shrinkage. The present methods involve the use of sodium chloride solutions that are about 25 mM to about 150 mM for reconstituting lyophilized cakes (or other non-liquid pharmaceutical formulations) into solution or for diluting pharmaceutical formulation solutions.
Owner:WYETH LLC
Recombinant galactose agglutinin-1 two-string protein and preparation method thereof
InactiveCN101830975AEasy to separate and purifyInexpensive separation and purification processPeptide preparation methodsFermentationChromatographic separationProkaryotic expression
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Determining method of content of antigen virus of inactivated vaccine of recombinant bird flu cellgen
ActiveCN102703602AReduce mistakesOperational benchmarks are reliableMicrobiological testing/measurementMicroorganism based processesCanine kidneyWater baths
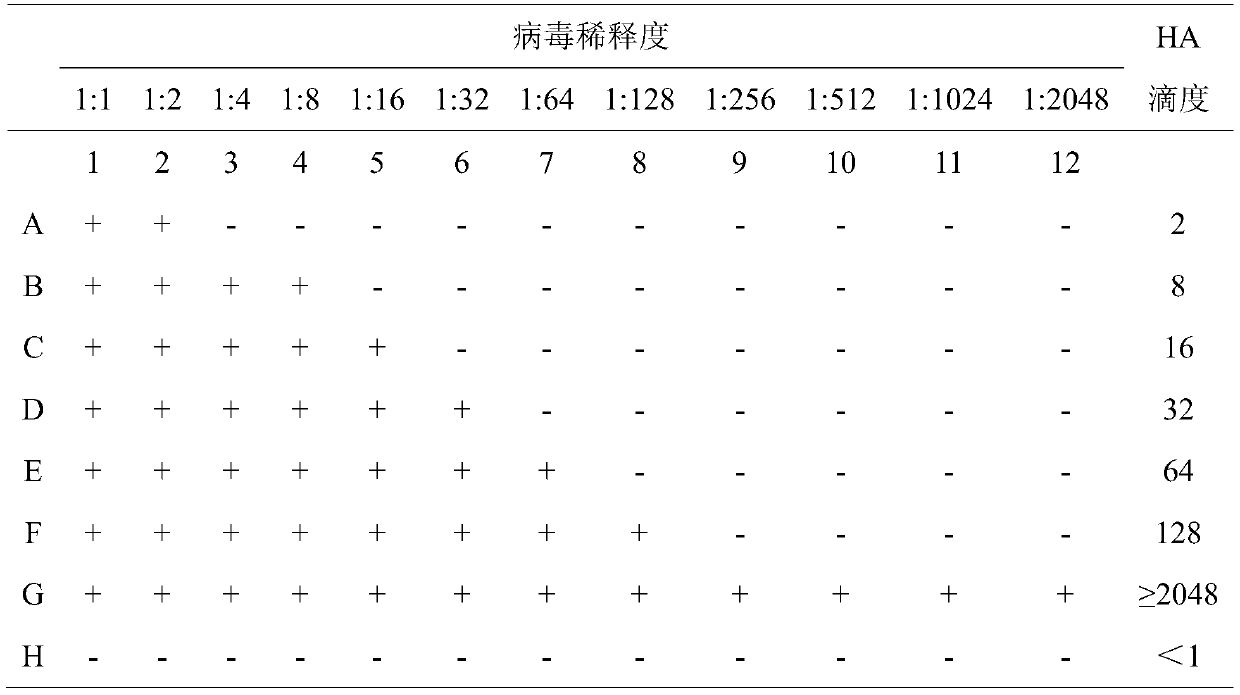
The invention provides a novel determining method of the content of antigen virus of an inactivated vaccine of a recombinant bird flu H5N1 cellgen. The method comprises the following steps of: (1) preparation of MDCK (madin darby canine kidney), wherein 1.5ml of MDCK frozen cell with density of 2.0*10<6> is extracted and put in a water bath at a temperature of 37 DEG C, 10 to 15ml of cell culturefluid is added to culture for 72 hours, the cell is digested with 0.25 percent EDTA (ethylene diamine tetraacetic acid)-pancreatin, the cell nutrient fluid is diluted into MDCK cell suspension of 4.0*10<5>, the MDCK cell suspension is spread to a 96-mesh cell plate in a cell density of 40,000 per pore (0.1ml per pore), the cell plate is put in a CO2 culture case at a temperature of 37 DEG C and humidity of 5 percent to culture for 24 hours to form a compact monolayer for later use; and (2) inoculation of a recombinant bird flu H5N1 antigen, wherein recombinant bird flu H5N1 cellgen antigens in different batches are extracted and respectively added into the compact monolayer with 100mu l in each pore, and 100mu l of cell maintenance fluid is added to culture for four days, and a TCID50 (50% tissue culture infection dose) value is calculated by the number of cytopathic pores in a Reed-Muench method. The method is applicable to a cellgen vaccine for detecting the virus content by an erythrocyte agglutination test, which means that different viruses use corresponding sensitive cells.
Owner:吉林冠界生物技术有限公司
Influenza hemagglutination inhibition test detection method
PendingCN111323581ASolve the difficulty that the experiment cannot be carried outAvoid difficultiesBlood/immune system cellsBiological testingAntigenic analysisSpecific lectin
The invention belongs to the technical field of microbiological detection, and discloses an influenza hemagglutination inhibition test detection method which comprises the following steps: pretreatingstandard reference antiserum to remove non-specific inhibin and non-specific lectin in the serum; preparing an erythrocyte suspension; determining the hemagglutination titer of the influenza virus strain; preparing four hemagglutination unit antigens for an erythrocyte agglutination inhibition test; rechecking and titrating four hemagglutination unit antigens; and performing influenza virus identification and antigen analysis by using an HAI method to obtain the serum titer of the detected serum. According to the detection method, chicken erythrocyte, turkey erythrocyte and guinea pig erythrocyte are used as raw materials to prepare erythrocyte suspension, animal erythrocyte is used for replacing human erythrocyte, and the difficulty that an experiment cannot be conducted due to lack of erythrocyte is solved.
