Microhyla pulchra antioxidation peptide and gene thereof, and application of microhyla pulchra antioxidation peptide to pharmacy
A technology for antioxidant peptides and drugs, which is applied in the field of biomedicine to achieve significant red blood cell agglutination and antioxidant effects, convenient artificial synthesis and simple structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1, Cloning of Antioxidant Peptide Gene of Rana japonica:
[0036] 1. Extraction of total RNA from the skin of Frog japonica: the live Frog japonica was cleaned with water, put into liquid nitrogen and quick-frozen for 4h, got skin tissue, weighed, got 300mg skin tissue, added 10m1 total RNA extraction buffer (Trizol solution, the U.S. GIBCOBRL company product), homogenized in 20m1 glass homogenizer for 30min. Add an equal volume of phenol / chloroform solution, mix vigorously, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, and discard the precipitate. Add an equal volume of isopropanol to the supernatant, place at room temperature for 10 minutes, centrifuge at 12,000 rpm for 10 minutes at 4°C, wash the precipitate once with 75% ethanol, and dry it.
[0037] II. Purification of Frog skin mRNA: The mRNA of Frog skin was isolated and purified by the American PROMEGA company. mRNA Isolation Systems Kit. The details are a...
Embodiment 2
[0049] Example 2, preparation of antioxidative peptides of Rana japonica:
[0050] Ⅰ. The preparation method of the anti-oxidative peptide of Rana japonica: according to the anti-oxidative peptide gene of Rana japonica, the amino acid sequence of the mature live secreted peptide encoded by the function is deduced, and then the polypeptide is synthesized by an automatic polypeptide