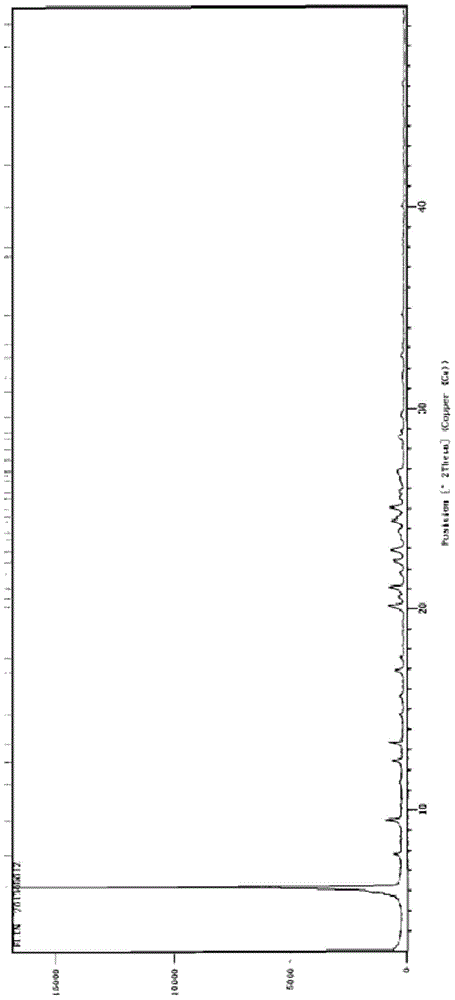
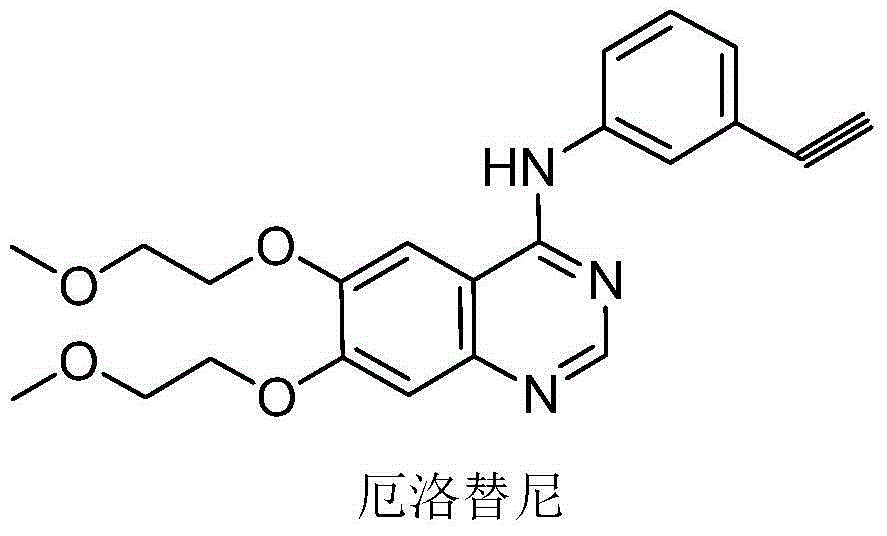
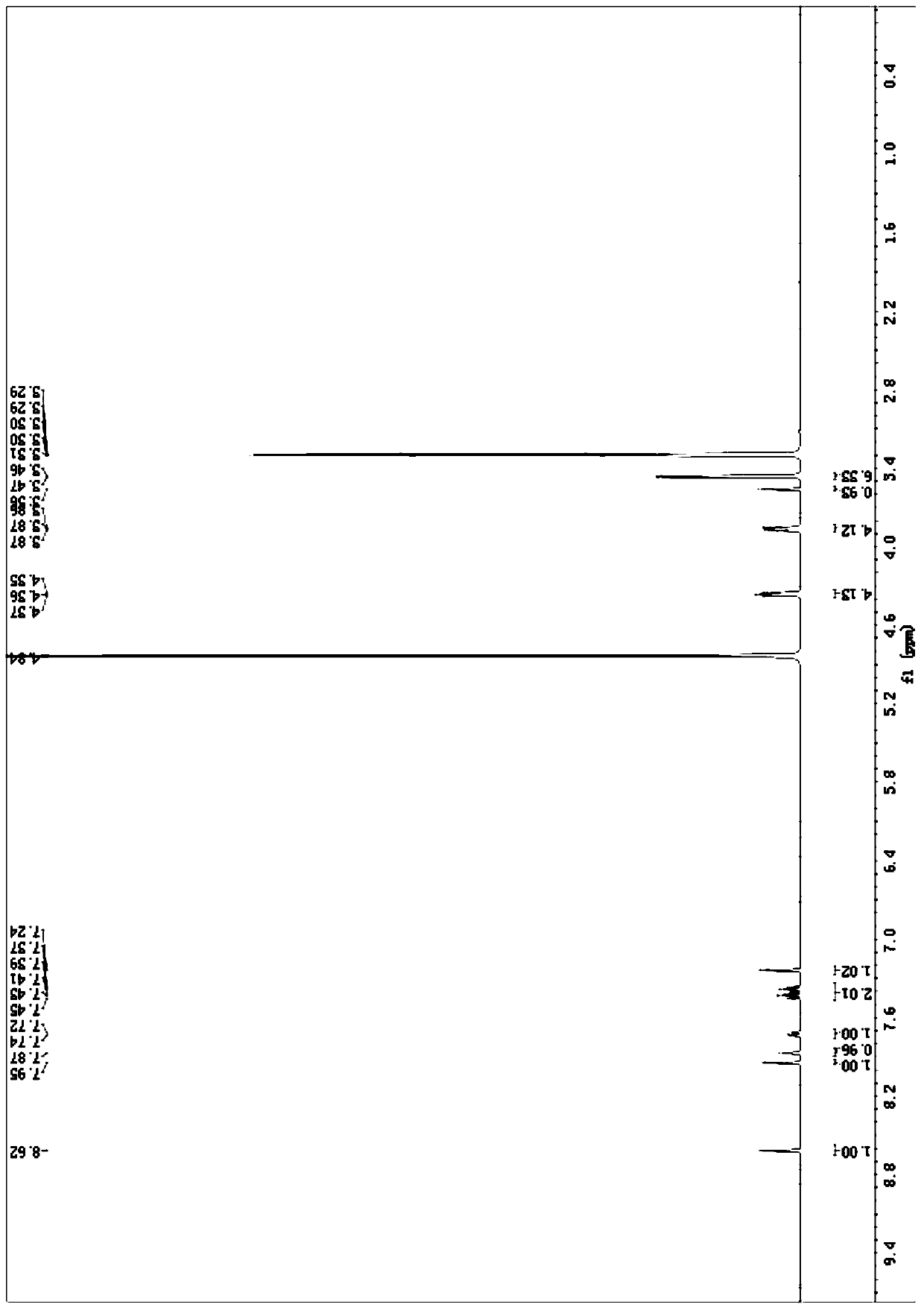
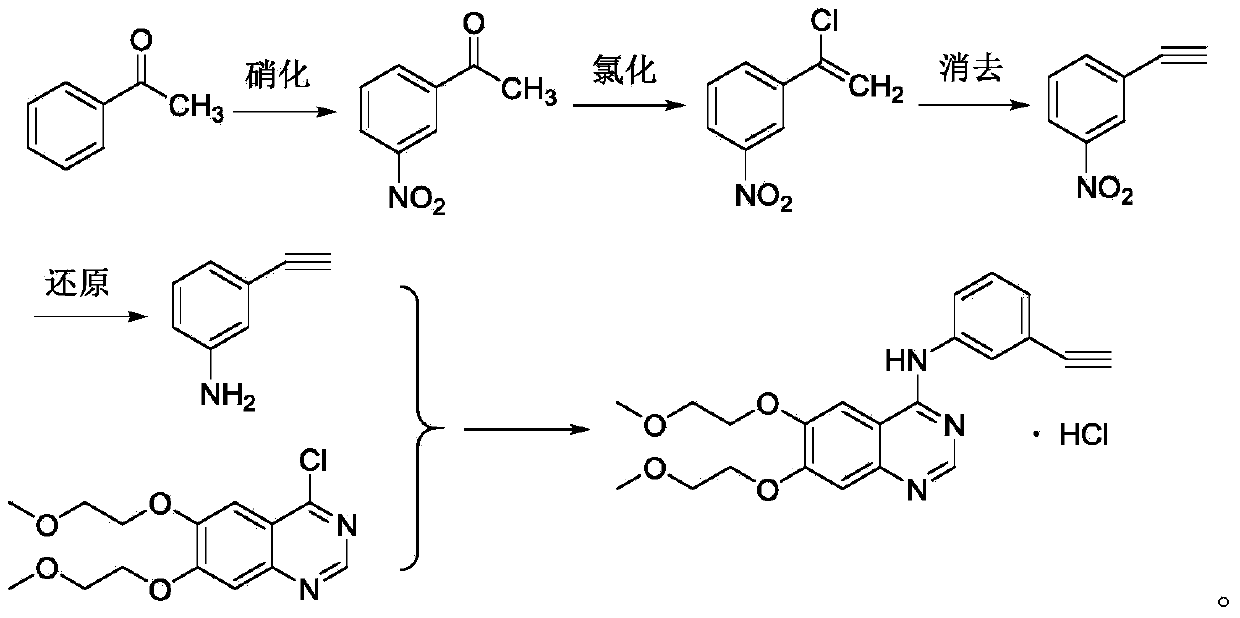
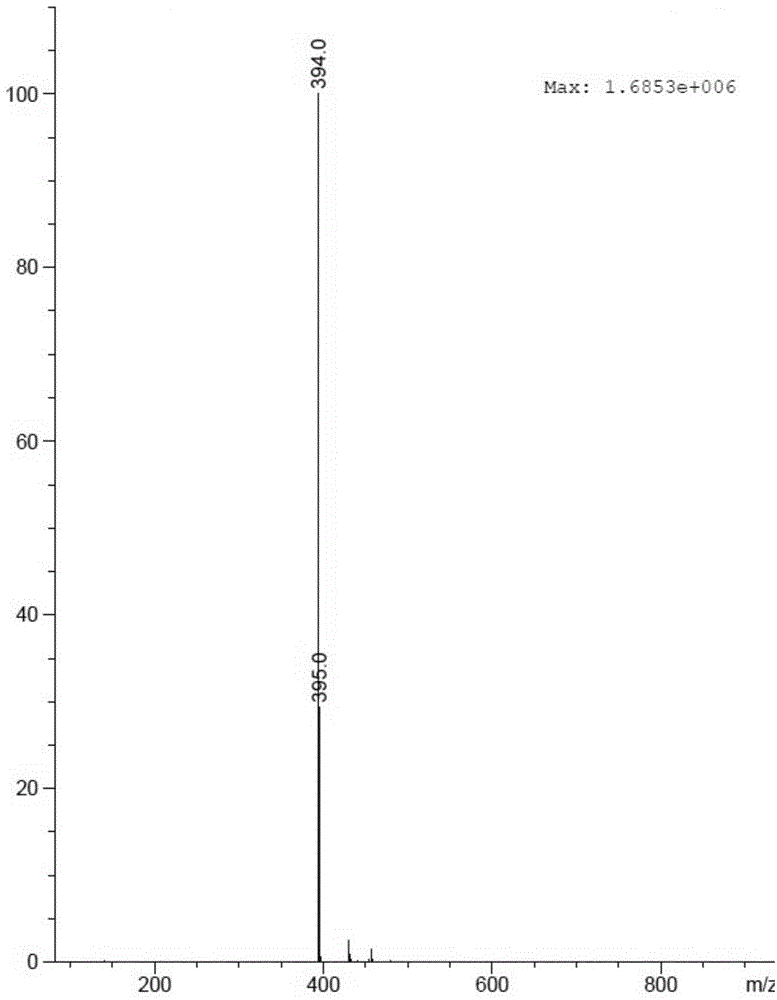
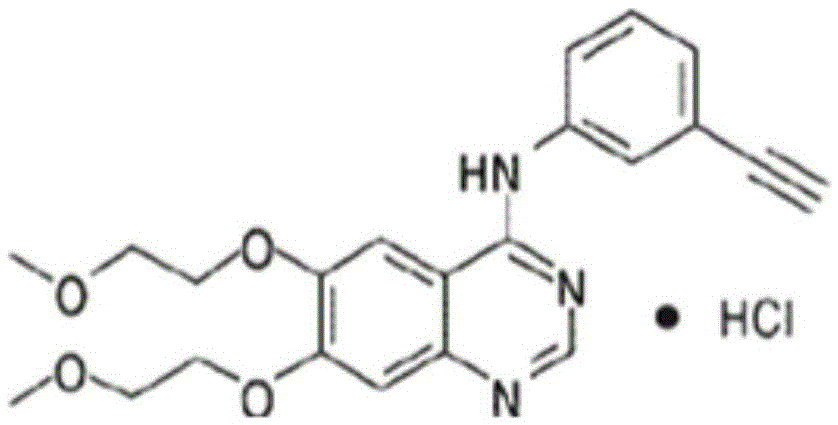
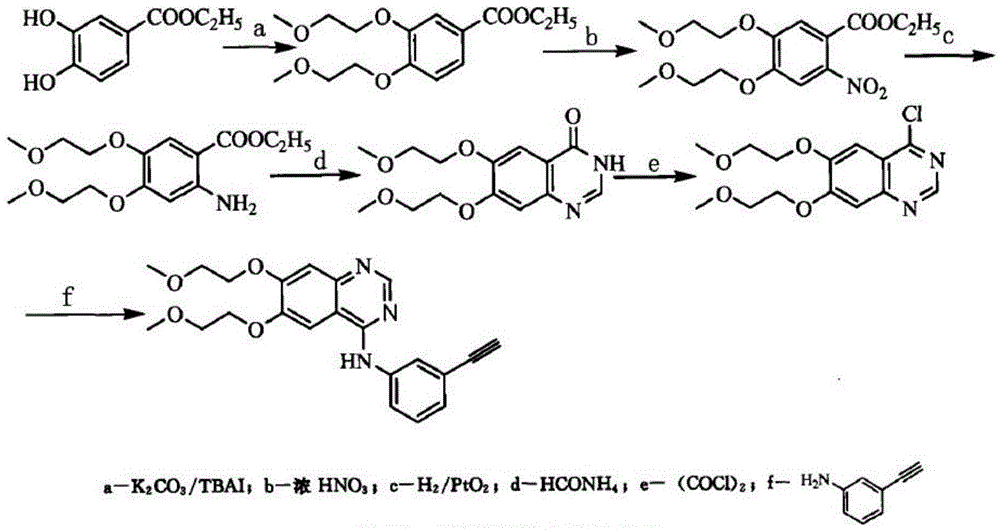
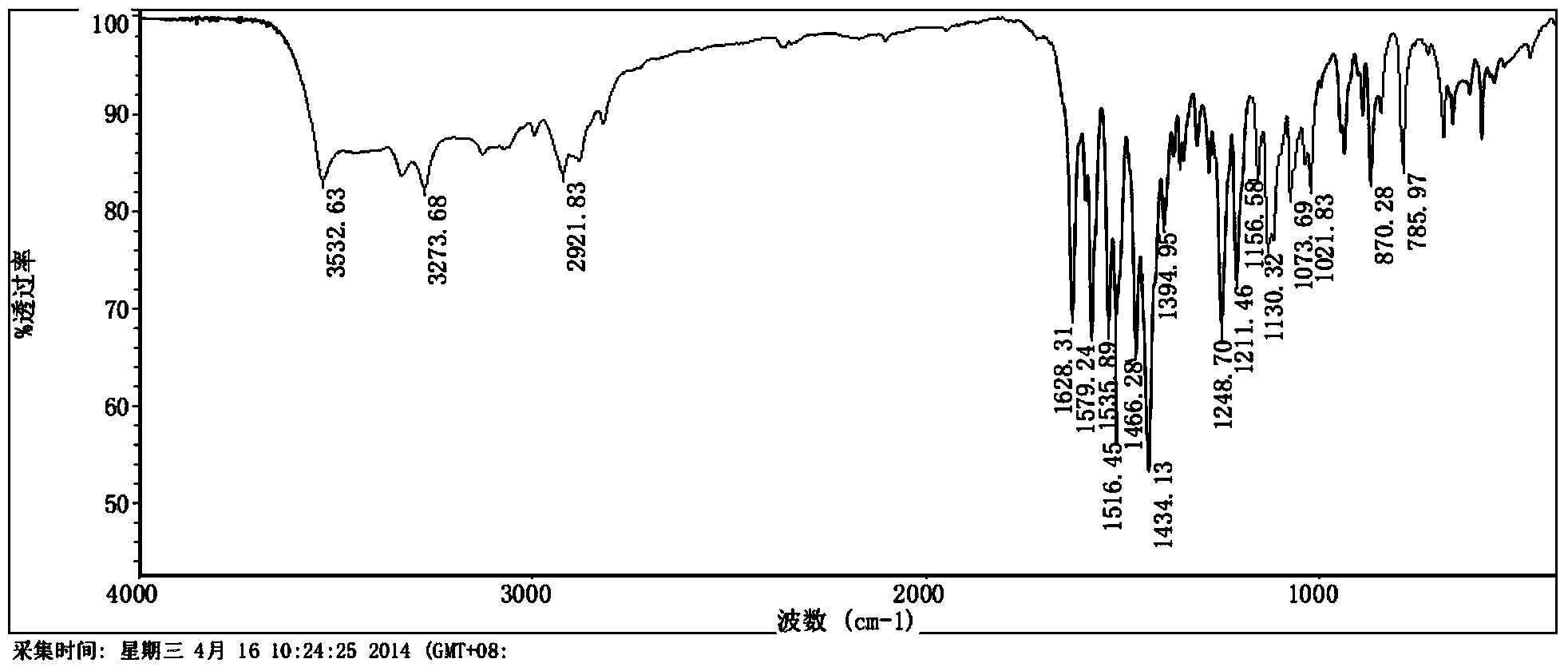
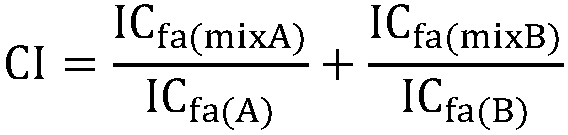Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Erlotinib Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
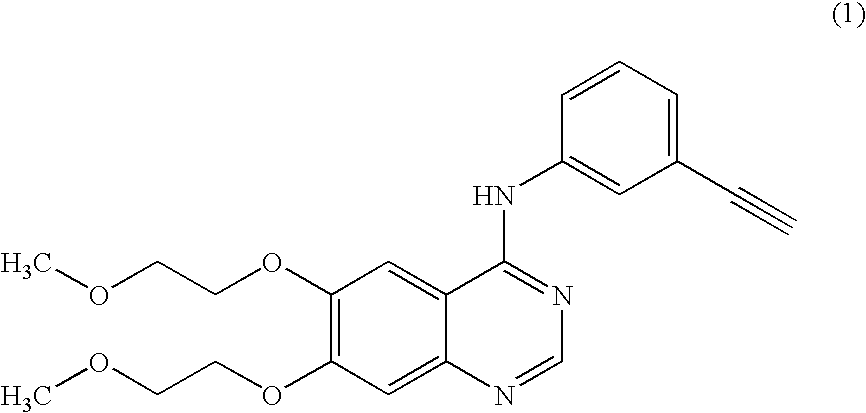
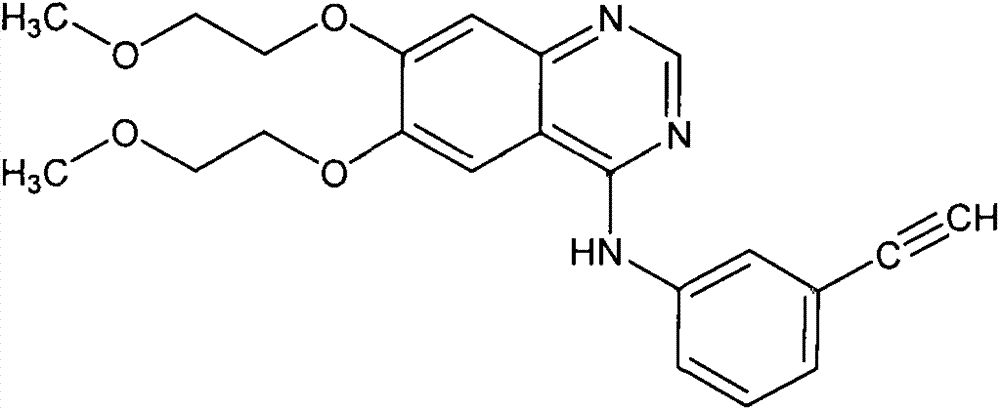
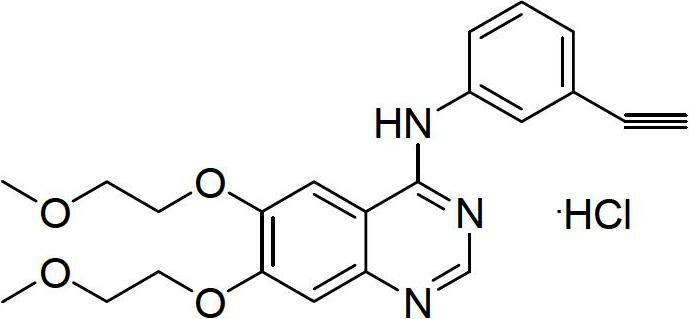
A quinazoline derivative and ANTINEOPLASTIC AGENT that functions as a PROTEIN KINASE INHIBITOR for EGFR associated tyrosine kinase. It is used in the treatment of NON-SMALL CELL LUNG CANCER.