Owner:广州鸿泉生物科技有限公司
Cell analyzer, erythrocyte agglutination amount measuring method and erythrocyte agglutination amount measuring system of the cell analyzer
ActiveCN104515726AImprove measurement accuracyParticle size analysisIndividual particle analysisTest sampleRed blood cell
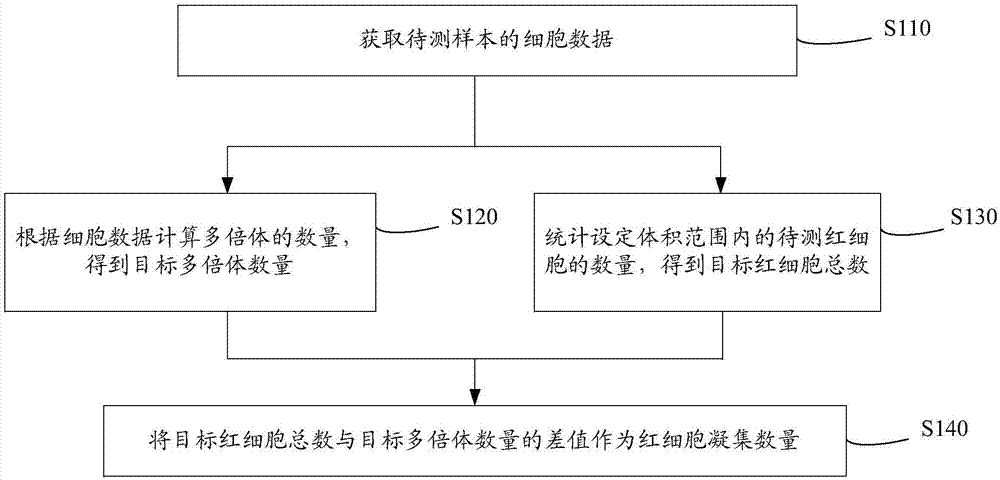
A cell analyzer, an erythrocyte agglutination amount measuring method and an erythrocyte agglutination amount measuring system of the cell analyzer. The erythrocyte agglutination amount measuring method comprises following steps: (1) obtaining cell data of a to-be-test sample; (2) calculating the amount of polyploidies according to the cell data to obtain the amount of the targeted polyploidies; (3) counting the amount of to-be-test erythrocytes in a preset volume to obtain a total amount of the targeted erythrocytes; and (4) calculating a different value between the total amount of the targeted erythrocytes and the amount of the targeted polyploidies. According to the method, the erythrocyte agglutination amount in the to-be-test sample is obtained by calculating the different value between the total amount of the targeted erythrocytes and the amount of the targeted polyploidies, so that determination according to the contents of certain components in the cells is unnecessary. The method, compared with a conventional erythrocyte agglutination amount measuring method, is improved in measurement accuracy and is simple, convenient and quick.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
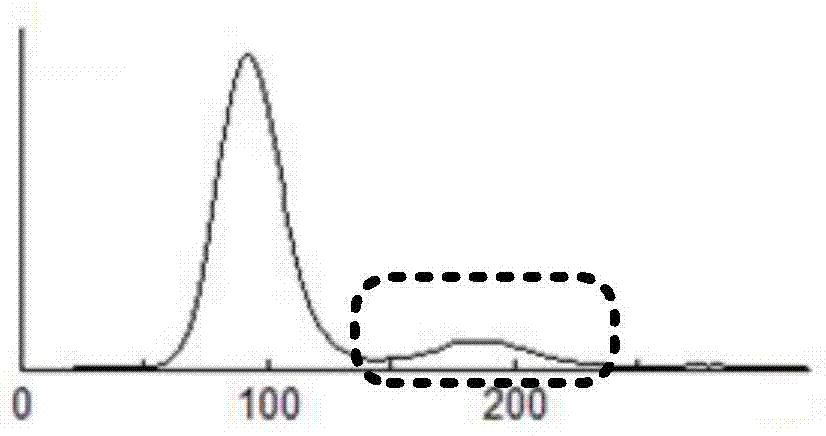
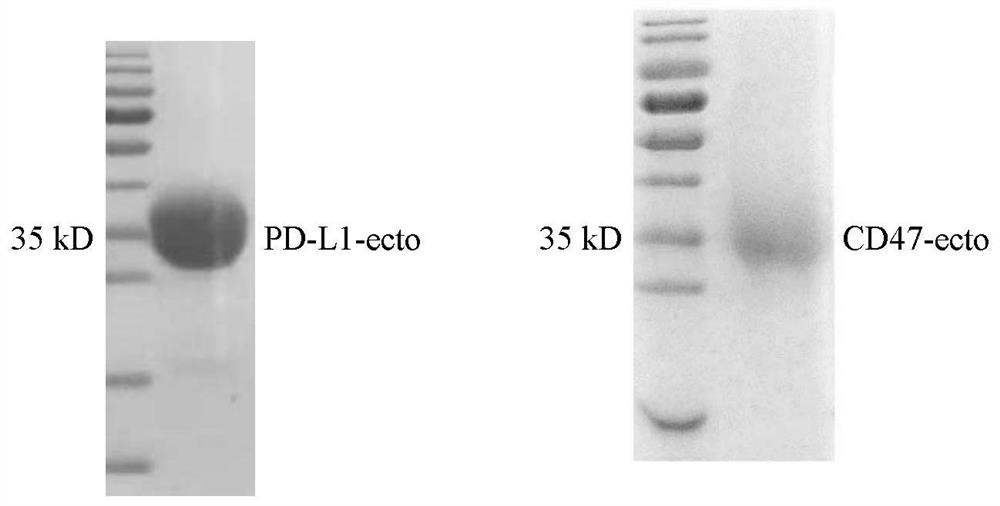
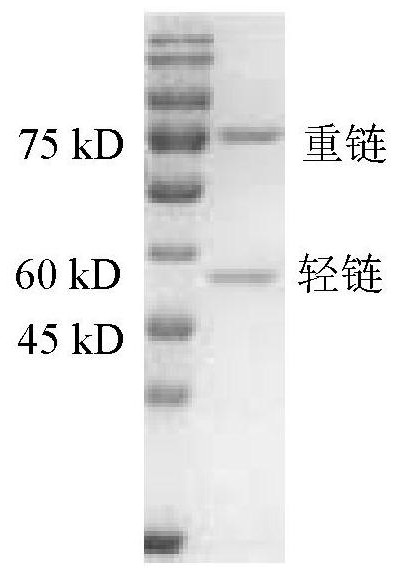
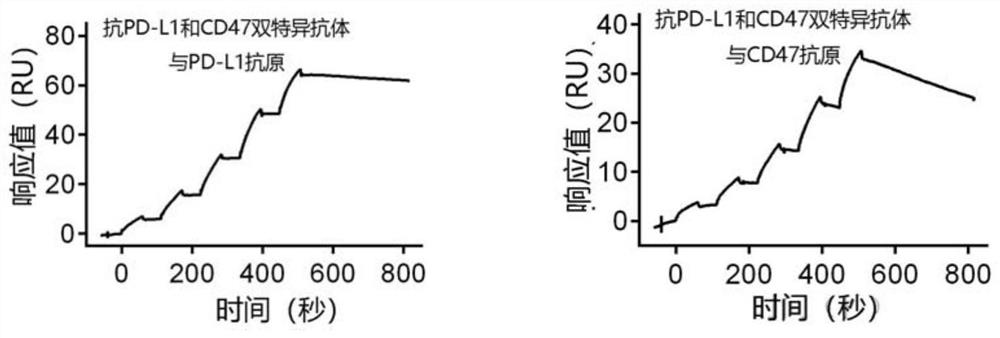
Non-erythrocyte agglutination anti-PD-L1/CD47 bispecific antibody and application thereof in anti-tumour treatment
ActiveCN113372449ADoes not cause agglutinationActivate phagocytosisHybrid immunoglobulinsAntibody ingredientsAntigenAntiendomysial antibodies
The invention relates to a non-erythrocyte agglutination anti-PD-L1 / CD47 bispecific antibody and application thereof in anti-tumour treatment. Specifically, the invention provides an anti-PD-L1 / CD47 bispecific antibody or an antigen binding fragment thereof; the anti-PD-L1 / CD47 bispecific antibody can be specifically bound with PD-L1 and CD47 molecules; binding of PD-L1 and PD-1 and binding of CD47 and SIRP alpha can be blocked at the same time after binding; T cells and macrophages are further activated to achieve the biological effects of resisting tumours and the like; and meanwhile, the antibody does not cause erythrocyte agglutination.