synthesizer. The formation of disulfide bond adopts the air oxidation method, specifically dissolving the polypeptide in the flask according to 0.1mg / ml in 0.1% acetic acid solution, titrating with ammonium hydroxide to pH 7.8, and then stirring overnight at room temperature. Desalted and purified by HPLC reverse phase C18 column chromatography. Liquid A is 0.05% TFA+2% CH during purification 3 CN, liquid B is 0.05% TFA+90% CH 3 CN, the B liquid gradient is 30-50% within 20 minutes, the detection wavelength is 220nm, and the polypeptide appears at 12.962 minutes.
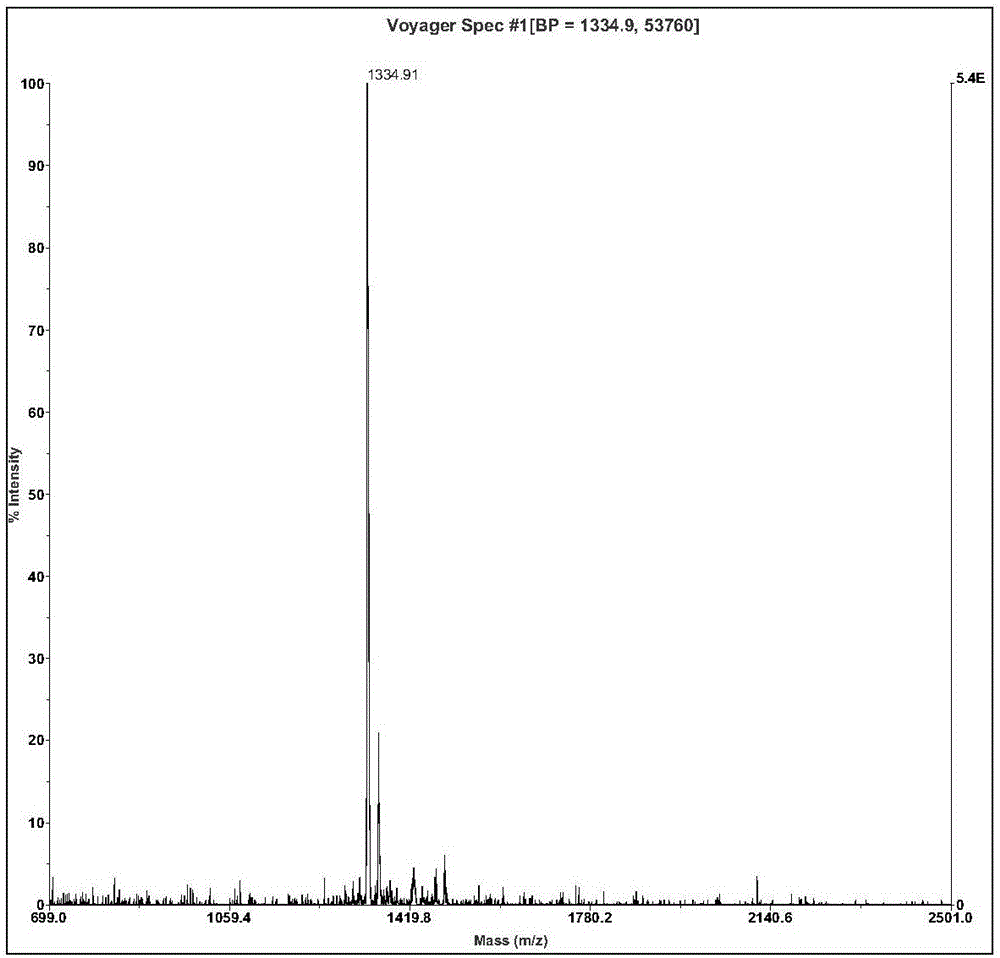
[0051] Ⅱ. The molecular weight i...
Embodiment 3
[0054] Example 3, Activity Experiment of Antioxidant Peptide of Frog Rana
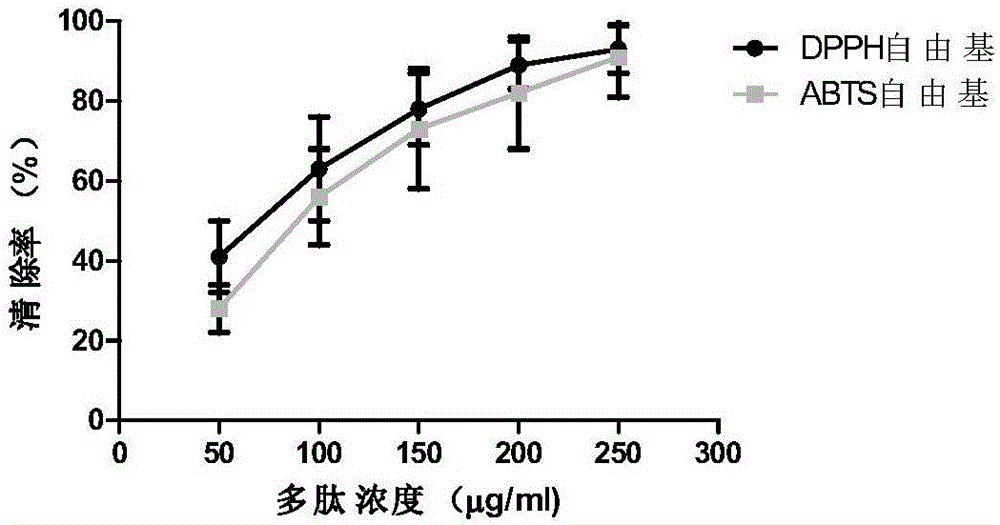
[0055] Ⅰ. Determination of antioxidant capacity
[0056] 1) Determination of DPPH free radical scavenging ability
[0057] DPPH (1,1-diphenyl-2-picryl-hydrazyl) free radical scavenging rate assay was used to study antioxidant peptides. Prepare a DPPH ethanol solution with a concentration of 1×10-5 mol / L, and store it in the dark. Add 2ml, 0.1mM DPPH absolute ethanol solution into a clean test tube containing 2ml of different enzymatic hydrolysis samples, and mix well. After standing at room temperature for 30 minutes, measure the absorbance at 517nm, the smaller the absorbance value, the stronger the ability to scavenge free radicals.
[0058] Clearance (%) = [1-(A i -A j ) / A 0 】*100%
[0059] In the formula, A 0 2ml, 0.1mM DPPH absolute ethanol solution + 2ml sample reagent, blank control, A i 2ml, 0.1mM DPPH absolute ethanol solution + 2ml sample, A j 2ml of absolute ethanol + 2ml of sample...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com