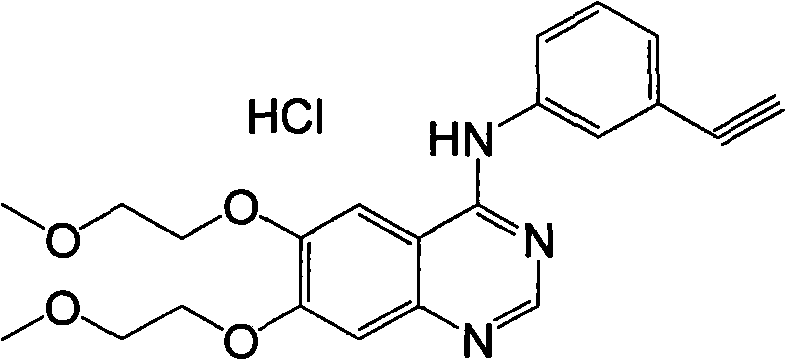
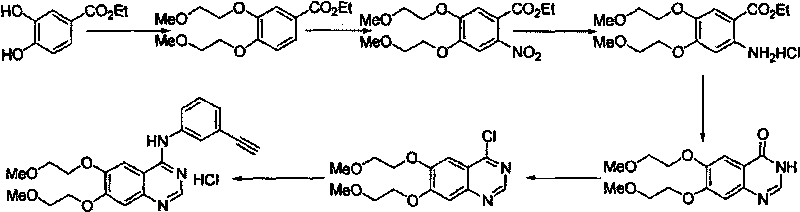
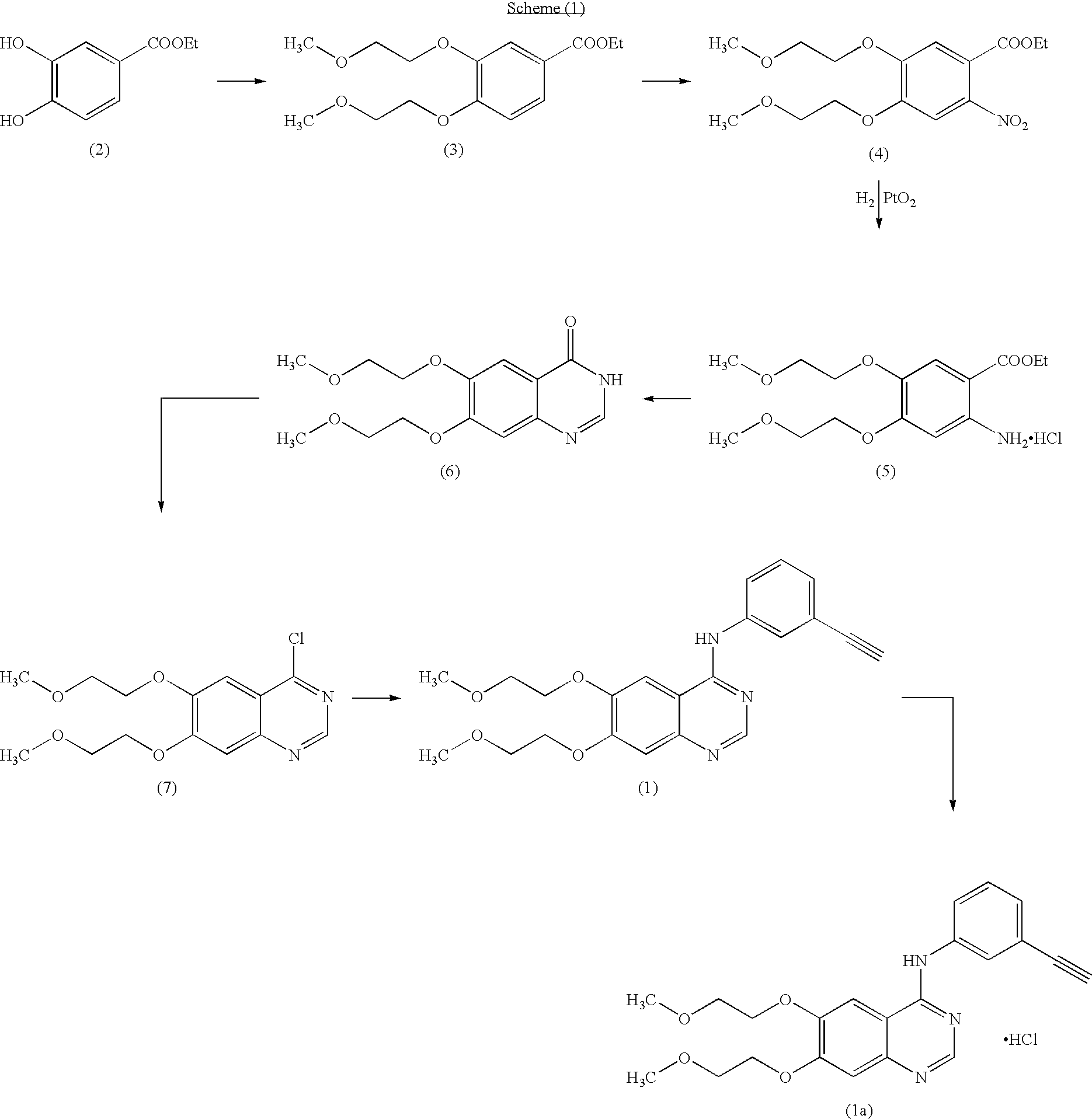
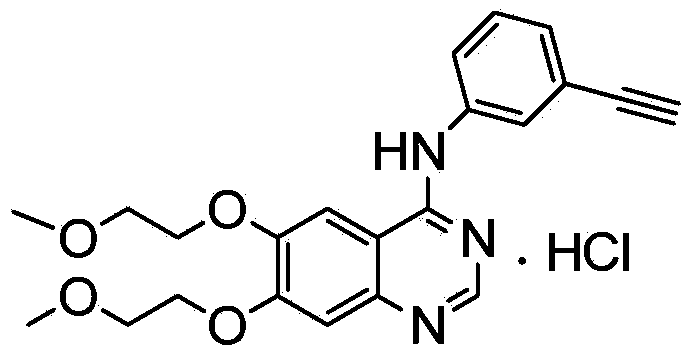
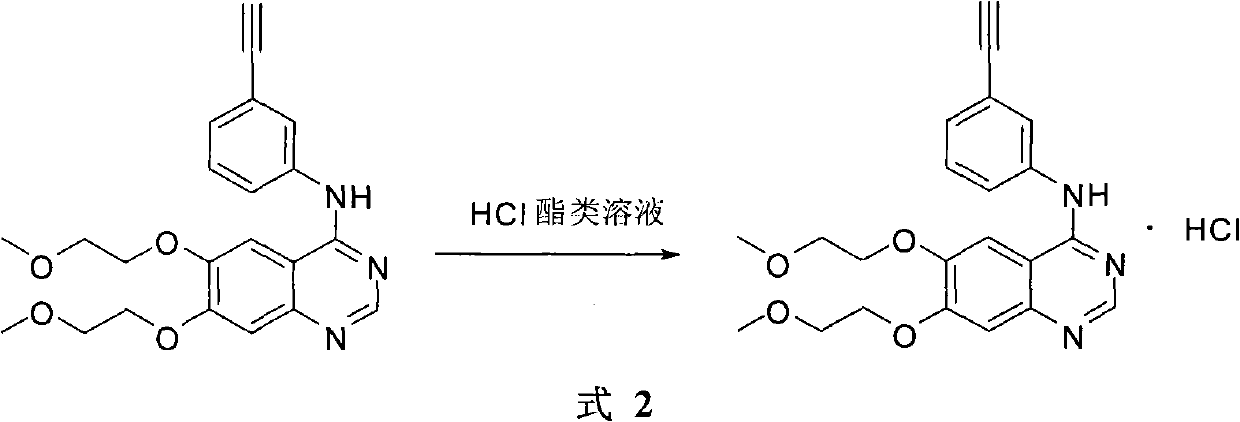
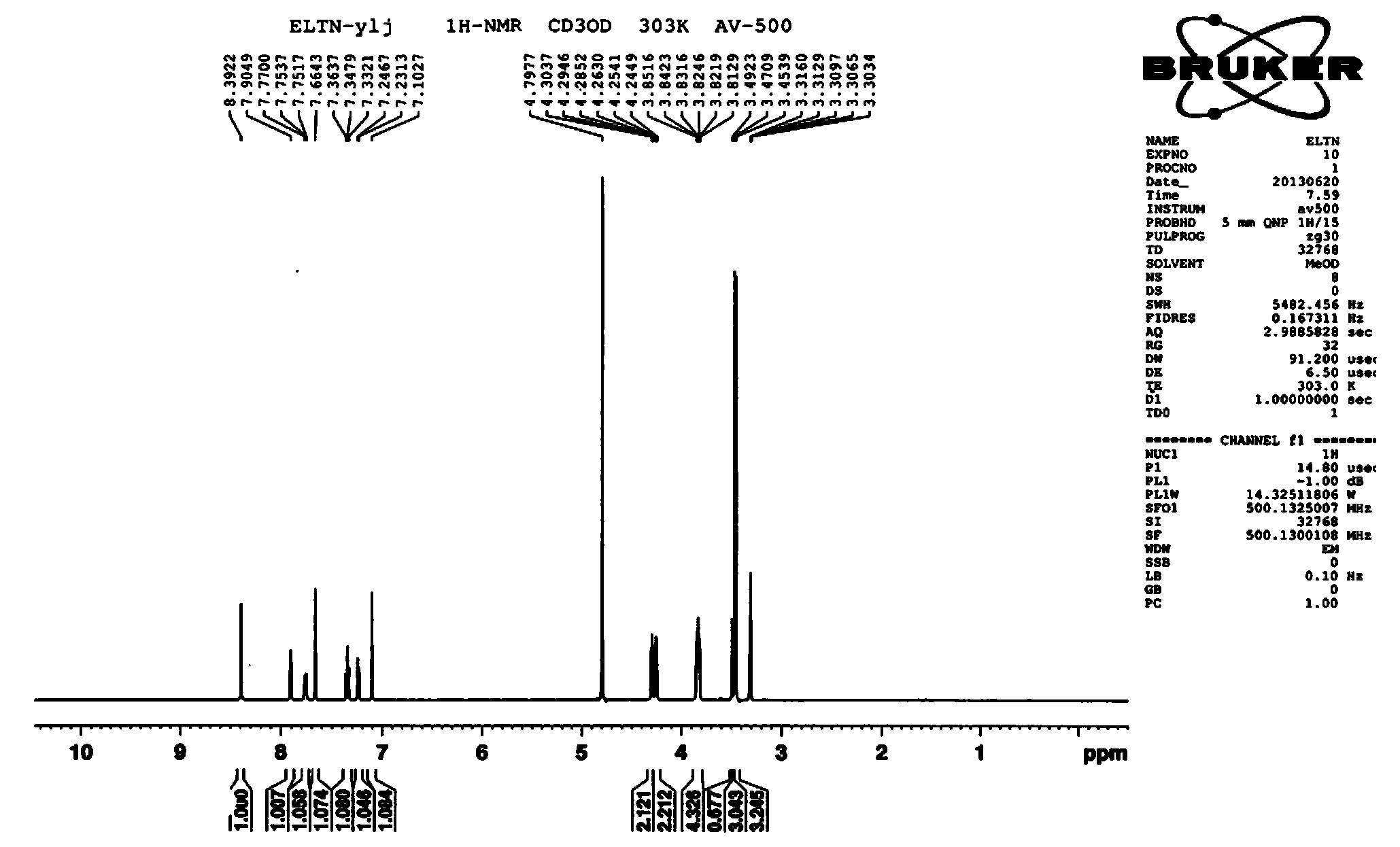
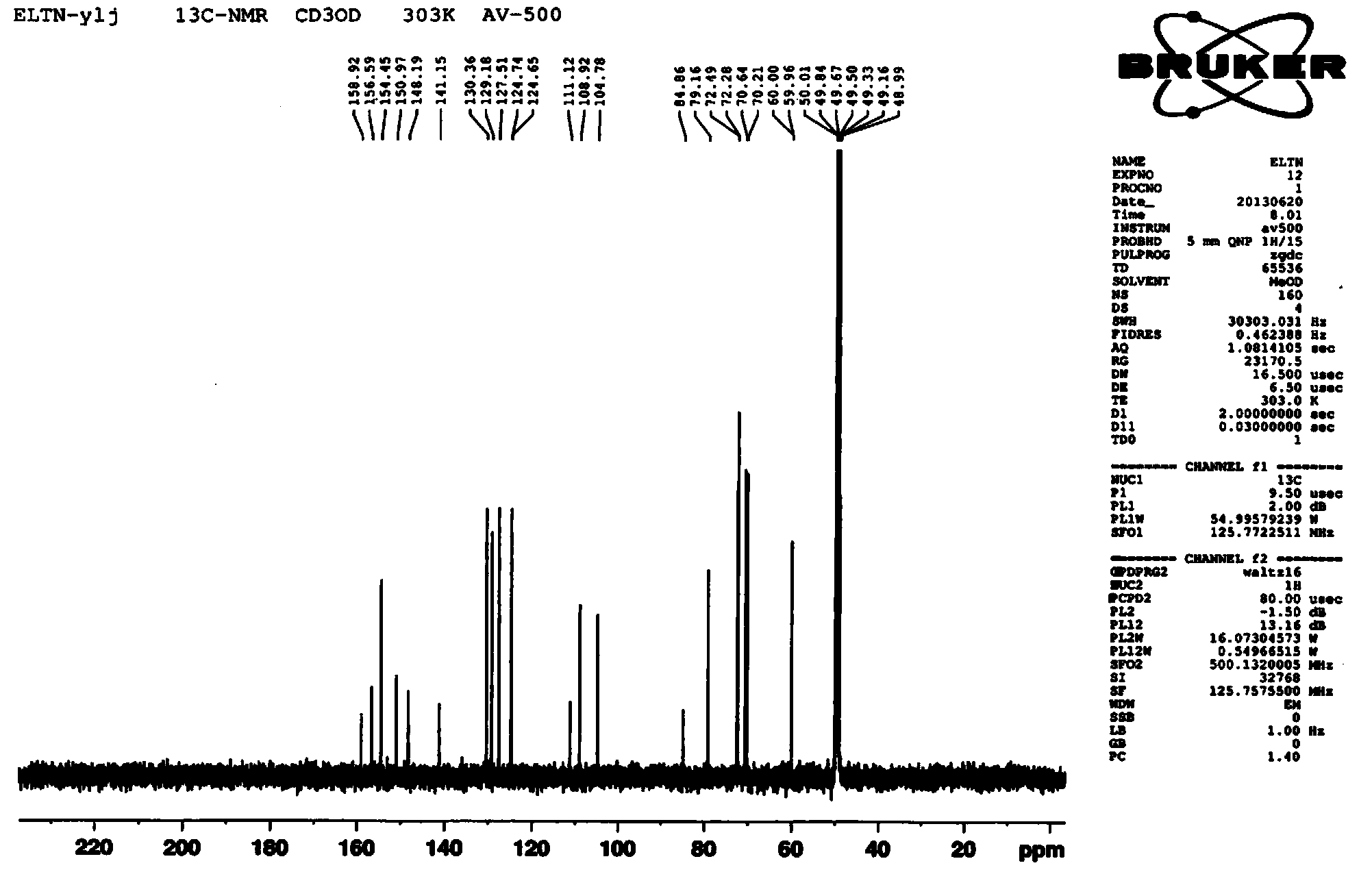
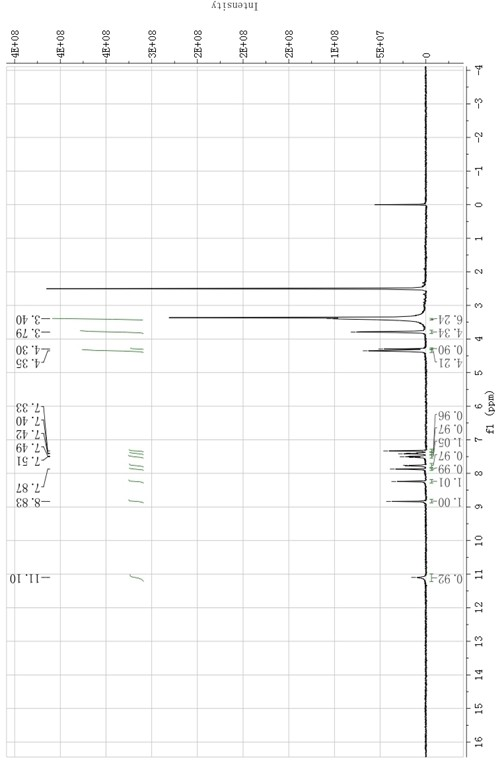
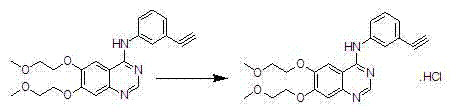
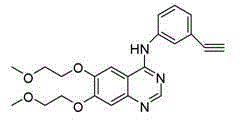
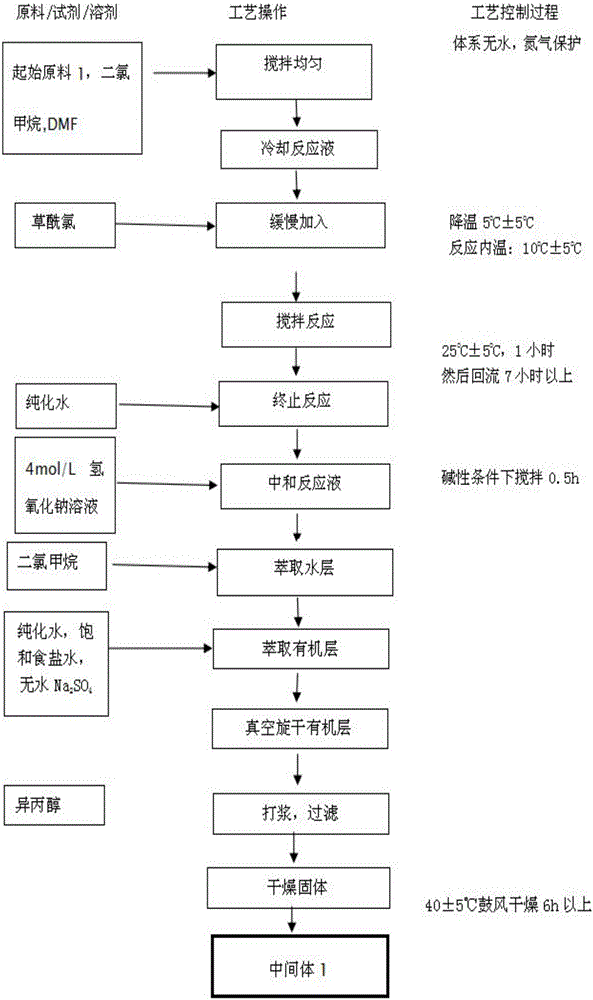
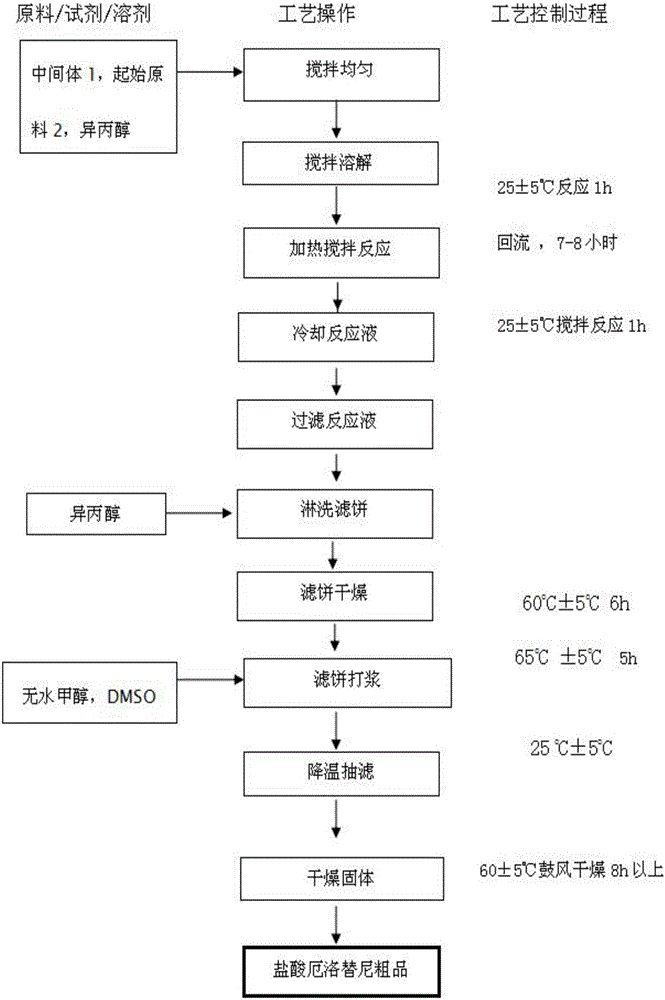
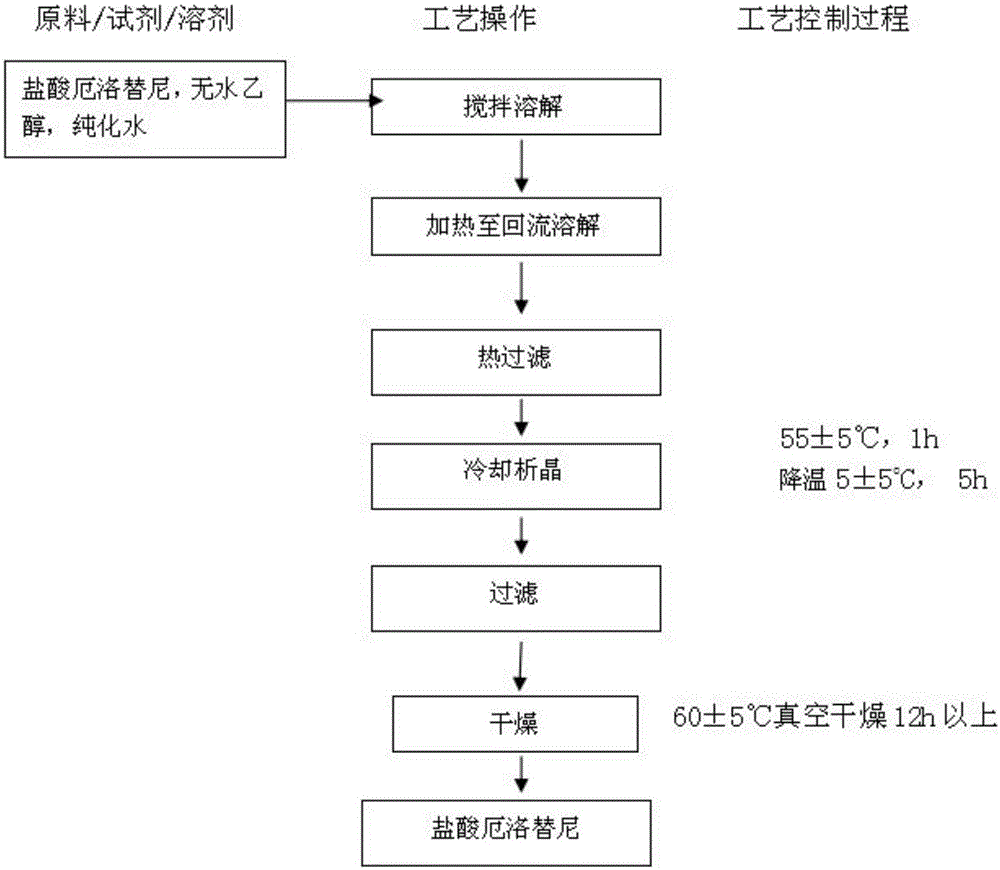
Preparation method of erlotinib hydrochloride
The invention provides a preparation method of erlotinib hydrochloride. The method comprises the following steps: taking 6,7-dimethoxy-3,4-dihydro-quinazoline as an initial raw material, chlorinating directly, then reacting with 3-aminophenylacetylene, afterwards removing methyl on the positions of 6 and 7 and finally introducing a methoxyethyl side chain to generate the erlotinib hydrochloride. The invention has the technological lines of mild reaction condition, no need of high temperature, deep cooling, energy saving and environment friendliness; the whole line has better yield and quality; and the reaction steps are little, the reaction is stable and controllable, the postprocessing is very simple and convenient, and the industrialized production is easy.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
Hydrates of erlotinib hydrochloride
Erlotinib hydrochloride hydrate is formed from an aqueous solution and is useful as a pharmaceutical and as a purification intermediate.