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Humanized CD47 antibody or antigen binding fragment thereof and application
ActiveCN112679611ASpecific target specificityGrowth inhibitionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntiendomysial antibodies
The invention provides a humanized CD47 antibody or an antigen binding fragment thereof and application, the humanized CD47 antibody or the antigen binding fragment thereof is low in toxicity and efficient, erythrocyte agglutination does not occur in vitro, and erythrocyte removal cannot be caused. In addition, the humanized CD47 antibody provided by the invention shows extremely weak level of low binding or non-binding with platelets and red blood cells, and shows more specific targeting specificity on CD47+ tumor cells. The humanized CD47 antibody or the antigen binding fragment thereof provided by the invention can effectively block the binding of CD47 and SIRP alpha in function, and activate and mediate the phagocytic activity of macrophages to tumor cells.
Owner:BETA PHARM SUZHOU LTD
Method of detecting red cell antigen-antibody reactions
InactiveUS7919266B2High test sensitivityAntibody uptake by the red cells is noticeably increasedBioreactor/fermenter combinationsBiological substance pretreatmentsSerum igeTest sample
A process for the detection of antibodies in a test sample by preparing a suspension of erythrocytes with a test serum or plasma by mixing a test serum or plasma with erythrocytes; incubating the suspension of erythrocytes at a temperature of from 37° C. to 45° C. to bind any antibodies in the test serum or plasma to the surface of said erythrocytes; combining the suspension of erythrocytes with an amount of a solution of a macromolecule which is effective to agglutinate the erythrocytes; packing the resultant red cell agglutinates by centrifuging the suspension of erythrocytes; and, determining the presence of anti-erythrocyte antibodies by observing if antibody-dependent erythrocyte agglutination has occurred.