Owner:SYNTHON BV
Synthesis intermediate of erlotinib and preparation method thereof
ActiveCN102557977ALow raw material costThe synthesis method is simpleOrganic active ingredientsOrganic compound preparationChemical synthesisErlotinib Hydrochloride
The invention relates to a process of a chemical synthesis medicament, in particular to a novel synthesis process of erlotinib hydrochloride serving as an antitumor medicament, and a novel intermediate. The process has the advantages of low-price and readily-available raw materials, mild conditions in a reacting process, no device with special requirements and suitability for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
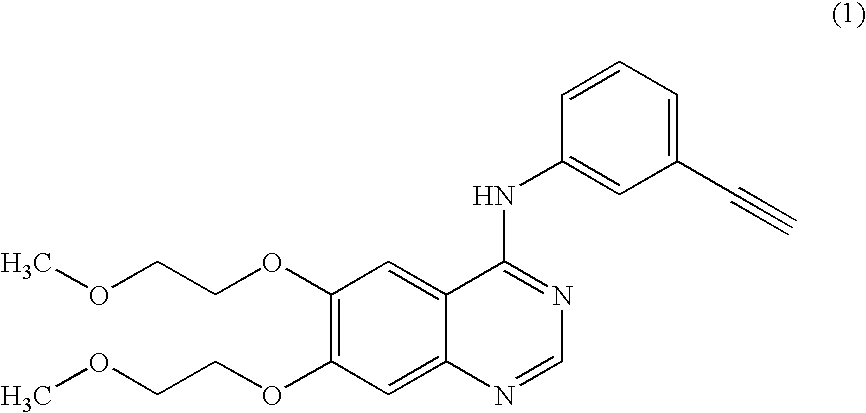
Novel process for the prepartion of erlotinib
InactiveUS20090306377A1Economical and simpleSpeed up the processOrganic chemistryLeaving groupAcetic anhydride
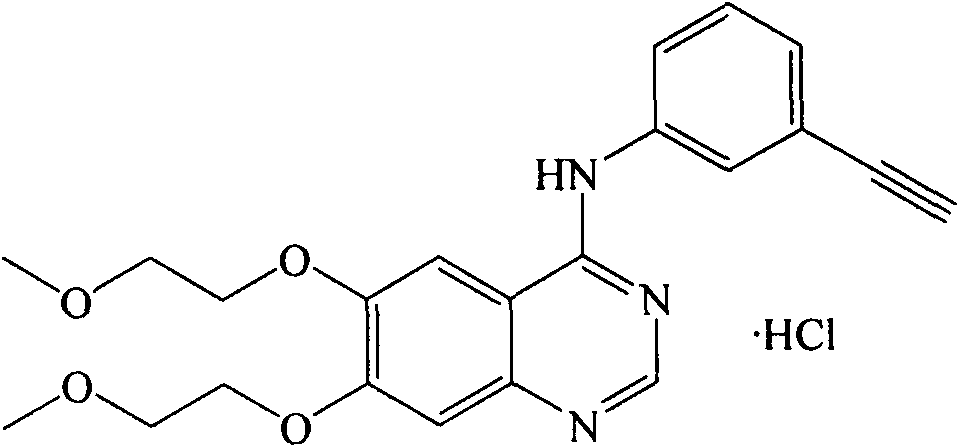
The present invention discloses an improved and novel process for the preparation of erlotinib (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine) of formula (1), which comprises: (i) demethylation of commercially available 6,7-dimethoxy-4(3H)-quinazolinone of formula (8); acetylation using acetic anhydride; (iii) introduction of a leaving group at C-4 position in quinazolinone; (iv) condensation with 3-ethynylaniline to get novel compound of formula (12); (v) deacetylation to get novel dihydroxy compound of formula (13); and (vi) O-alkylation with 2-iodoethylmethyl ether to get the erlotinib base of formula (1). Erlotinib base is purified by recrystallization from ethyl acetate to get a HPLC purity of >99.5%. Salt formation of this base with hydrogen chloride gave pharmaceutically acceptable erlotinib hydrochloride of formula (1a) with a HPLC purity of >99.8%. Erlotinib hydrochloride is useful for the treatment of proliferative disorders, such as cancers, in humans.
Owner:NATCO PHARMA LTD
Process for the prepartion of erlotinib
InactiveUS7960545B2Economical and simpleSpeed up the processOrganic chemistryAcetic anhydrideEthyl group
The present invention discloses an improved and novel process for the preparation of erlotinib (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine) of formula (1), which comprises: (i) demethylation of commercially available 6,7-dimethoxy-4(3H)-quinazolinone of formula (8); acetylation using acetic anhydride; (iii) introduction of a leaving group at C-4 position in quinazolinone; (iv) condensation with 3-ethynylaniline to get novel compound of formula (12); (v) deacetylation to get novel dihydroxy compound of formula (13); and (vi) O-alkylation with 2-iodoethylmethyl ether to get the erlotinib base of formula (1). Erlotinib base is purified by recrystallization from ethyl acetate to get a HPLC purity of >99.5%. Salt formation of this base with hydrogen chloride gave pharmaceutically acceptable erlotinib hydrochloride of formula (1a) with a HPLC purity of >99.8%. Erlotinib hydrochloride is useful for the treatment of proliferative disorders, such as cancers, in humans.
Owner:NATCO PHARMA LTD
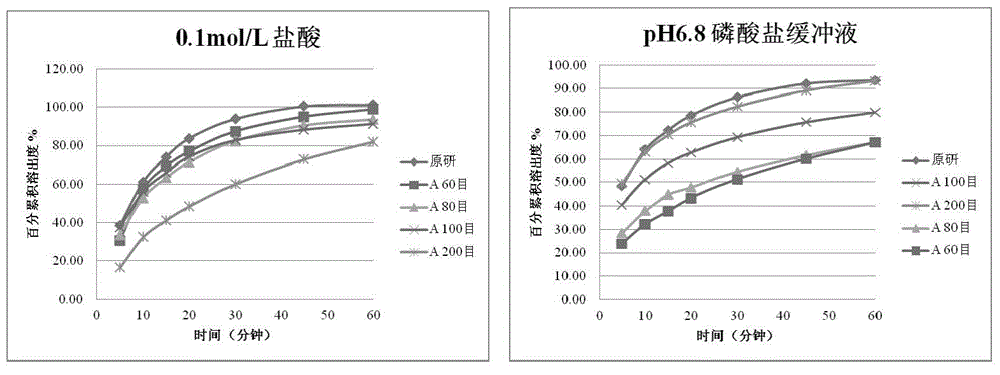
Tablets containing erlotinib hydrochloride and preparation method thereof
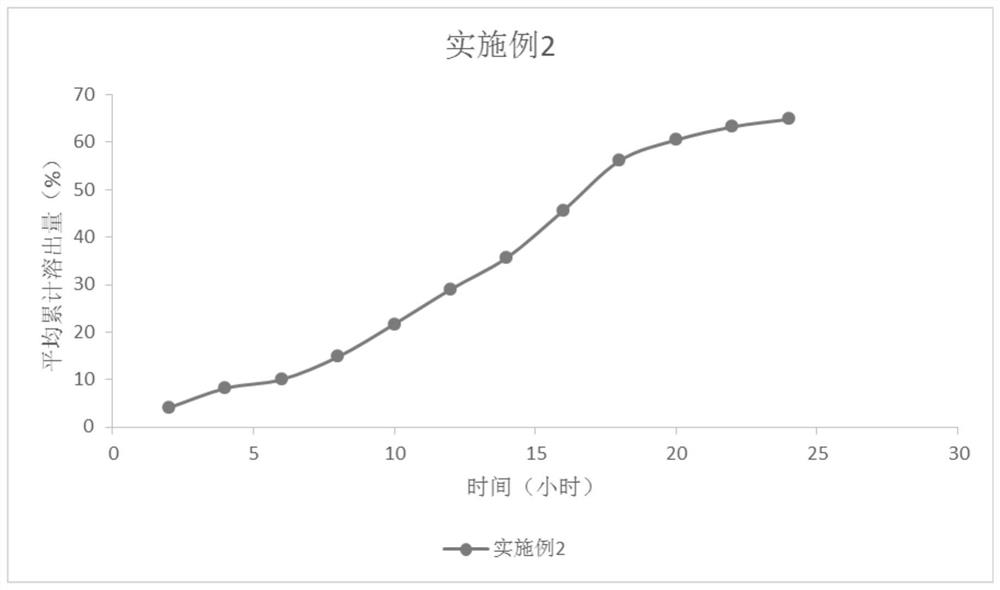
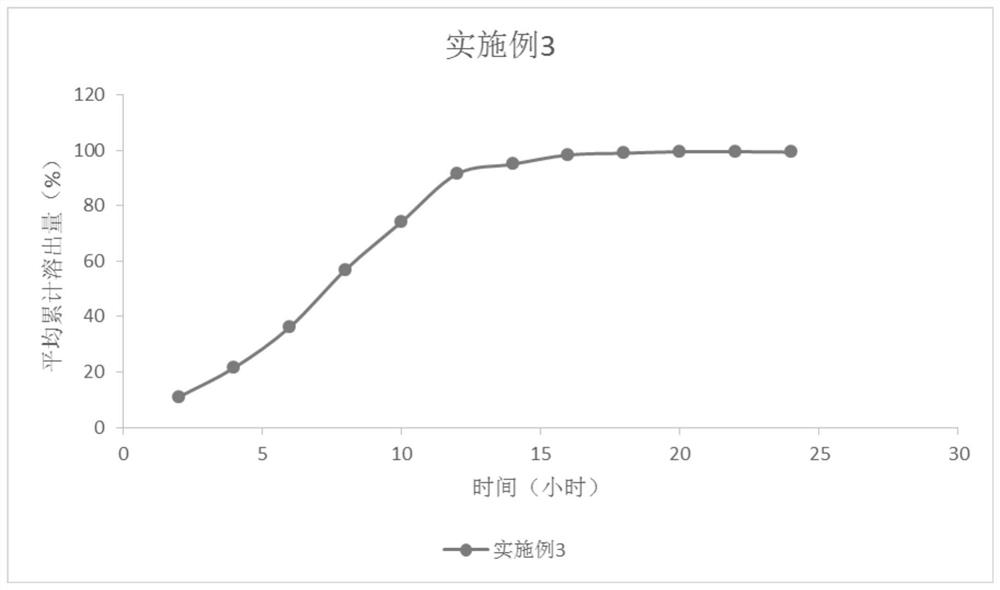
InactiveCN103705477AHigh dissolution rateNo longer affects dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionMedical prescription
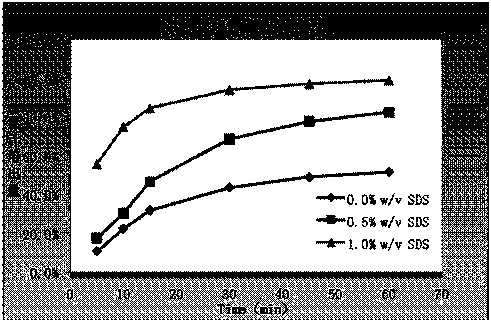
The invention discloses erlotinib hydrochloride tablets which contain silica which accounts for 2-12 percent of the total weight of the erlotinib hydrochloride tablets. The dissolution can be promoted. The invention also relates to a method for preparing the tablets. The process flow is simple, special requirements on the granularity of the raw materials are avoided, and the dissolution property of the product is good.