Owner:CLAVINA DIAGNOSTICS
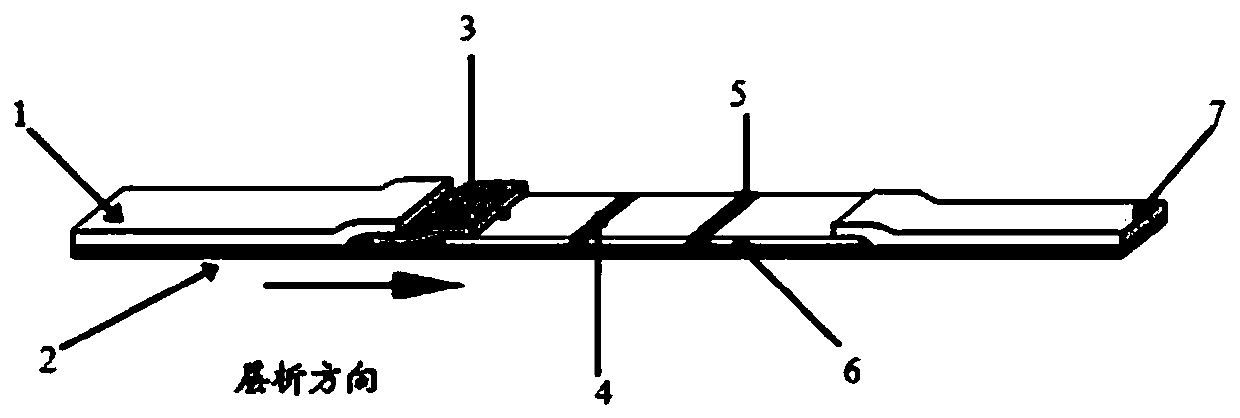
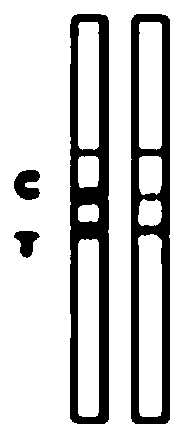
Colloidal gold detection card for quickly detecting rabbit hemorrhagic disease virus (RHDV) and preparation method thereof
ActiveCN109856397ASolve the problem of not being able to effectively distinguish between seropositives caused by rabbit plague virus infection and seropositives after vaccinationEffective controlMaterial analysisLiver and kidneyTest sample
The invention relates to a colloidal gold detection card for quickly detecting rabbit hemorrhagic disease virus (RHDV) and a preparation method thereof. The method comprises the following steps: 1) preparation of paired monoclonal antibodies against RHDV, wherein the preparation processes comprise immune procedure, cell fusion, hybridoma screening and cloning and antibody preparation and purification; and 2) preparation of the colloidal gold detection card for quickly detecting rabbit hemorrhagic disease virus (RHDV), wherein the preparation processes comprise preparation of a colloidal gold monoclonal antibody, sample pad treatment, membrane spraying, determination of C and T lines, treatment of tested samples and performance determination. The method can quickly, sensitively and accurately detect RHDV infection in the liver and kidney of a rabbit, and overcomes the problem that erythrocyte agglutination test is the main method to detect the RHDV in China, thereby greatly reducing detection time and providing convenience for on-site detection.
Owner:SHANDONG LVDU BIO SICIENCE & TECH
Chicken embryo virus liquid virus titer measurement method
ActiveCN104774973AReduce mistakesImprove accuracyMicrobiological testing/measurementMicroorganism based processesYolkEmbryo
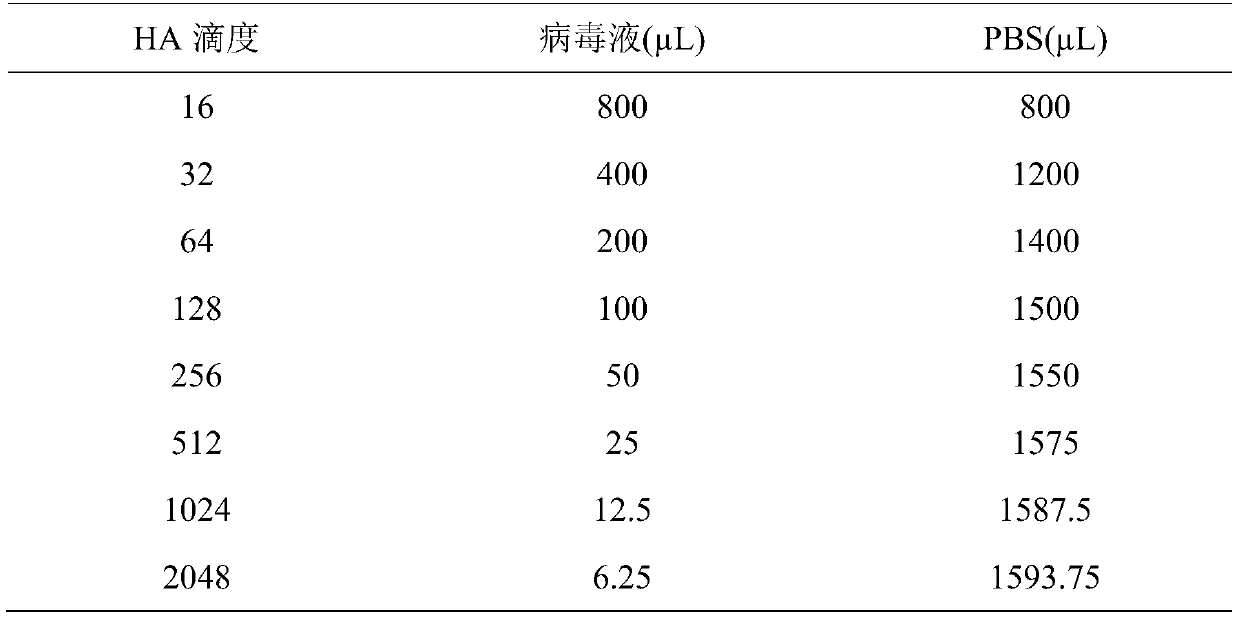
The invention discloses a chicken embryo virus liquid virus titer measurement method. The method includes the following steps of chicken embryo inoculation position determination, sample dilution, inoculation, hole sealing, chicken embryo post-incubation, cold egg acquisition of chicken embryos, chicken embryo erythrocyte agglutination titer measurement and EID50 calculation. By the adoption of the method, the chicken embryo inoculation position is changed, the amount of virus liquid injected in an allantoic cavity is more accurate, measurement result errors are reduced, in the hole sealing process, quick-setting glue is used for replacing wax adopted for hole sealing traditionally, the hole sealing speed is high, hole sealing operation is safe, in the cold egg acquisition process, a method of gradual transition of different temperatures is selected, the situation that yolks are broken due to chicken embryo struggles caused by the large temperature difference is avoided, and therefore the accuracy of a virus titer measurement result is improved.
Owner:兆丰华生物科技(南京)有限公司
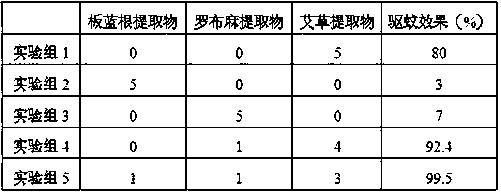
Mosquito repelling and antibacterial cellulose fiber containing wormwood component and preparation method thereof
ActiveCN106906530BReduce churnReduce alkali decompositionArtificial filaments from viscoseArtificial filaments from cellulose derivativesSilicic acidCandida albicans
Owner:青岛邦特生态纺织科技有限公司
Drug- or gene-carrier composition having lowered hemagglutinin activity
InactiveCN100358581CEasy to useImprove stabilitySsRNA viruses negative-senseVectorsDrug carrierRed Cell
The present invention provides pharmaceutical- or gene-carrier compositions with reduced hemagglutinating activity. By attaching a compound to a minus-strand RNA virus envelope protein having hemagglutinating activity, a pharmaceutical- or gene-carrier composition with lower hemagglutinating activity than a composition to which the compound has not been attached can be successfully constructed. For example, an embodiment of the present invention provides a viral vector whose erythrocyte agglutination activity and hemolytic activity are significantly lowered, and whose stability in blood is remarkably elevated. The pharmaceutical- or gene-carrier compositions provided in this invention can be preferably used for transferring pharmaceuticals or genes in vivo.
Owner:DNAVEC RES