Owner:SHANDONG BOMAIKANG PHARMA RES
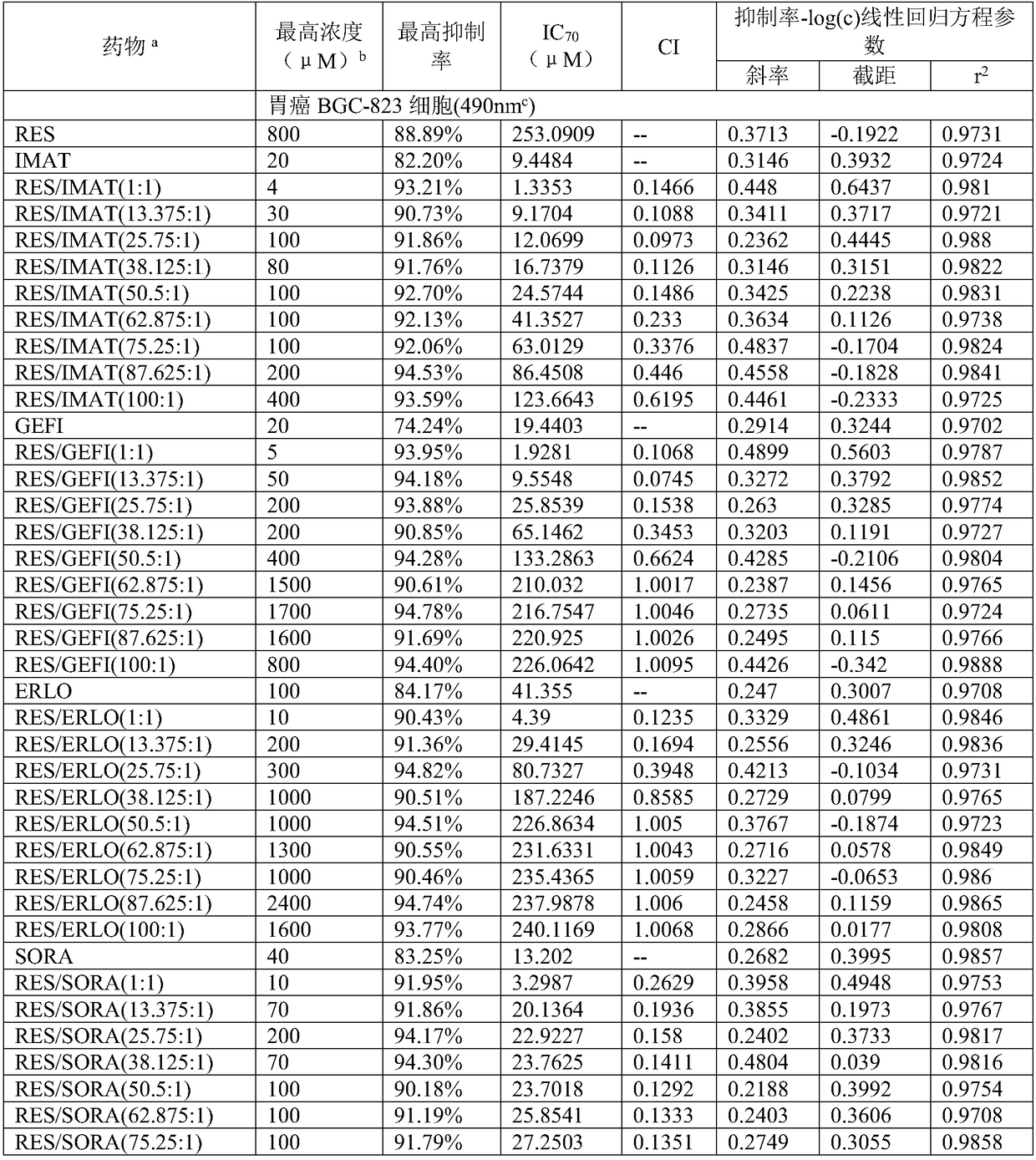
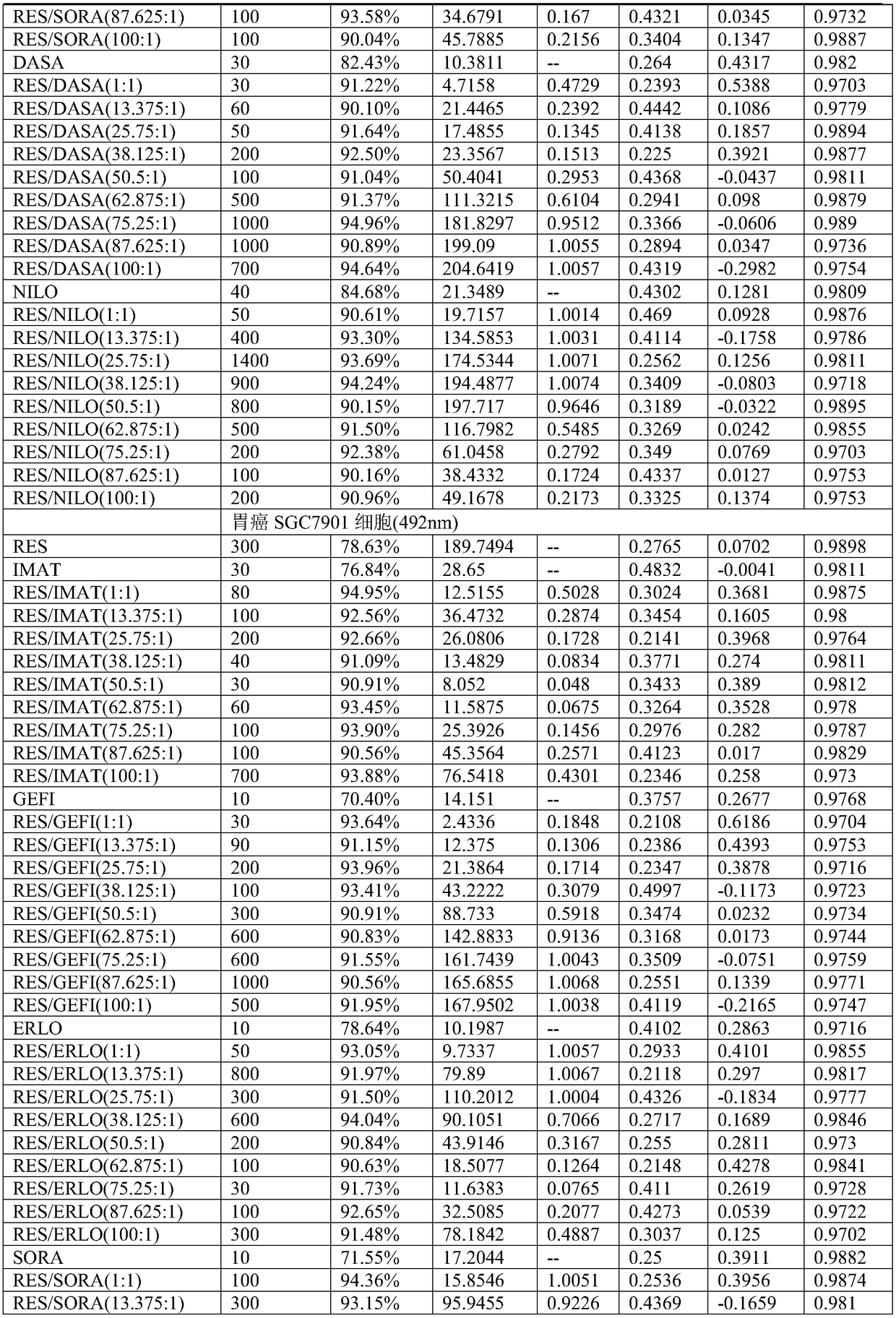
Application of compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs
InactiveCN108939080AGood synergyHydroxy compound active ingredientsAntineoplastic agentsTyrosine-kinase inhibitorDasatinib
The invention provides an application of a compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs. The application is characterized in that the tyrosine kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib hydrochloride tablets, sorafenib, dasatinib tablets and tasigna or pharmaceutically acceptable salts or solvates or pharmaceutically acceptable salt solvents thereof; the tumor is selected from one of gastric cancer, liver cancer, lung cancer, kidney cancer, cervical cancer, pancreatic cancer, breast cancer, esophagus cancer, nasopharynxcancer and ovarian cancer; the mole ratio of resveratrol to tyrosine kinase inhibitor is (1-100):1.
Owner:黄泳华
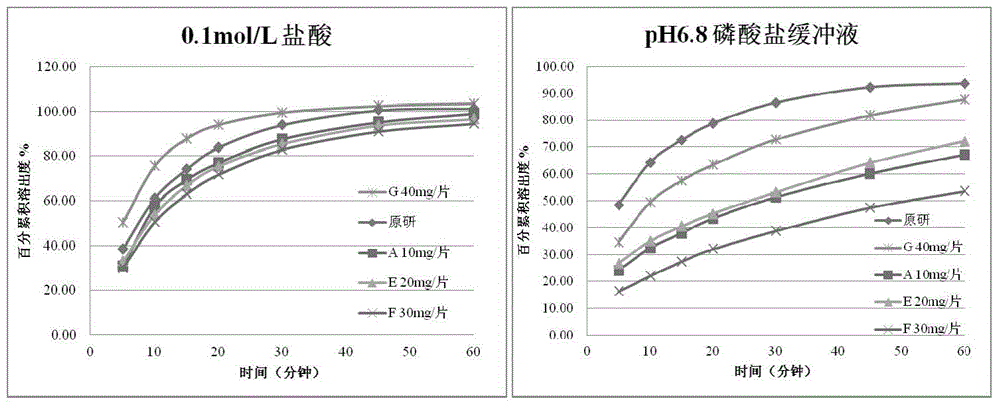
Erlotinib hydrochloride tablets and preparation method thereof
The invention discloses erlotinib hydrochloride tablets. The erlotinib hydrochloride tablets contain a surfactant with the HLB (hydrophile-lipophile balance) value of between 10 and 20 serving as a solubilizing agent which can effectively promote medicament dissolution. The invention also relates to a preparation method of the erlotinib hydrochloride tablets, which is simple in technological process, has no special requirement on granularity of raw materials, and is good in product dissolution.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
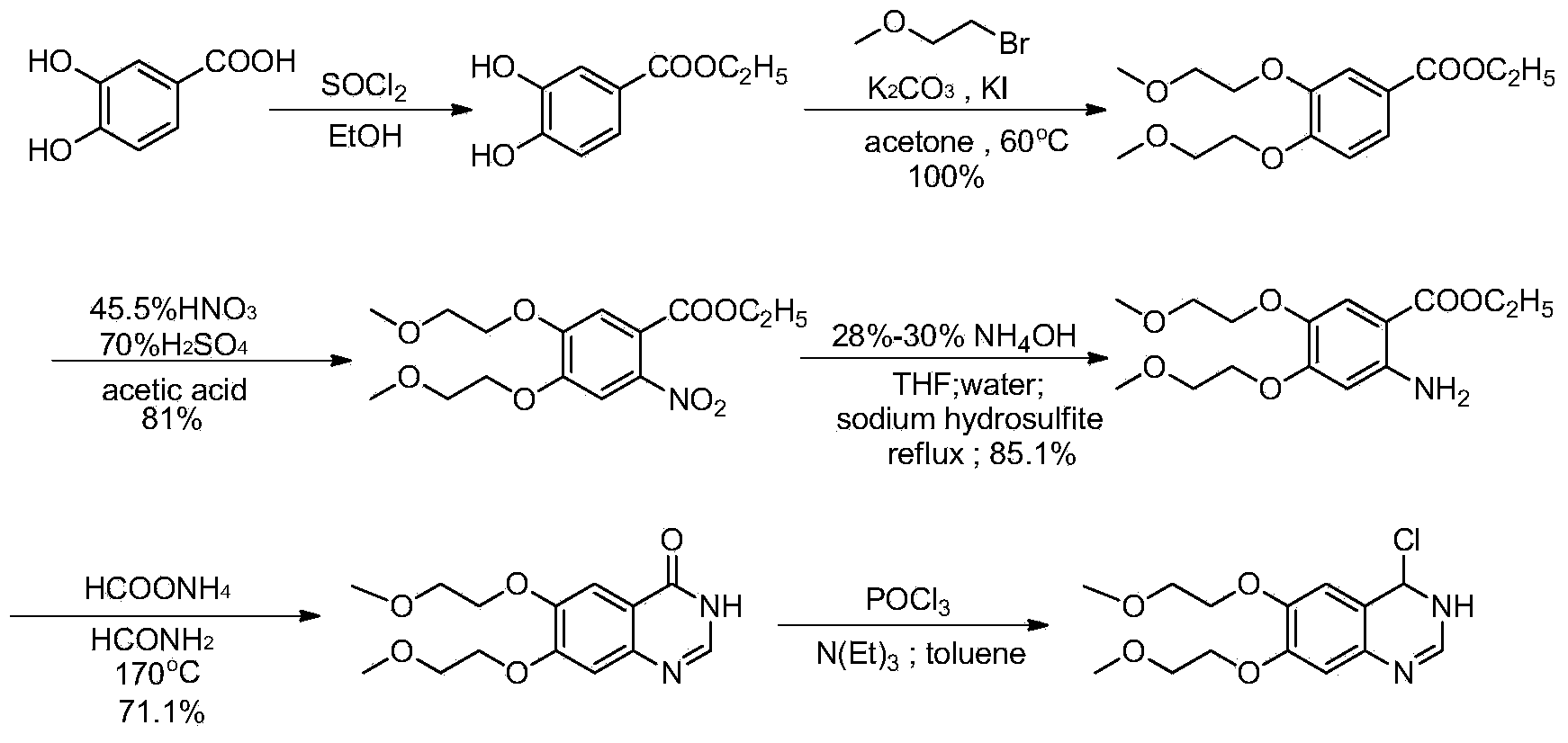
Preparation method of erlotinib hydrochloride key intermediate