Cell analyzer and its red blood cell agglutination alarm method and system
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Antioxidant Peptides and Their Genes of Frog Rana and Their Application in Pharmaceuticals
ActiveCN106432425BSimple structureEasy to synthesizeCosmetic preparationsNervous disorderCyclic peptideDisulfide bonding
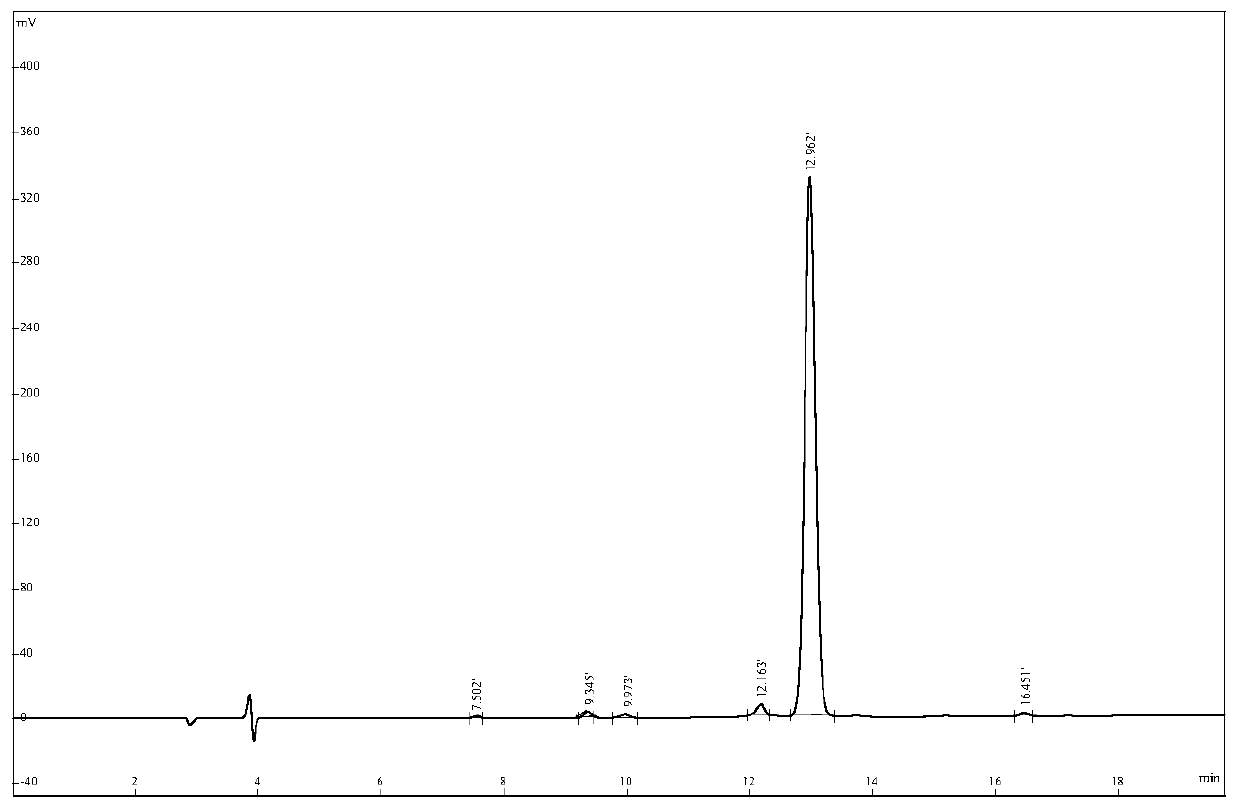
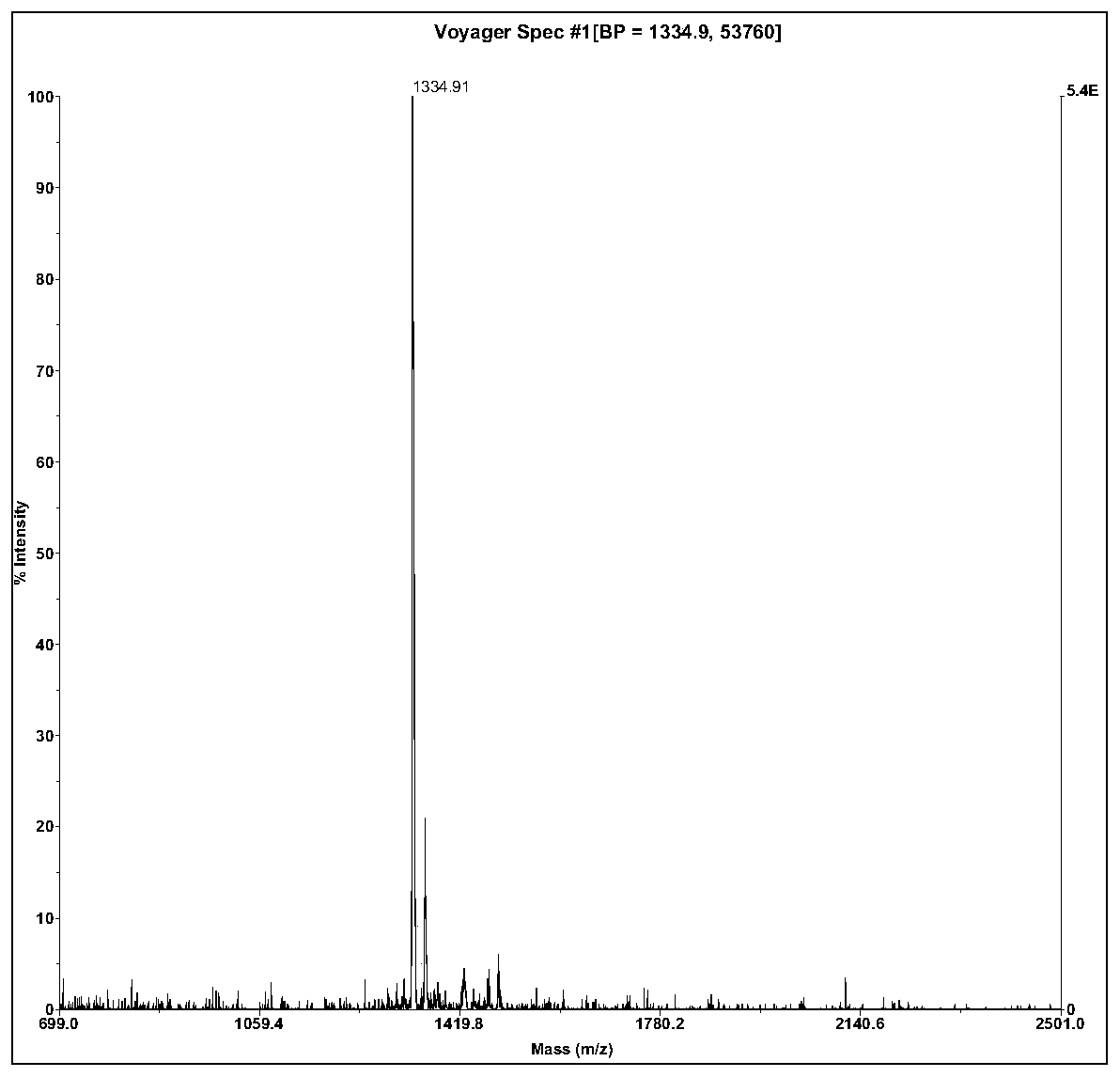
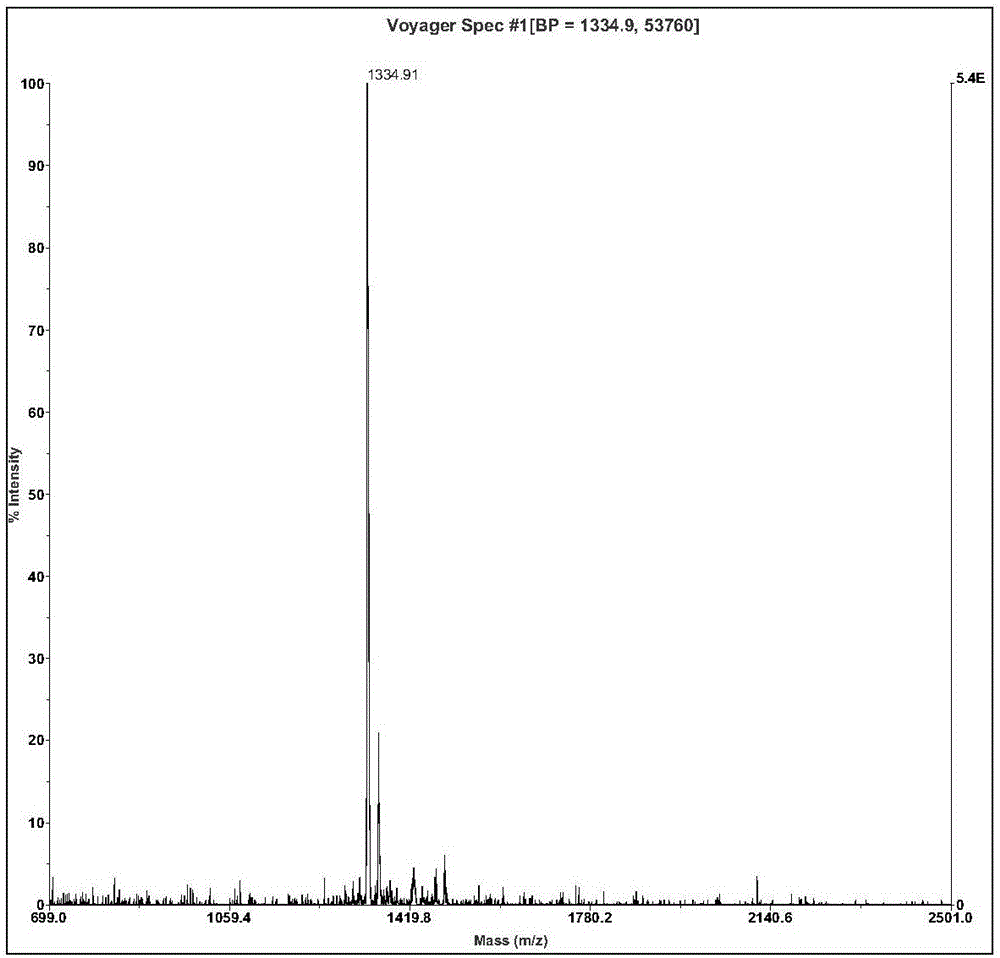
The invention relates to active polypeptide and a gene thereof, and application of the active polypeptide to pharmacy. Microhyla pulchra antioxidation peptide is cyclopeptide consisting of 12 pieces of amino acid, wherein the molecular weight is 1334.91 daltons; the isoelectric point is 8.002; the amino acid sequence is SEQ ID NO.1; and the third-position cysteine and the eleventh-position cysteine of the polypeptide form intramolecular disulfide bonds. The gene sequence of the microhyla pulchra antioxidation peptide consists of SEQ ID NO.4, and the gene encoded with the functional mature microhyla pulchra antioxidation peptide is the 187th to the 222nd position nucleotide. The gene of the microhyla pulchra antioxidation peptide deduces the mature functional polypeptide amino acid sequence, and the synthesized microhyla pulchra antioxidation peptide has strong erythrocyte agglutination and anti-oxidation functions.
Owner:SOUTHERN MEDICAL UNIVERSITY
Novel coronavirus COVID-2019 detection card and preparation method thereof
PendingCN111474352AImprove accuracyEasy to detectBiological material analysisAgainst vector-borne diseasesSpecific iggEngineering
The invention discloses a novel coronavirus COVID-2019 detection card and a preparation method thereof. The novel coronavirus COVID-2019 detection card comprises a card supporting layer attached to detection test paper, a sample unit positioned on the card supporting layer, a preprocessing unit positioned on the card supporting layer, a marker unit positioned on the card supporting layer, a detection unit positioned on the card supporting layer, and a recycling unit positioned on the card supporting layer; the sample units correspond to the sample holes; the pretreatment unit corresponds to the pretreatment hole; a hollow or transparent sealed detection window is arranged at the position, corresponding to the detection unit, of the shell; the erythrocyte agglutination layer is provided with erythrocyte agglutinin; the endogenous interfering substance treatment layer is provided with an endogenous interfering substance conjugate; the exogenous interfering substance treatment layer is provided with an exogenous interfering substance chelating agent; a non-specific IgM antibody treatment layer is provided with an IgM chelating agent; a non-specific IgG antibody treatment layer has anIgG precipitant. The problem that interfering substances influence the detection result of the novel coronavirus is solved, and the detection accuracy is improved.
Owner:北京乐普诊断科技股份有限公司
Microhyla pulchra antioxidation peptide and gene thereof, and application of microhyla pulchra antioxidation peptide to pharmacy
ActiveCN106432425ASimple structureSignificant erythrocyte agglutinationCosmetic preparationsNervous disorderNucleotideMicrohyla pulchra
The invention relates to active polypeptide and a gene thereof, and application of the active polypeptide to pharmacy. Microhyla pulchra antioxidation peptide is cyclopeptide consisting of 12 pieces of amino acid, wherein the molecular weight is 1334.91 daltons; the isoelectric point is 8.002; the amino acid sequence is SEQ ID NO.1; and the third-position cysteine and the eleventh-position cysteine of the polypeptide form intramolecular disulfide bonds. The gene sequence of the microhyla pulchra antioxidation peptide consists of SEQ ID NO.4, and the gene encoded with the functional mature microhyla pulchra antioxidation peptide is the 187th to the 222nd position nucleotide. The gene of the microhyla pulchra antioxidation peptide deduces the mature functional polypeptide amino acid sequence, and the synthesized microhyla pulchra antioxidation peptide has strong erythrocyte agglutination and anti-oxidation functions.
Owner:SOUTHERN MEDICAL UNIVERSITY
A kind of avian influenza h9n2 subtype virus strain and application thereof
ActiveCN103789273BStrong specificityImprove featuresSerum immunoglobulinsImmunoglobulins against virusesFowlSerum ige
The invention provides an avian influenza H9N2 subtype virus strain with the preservation number of CGMCC No.6757. The avian influenza H9N2 subtype virus strain provided by the invention has favorable specificity and immunogenicity, the erythrocyte agglutination effect of allantoic fluid can not be inhibited by anti-NDV (Newcastle Disease Virus) positive serum, anti-EDS76 positive serum, anti-M41 positive serum, anti-H5 subtype avian influenza virus positive serum and anti-H7 subtype avian influenza virus positive serum, but can be inhibited by H9 subtype avian influenza virus positive serum, and immunized chicken can generate an HI (Hemagglutination Inbition) antibody which is specific for H9. The avian influenza H9N2 subtype virus strain provided by the invention can be used as a vaccine strain for preventing H9 subtype avian influenza of birds, and can be used for authenticating avian influenza viruses and researching epidemiology so as to have favorable market application prospects.
Owner:诗华动保科技(北京)有限公司
Recombinant galactose agglutinin-1 two-string protein and preparation method thereof
InactiveCN101830975BImprove biological activityHigh agglutination activityPeptide preparation methodsFermentationChromatographic separationProkaryotic expression
The invention discloses a recombinant galactose agglutinin-1 two-string protein and a preparation method thereof. The method specifically comprises the following steps of: providing a recombinant Galectin-1 protein with bioactivity through a biotechnological method; respectively transfecting BL21 to construct engineering bacteria through constructing prokaryotic expression vectors pET-22b(+)-Gal-1 and pET-22b(+)-Gal-1 (2); and then obtaining the recombinant Galectin-1 monomer protein or a recombinant Galectin-1 two-string protein through IPTG (Isopropyl Beta-D-1-Thiogalactopyranoside) induction expression and the chromatographic separation and passivation after dividing the bacteria. Through an erythrocyte agglutination test, it is proved that both the two recombinant Galectin-1 proteins have obvious bioactivity, and the bioactivity of the Galectin-1 two-string protein is higher than that of the Galectin-1 monomer protein.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Monoclonal antibody specifically binding to human CD47 and application thereof
PendingCN114539404AImprove anti-tumor efficacyHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMouse tumorErythroid cell
The invention provides a monoclonal antibody specifically binding to human CD47 or a fragment thereof, which can bind to CD47 on the surface of a cell and block binding of SIRP alpha to CD47 on the surface of the cell. The affinity KD value on the recombinant human CD47 is between 1 * 10 <-10 > and 8 * 10 <-8 >, and the tumor inhibition activity and the adverse reaction activity can be balanced. In the aspect of tumor inhibition activity, the antibody can inhibit tumor growth in a CD47 / SIRP alpha double-transgenic mouse tumor-bearing hCD47-MC38 subcutaneous transplanted tumor model, and can enhance in-vivo cell phagocytosis and prolong the lifetime of a mouse in a mouse leukemia model of transplanted human acute B lymphocytic leukemia; in the aspect of adverse reaction activity, the antibody does not have or only has reduced erythrocyte agglutination activity, and does not have a significant effect or only has an over-sexual effect on erythrocytes, platelets and hemoglobin.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD
Chicken Newcastle Disease Virus Attenuated Strain, Inactivated Vaccine and Its Application
Owner:NORTHEAST FORESTRY UNIVERSITY
CD47 antibody or its immunologically active fragment and its application
ActiveCN111454359BDoes not cause clearingSpecific target specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMedicine
The present invention provides a novel CD47 antibody or immunologically active fragment thereof, or a composition comprising the antibody / immunoactive fragment. The CD47 antibody does not cause erythrocyte agglutination, and exhibits a very weak level of low or no binding to erythrocytes and platelets. The present invention provides nucleic acid, expression vector and host cell encoding the CD47 antibody or its immunologically active fragment. In addition, the present invention provides the use of the CD47 antibody or the pharmaceutical composition containing the CD47 antibody / immune active fragment for preparing a medicine for treating CD47-mediated diseases.