InactiveCN103709110ALow pricePromote environmental protectionOrganic chemistryEthyl hydroxybenzoateEthyl ester
The invention discloses a preparation method of an erlotinib hydrochloride key intermediate 4-chloro-6,7-di(2-methoxyethoxy)quinazoline, which comprises the following steps: reacting the raw material ethyl 3,4-dihydroxybenzoate with ethyl 2-methoxysulfonate, nitrating, reducing, cyclizing and chlorinating to obtain the key intermediate 4-chloro-6,7-di(2-methoxyethoxy)quinazoline. The method has the advantages of mild reaction conditions, low cost, high purity and high total yield (up to 74.8%), and can easily implement industrial production.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
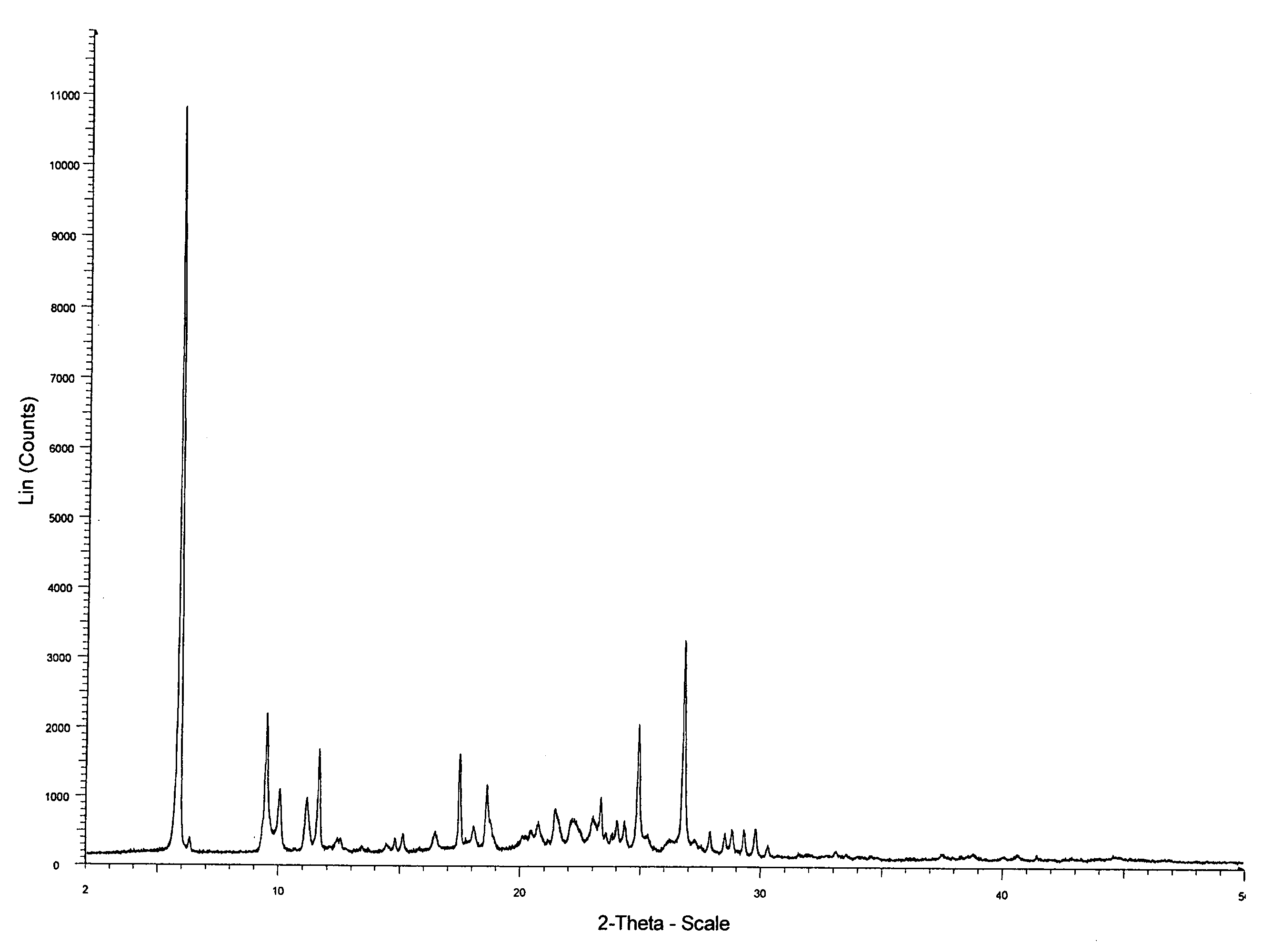
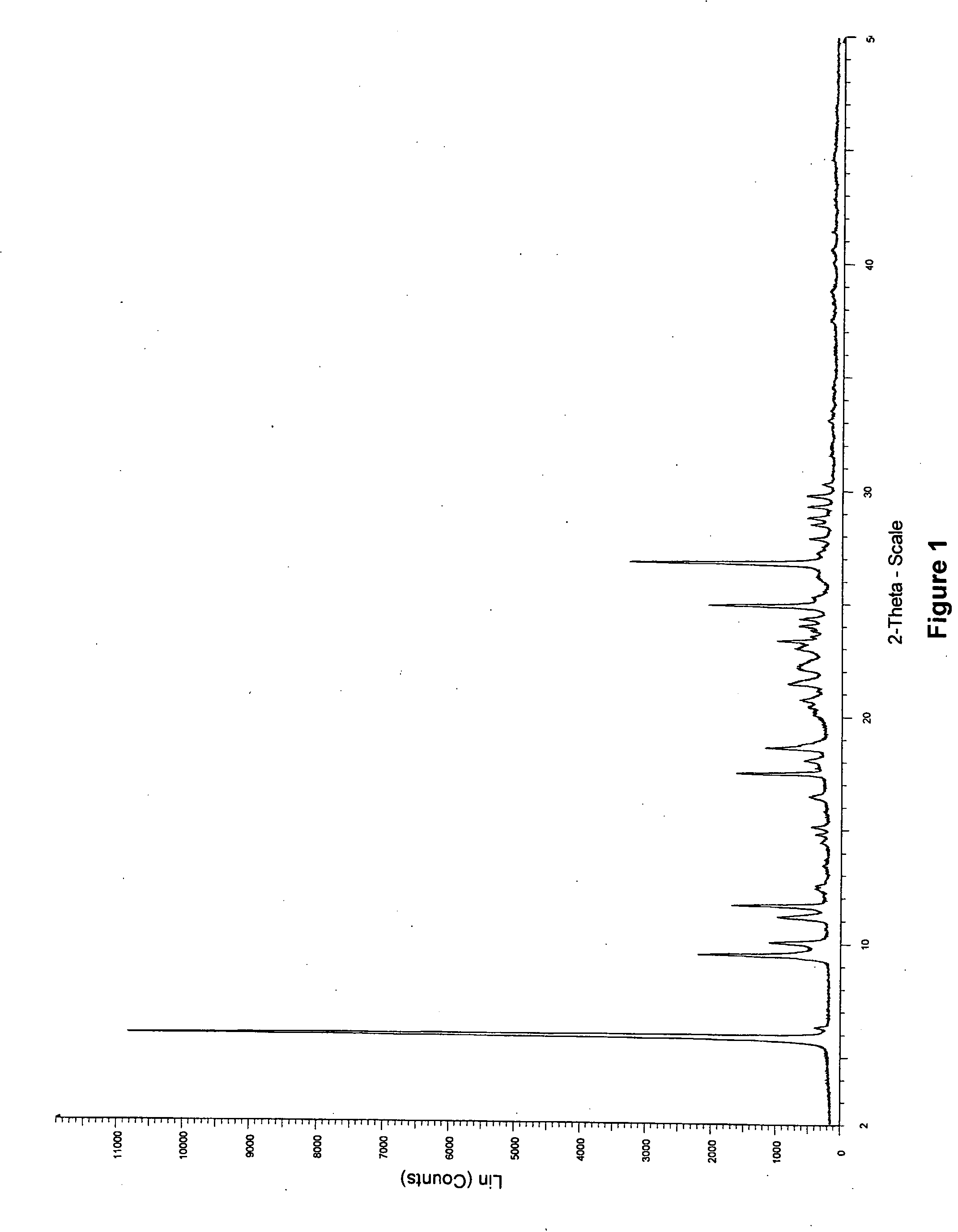
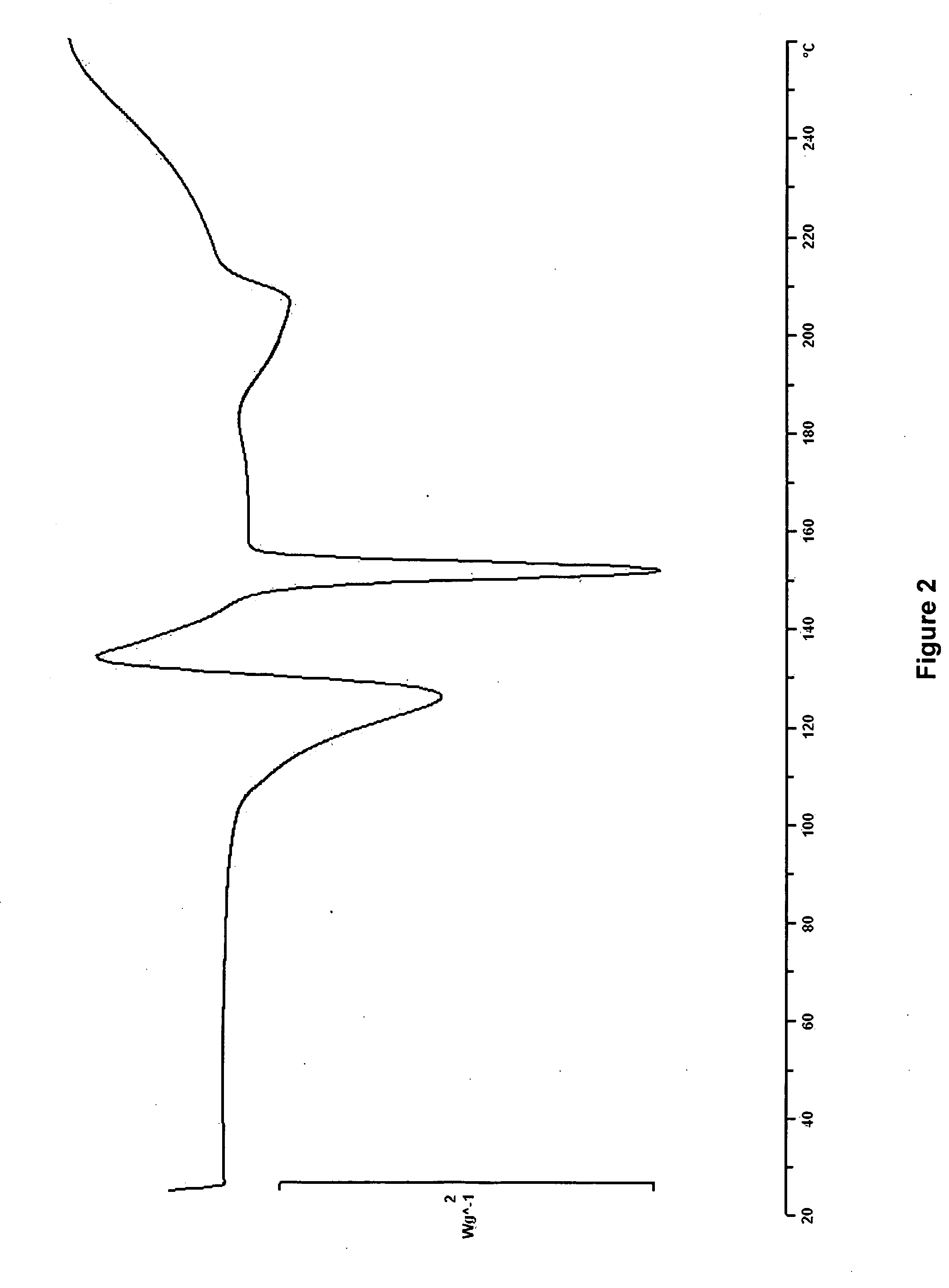
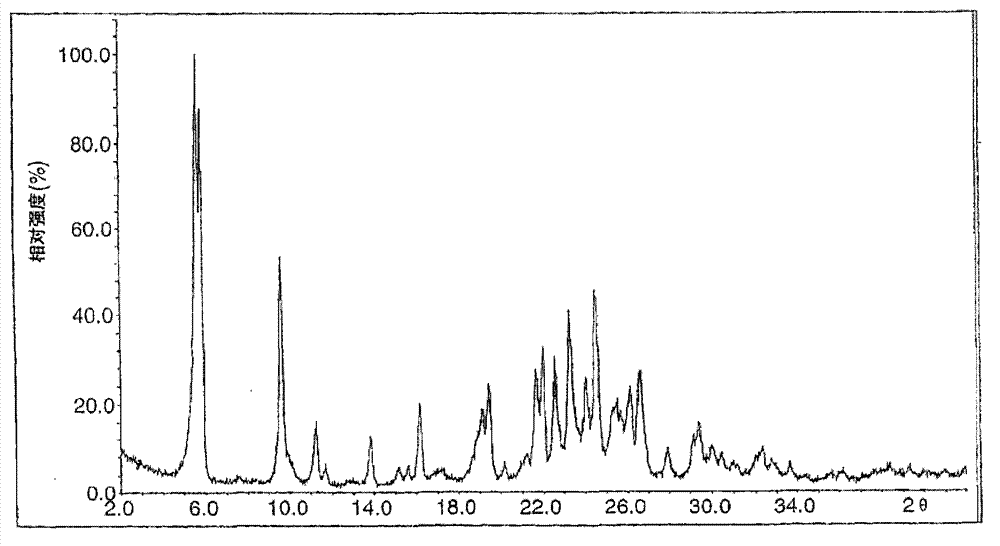
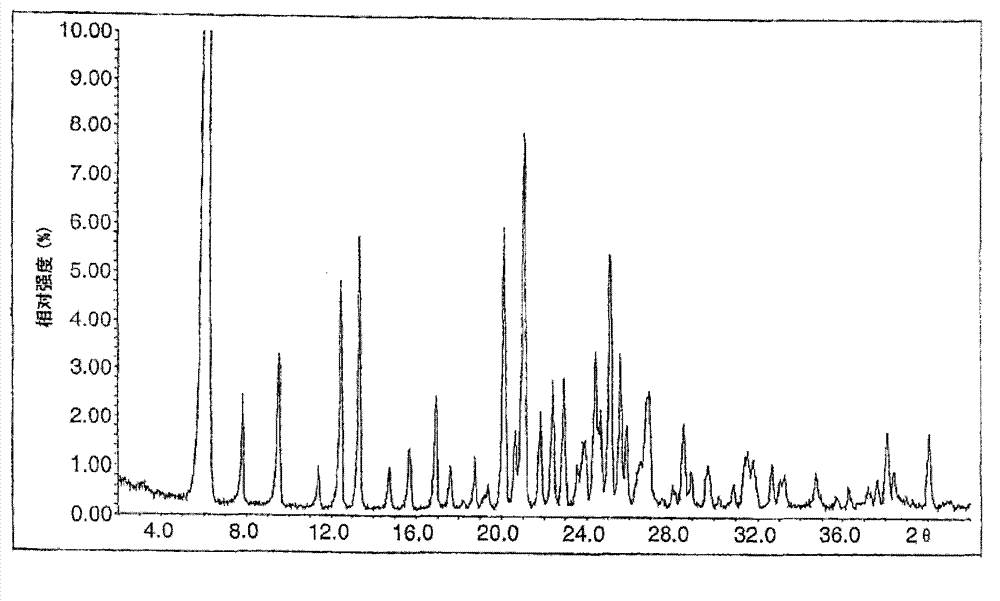
Novel preparation method of Erlotinib hydrochloride with crystal form A
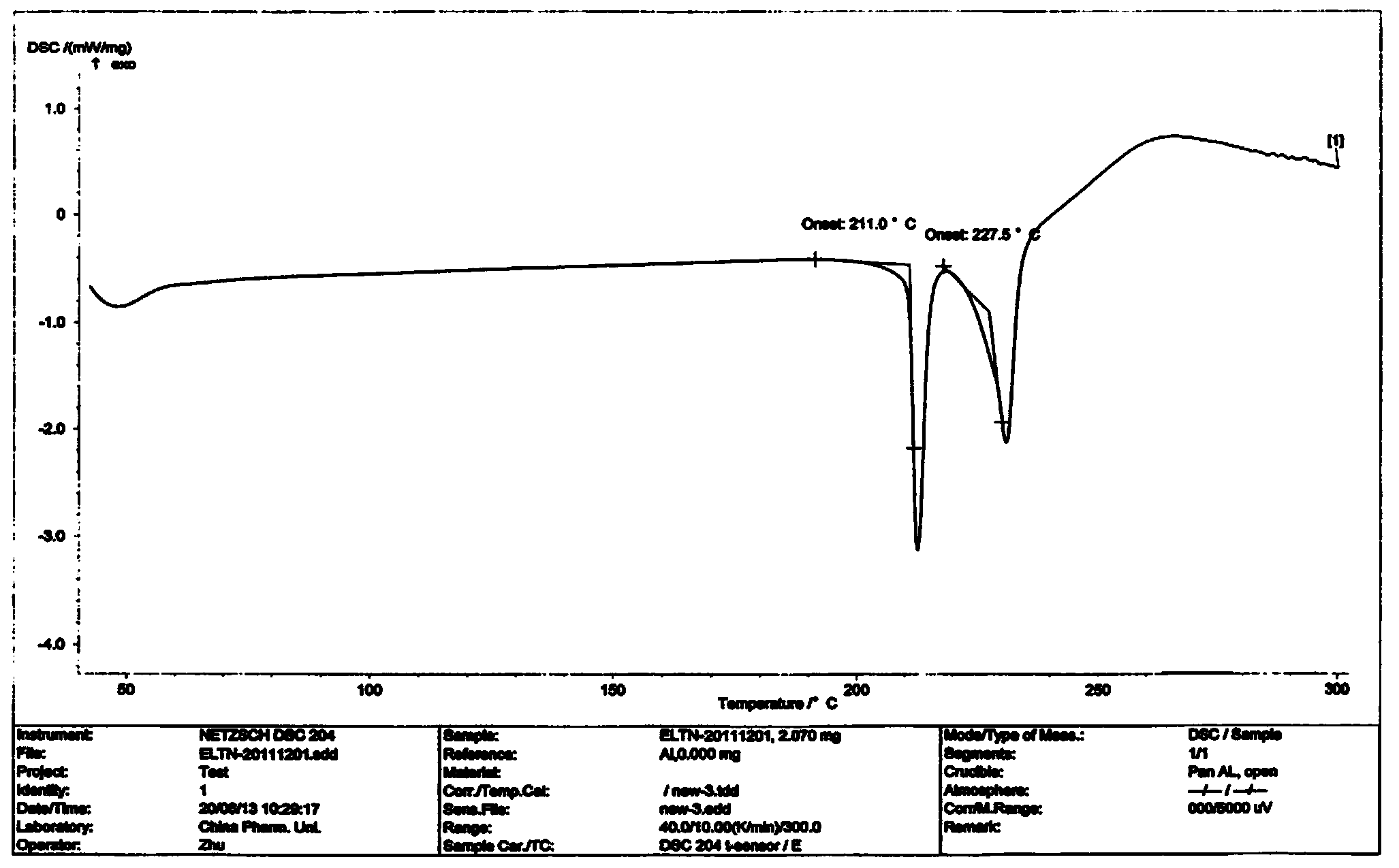
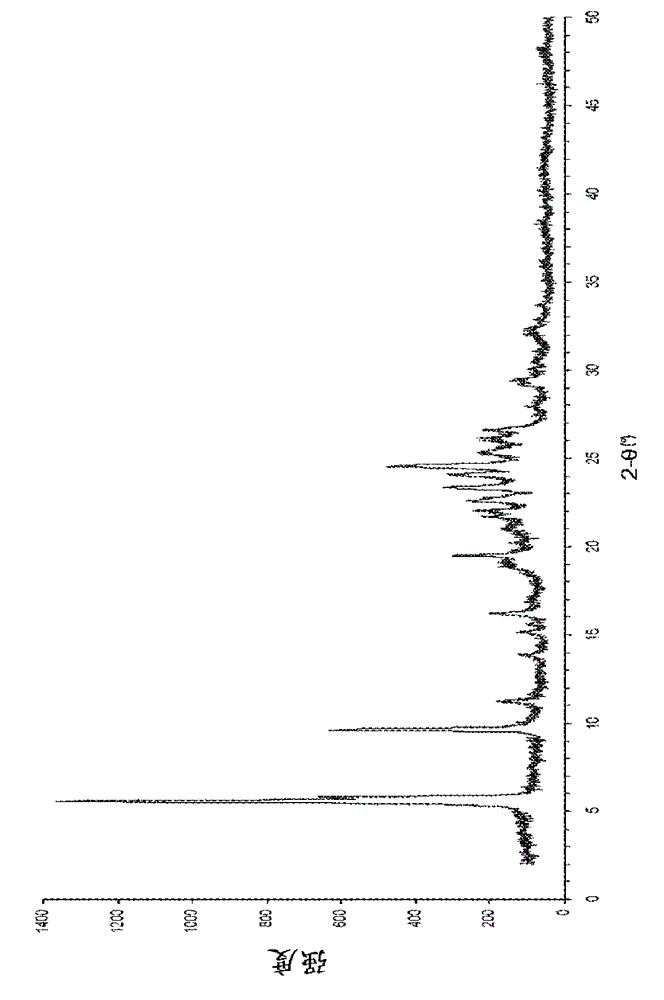
ActiveCN101948441AOvercome the disadvantage of being unstable and prone to mixed crystalsRaise the reaction temperatureOrganic chemistryOrganic solventMedicinal chemistry
The invention provides a preparation method of Erlotinib hydrochloride with the crystal form A. The method comprises the following steps: mixing Erlotinib free alkali with organic solvent, and then dropping the hydrochloric acid solution of ester to obtain the Erlotinib hydrochloride with the crystal form A.
Owner:HAINAN SIMCERE PHARMA CO LTD
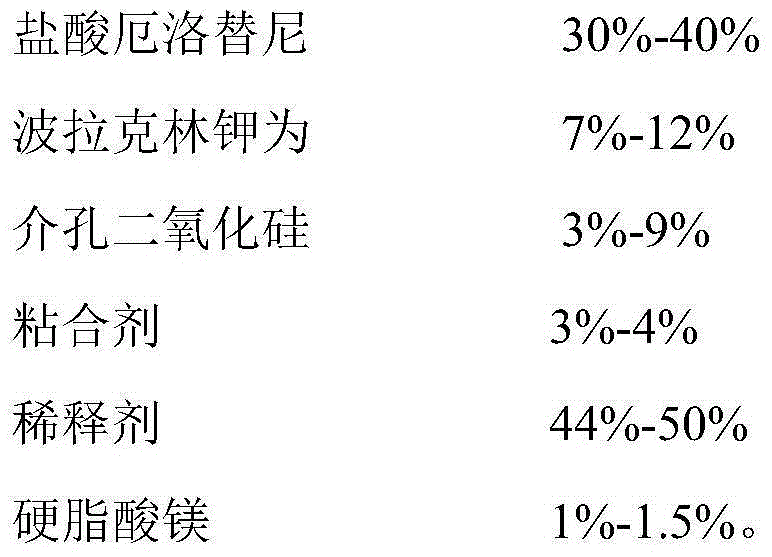
Anti-cancer drug erlotinib hydrochloride tablet and preparation method thereof
ActiveCN105030705AGuaranteed stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsPrillFluidized bed
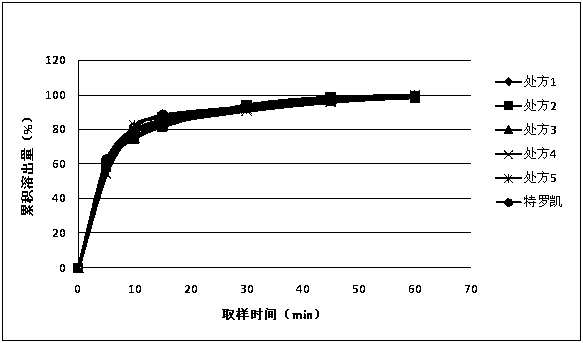
The invention discloses an anti-cancer drug erlotinib hydrochloride tablet and a preparation method thereof. A preparation of the drug is prepared from raw materials and auxiliary materials. The auxiliary materials contain polacrilin potassium and mesoporous silicon dioxide. The preparation method comprises the following steps: (1) dissolving a binder in an acidic solution, adding erlotinib hydrochloride into the acidic solution, stirring and grinding for standby application; (2) measuring the polacrilin potassium, mesoporous silicon dioxide and a diluting agent, uniformly mixing in a fluidized bed, then spraying a mixed solution obtained in the step (1), granulating, drying, and size stabilizing; (3) uniformly mixing dry particles and a lubricating agent, and tabletting. The preparation has the advantages of fast dissolution, good dissolution stability and simple preparation process.
Owner:QINGDAO TUMOR HOSPITAL
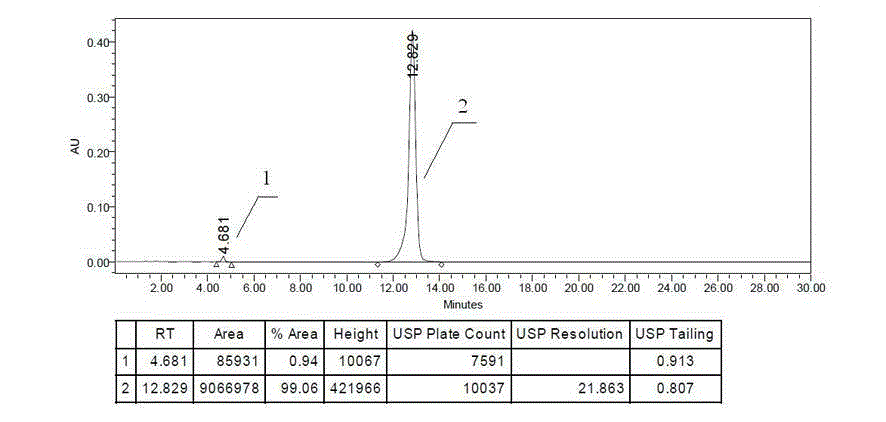
Impurity of erlotinib hydrochloride as well as preparation method and detection method thereof
The invention discloses a process impurity of erlotinib hydrochloride. The impurity is produced in a secondary reaction during the synthesis of erlotinib hydrochloride, due to the impurity, the quality of an erlotinib hydrochloride product is influenced and meanwhile an adverse reaction in the human body can be produced. The invention also provides a preparation method of the impurity and application of the impurity in detection of the erlotinib hydrochloride crude drugs and the quality of a preparation.