Owner:BETA PHARM SUZHOU LTD
Application of nicotinamide in preparation of medicine for resisting porphyromonas gingivalis
ActiveCN113908157AWide variety of sourcesEasy to manufactureAntibacterial agentsOrganic active ingredientsERYTHROCYTES AGGLUTINATIONNicotinamide
The invention discloses an application of nicotinamide in preparation of a medicine for resisting porphyromonas gingivalis. The nicotinamide disclosed by the invention is amide of nicotinic acid, is a clinical common medicine, is low in cost, wide in source and easy to prepare, and has good biological safety performance; and according to the application disclosed by the invention, the growth of the porphyromonas gingivalis, the erythrocyte agglutination capability and the activities of arginine-specific gingival protease and lysine-specific gingival protease of the nicotinamide serving as the inhibitor are measured, and experiments prove that the nicotinamide has an obvious inhibition effect on the periodontal disease toxicity caused by the porphyromonas gingivalis, is good in biological safety, tcan be applied to preparation of drugs for inhibiting porphyromonas gingivalis, especially to preparation of drugs for preventing and treating periodontitis, and can be used as a novel drug for preventing and treating periodontitis.
Owner:SICHUAN UNIV
A kind of assay method of virus titer of chicken embryo virus liquid
ActiveCN104774973BReduce mistakesImprove accuracyMicrobiological testing/measurementMicroorganism based processesYolkEmbryo
The invention discloses a chicken embryo virus liquid virus titer measurement method. The method includes the following steps of chicken embryo inoculation position determination, sample dilution, inoculation, hole sealing, chicken embryo post-incubation, cold egg acquisition of chicken embryos, chicken embryo erythrocyte agglutination titer measurement and EID50 calculation. By the adoption of the method, the chicken embryo inoculation position is changed, the amount of virus liquid injected in an allantoic cavity is more accurate, measurement result errors are reduced, in the hole sealing process, quick-setting glue is used for replacing wax adopted for hole sealing traditionally, the hole sealing speed is high, hole sealing operation is safe, in the cold egg acquisition process, a method of gradual transition of different temperatures is selected, the situation that yolks are broken due to chicken embryo struggles caused by the large temperature difference is avoided, and therefore the accuracy of a virus titer measurement result is improved.
Owner:兆丰华生物科技(南京)有限公司
Newcastle disease attenuated sugar vitrified vaccine and preparation method thereof
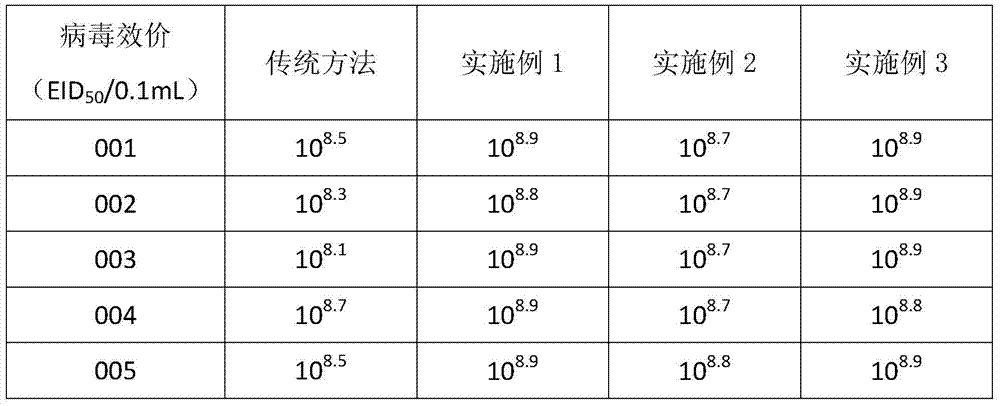
PendingCN111588844AGuaranteed performanceSsRNA viruses negative-sensePowder deliveryPenicillinVeterinary Drugs
The invention belongs to the technical field of veterinary drugs, and particularly relates to a Newcastle disease attenuated sugar vitrified vaccine and a preparation method thereof. The preparation raw materials of the vaccine include hatching eggs, reagents and seed virus. The reagents comprise any one or more of PBS (phosphate buffer solution) or normal saline and any one or more of a penicillin solution or a streptomycin solution; and the seed virus comprises any one or more of an HB1 strain, an F strain, a LaSota strain, a LaSota-Clone30 strain or an N79 strain, and propiolactone. The vaccine also comprises a raw material reactant for preparing the vitrified vaccine, and the reactant is specifically one or more of trehalose or agarose. According to the present invention, an erythrocyte experiment is directly performed on the generated vaccine, and the optimal attenuated virus can be found according to the optimal erythrocyte agglutination point so as to effectively ensure the performance of the vaccine.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com