Owner:CHENGDU SINO STRONG PHARMA
Medicine composition containing erlotinib hydrochloride and preparation method of medicine composition
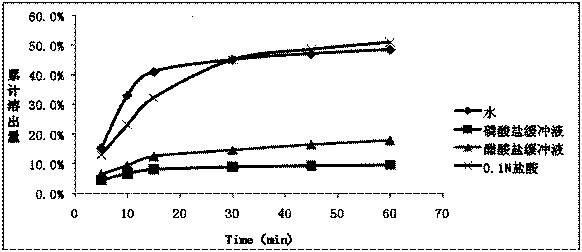
InactiveCN105362239APromote dissolutionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsTraditional medicineDigestion
The invention provides a medicine composition containing erlotinib hydrochloride. The medicine composition has the advantages of being good in digestion performance, high in stability, low in clinical pharmacy risk and the like. In the production and preparation process, raw materials and auxiliary materials do not need to be specially treated, and the production process is simple.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Method for synthesizing erlotinib hydrochloride
ActiveCN104193689ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryNitrationAcetophenone
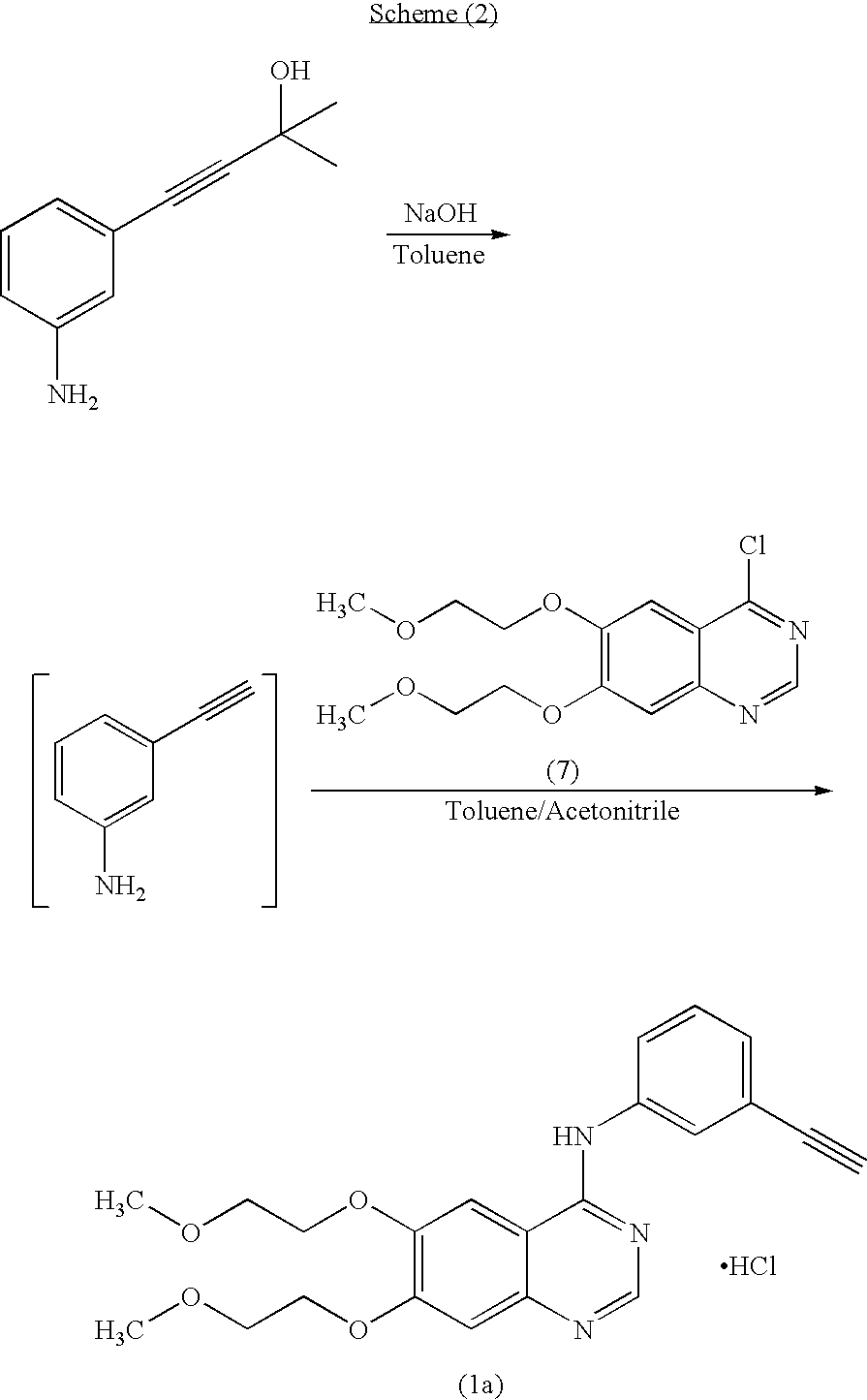
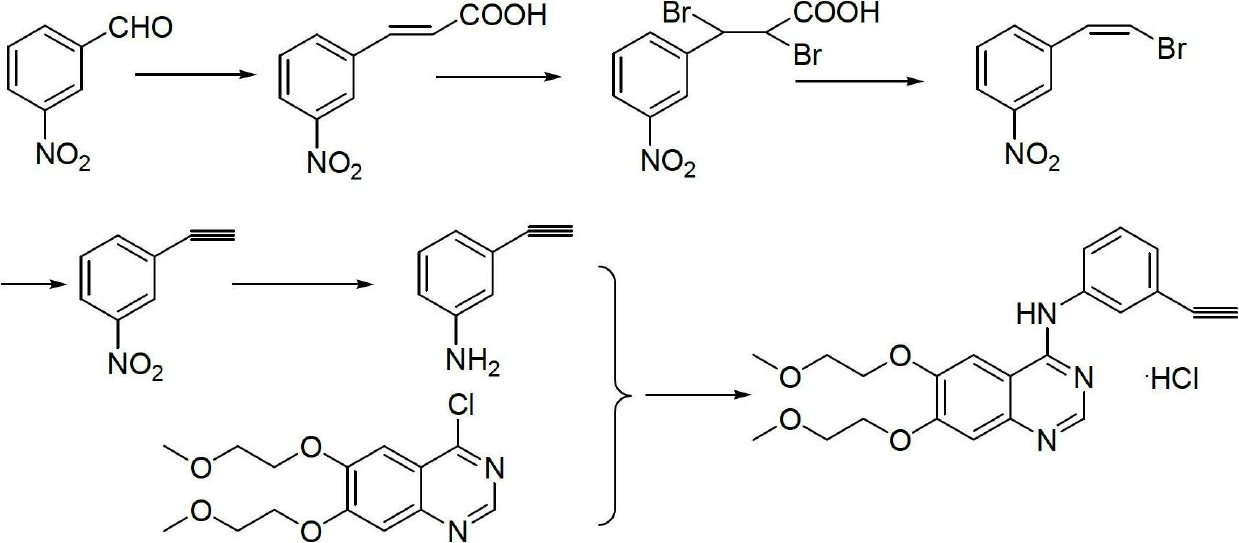
The invention relates to the technical field of medicines, and particularly relates to a novel method for synthesizing erlotinib hydrochloride. The method comprises the following steps: (1) carrying out a nitration reaction in mixed acid on acetophenone serving as an initial raw material to obtain 3-nitroacetophenone; (2) carrying out a chlorination reaction on 3-nitroacetophenone and a chloride agent in an organic solvent to obtain 1-chloro-1-(3-nitrophenyl) ethylene; (3) removing hydrogen chloride from 1-chloro-1-(3-nitrophenyl) ethylene in existence of the organic solvent and strong alkali to obtain m-nitrophenylacetylene; (4) carrying out selective reduction on m-nitrophenylacetylene by means of nitryl to obtain 3-aminophenylacetylene, wherein the reduction method performs reduction by a reducing agent or catalytic hydrogenation; (5) reacting 3-aminophenylacetylene and 4-chloro-6,7-bis-(2-methoxyethoxy) quinazoline in the organic solvent to obtain erlotinib hydrochloride. The method has the advantages of low production cost, mild reaction conditions and the like, is simple and convenient to operate, and is suitable for industrial production; and the raw materials are low in cost and easily available.
Owner:DALIAN UNIV OF TECH
Preparation method of erlotinib hydrochloride crystal form A
ActiveCN103396371AAvoid adjusting pHAvoid the extraction processOrganic chemistryChemical recyclingDimethyl acetalPharmaceutical Substances
The invention provides a preparation method of an erlotinib hydrochloride crystal form A, which belongs to the technical field of the preparation of a drug compound. The preparation method comprises the following steps: enabling 2-amino-4,5-di(2-methoxy ethyoxyl) cyanophenyl and N, N-dimethyl amide dimethyl acetal to react; re-crystallizing and purifying the obtained Schiff base intermediate, and then synthesizing with aminophenylacetylene to obtain erlotinib free alkali; and adding a hydrochloric acid solution, and recrystallizing to obtain the erlotinib hydrochloride crystal form A. According to the scheme of the invention, the process route is short, the product purity is high, the repeatability is good, and the operation is simple and easy to implement, and therefore, the preparation method is suitable for large-scale industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Synthesis method of erlotinib hydrochloride
InactiveCN102675225AEasy to separate and purifyMild reaction conditionsOrganic chemistryPropanoic acidSynthesis methods
The invention relates to a synthesis method of erlotinib hydrochloride, which comprises the following technological process of: a, synthesizing m-nitrocinnamic acid by taking m-nitrobenzaldehyde as raw material; b, carrying out bromine addition to obtain 2, 3-dibromo-3-(3'-nitrophenyl) propanoic acid; c, carrying out decarboxylation and dehydrobromination to obtain (Z)-beta-bromo-(3'-nitrophenyl) ethylene; d, enabling the (Z)-beta-bromo-(3'-nitrophenyl) ethylene to have reaction with metal hydride to obtain m-nitrobenzene acetylene; e, reducing to obtain m-aminophenyl acetylene; and f, enabling the m-aminophenyl acetylene to have reaction with 4-chloro-6, 7-bi-(2-methoxy-ethoxy)-quinazoline, to obtain the erlotinib hydrochloride. The raw materials of the synthesis method are easy to obtain and low in cost, and the synthesis method is mild in reaction conditions, simple and convenient in operation, higher in yield and suitable for amplification.
Owner:ZHEJIANG SCI-TECH UNIV
Process for erlotinib hydrochloride
The present invention provides an improved and commercially viable process for preparation of erlotinib substantially free of N-methoxyethyl impurity, namely N-[(3-ethynylphenyl)-(2-methoxyethyl)]-6,7-bis(2-methoxyethoxy)-4-quinazolinamine, and its pharmaceutically acceptable acid addition salts thereof in High purity and in high yield. According to the present invention, erlotinib or a pharmaceutically acceptable acid addition salt of erlotinib substantially free of N-methoxyethyl impurity is prepared by isolating erlotinib or a pharmaceutically acceptable salt of erlotinib from a solvent medium comprising dimethyl sulfoxide and an alcoholic solvent.
Owner:HETERO DRUGS LTD
Synthesis method of erlotinib hydrochloride
InactiveCN106957274AReasonable designRaw materials are easy to getOrganic chemistrySynthesis methodsHydroxylammonium chloride
The invention discloses a synthesis method of erlotinib hydrochloride. The synthesis method comprises the following steps: (1) at a first working section: preparing 3-methoxyl-4-hydroxybenzonitrile from vanillin and hydroxylammonium chloride; (2) at a second working section: synthesizing 3,4-dihydroxybenzonitrile; (3) at a third working section: synthesizing 3,4-di(2-methoxyethoxy)phenylacetonitrile; (4) at a fourth section: synthesizing 4,5-di(2-methoxyethoxy)-2-nitrophenylacetonitrile; (5) at a fifth working section: synthesizing 4,5-di(2-methoxyethoxy)-2-aminophenylacetonitrile hydrochloride; and (6) at a sixth working section: synthesizing the erlotinib hydrochloride. The synthesis method of the erlotinib hydrochloride, disclosed by the invention, has the advantages of reasonable design, easiness of obtaining raw materials, relatively low production cost, simplicity and easiness of operation and is suitable for industrial production.
Owner:YANCHENG TEACHERS UNIV +1
Process for preparing stable polymorphic form of erlotinib hydrochloride
The present invention discloses an improved and efficient process for preparing Erlotinib hydrochloride suitable as a cancer drug.
Owner:CADILA HEALTHCARE LTD
Preparation method for erlotinib hydrochloride impurity
ActiveCN104003946ARaise quality standardsShort synthetic routeOrganic chemistryPresent methodEthyl Chloride
The present invention relates to a preparation method for an erlotinib hydrochloride impurity N-(3-vinyl-phenyl)--quinolineamine hydrochloride. Firstly, 6,7-bis-(2-methoxyethoxy)-4(3H)-quinazolinone reacts with an acylating reagent to obtain 4-chloro-6,7-bis-(2-methoxyethoxy)-quinazoline, then the 4-chloro-6,7-bis-(2-methoxyethoxy)-quinazoline reacts with m-aminostyrene to generate the target product N-(3-vinyl-phenyl)--quinolineamine hydrochloride. The present method has characteristics of a short synthetic route, simple operation and high product purity up to 99.77%, and provides conditions for the qualitative and quantitative analysis of impurity in the production of erlotinib hydrochloride, thereby further improving the quality standards of erlotinib hydrochloride and providing guidance for safe medication of people.
Owner:NINGBO MENOVO PHARMA
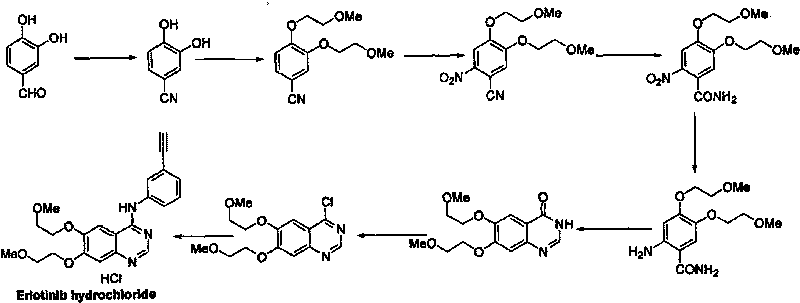
Preparation method of erlotinib hydrochloride
The invention discloses a preparation method of erlotinib hydrochloride. The preparation method comprises the following steps of using 3,4-dihydroxy benzaldehyde (compound I) as a starting raw material; converting an aldehyde group into a cyano group; undergoing six-step reactions of oxyalkylating a side chain, nitrating, hydrolyzing the cyano group, reducing a nitro group, cyclizing and performing salt formation to obtain the erlotinib hydrochloride. According to a synthetic method, the starting material is simple, low in price and easily-obtained; compared with an existing route of first closing ring, then chloridizing and finally ammonifying; the synthetic method disclosed by the invention has the advantages that steps are reduced, the yield is increased, the reaction process is easy in operation and the preparation cycle is shorter; meanwhile, the use of chlorinating agents such as phosphorus trichloride, phosphorus pentachloride, thionyl chloride, phosgene or phosphorus oxychloride with corrosivity is also avoided; the preparation method is suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method of erlotinib hydrochloride crystal form A
InactiveCN103360325ASolve poor crystallization effectImprove stabilityOrganic chemistryOrganosolvHydrogen chloride
The invention specifically relates to a preparation method of an erlotinib hydrochloride crystal form A. The preparation method comprises the following steps of: mixing erlotinib hydrochloride free alkaline monomer with an organic solvent; and dropwise adding a non-ether hydrogen chloride solution or a hydrogen chloride solution for reacting to obtain the erlotinib hydrochloride crystal form A under low-temperature reaction condition. The preparation method of the erlotinib hydrochloride crystal form A is incapable of affecting industrial production due to weather temperature changes, simple and convenient to operate, very good in safety, high in stability, good in repeatability and more suitable for the industrial production of the erlotinib hydrochloride.
Owner:CHONGQING PHARMA RES INST
Erlotinib hydrochloride tablet and preparation method thereof
InactiveCN105616374AReduce the risk of delaminationDoes not affect in vivo permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionSilicon dioxide
The invention provides an erlotinib hydrochloride tablet. The erlotinib hydrochloride tablet is prepared from 109-164 parts by weight of erlotinib hydrochloride, 69-104 parts by weight of lactose, 120-180 parts by weight of microcrystalline cellulose, 5-50 parts by weight of sodium carboxymethyl starch, 0.1-1 part by weight of sodium dodecyl sulfate, 10-30 parts by weight of silica, 0.5-10 parts by weight of magnesium stearate and 0-25 parts by weight of a coating agent. The invention also provides a preparation method of the erlotinib hydrochloride tablet. The preparation method solves the problem that the fine raw materials can easily form airborne dust and has high adhesion and a slow dissolution rate. The preparation method utilizes a wet granulation process, improves raw material granularity and utilizes a wetting agent so that material airborne dust is greatly reduced and sticking possibility is reduced. Through wet granulation, the raw materials and accessory materials are uniformly dispersed and bonded with each other and layering possibility is reduced. Through prescription adjustment of silica addition, a raw material dissolution rate is improved. The erlotinib hydrochloride tablet provides a novel choice for clinical application.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Erlotinib hydrochloride pharmaceutical composition without containing surfactant
ActiveCN104288114ADoes not affect bioavailabilityImprove securityOrganic active ingredientsPill deliveryActive agentPharmaceutical drug
The invention discloses an erlotinib hydrochloride pharmaceutical composition without containing a surfactant. The erlotinib hydrochloride pharmaceutical composition comprises the following components in parts by weight: 150-300 parts of erlotinib hydrochloride, 40-250 parts of diluents, 80-190 parts of disintegrants and 5-10 parts of lubricants. The invention also provides a preparation method of the pharmaceutical composition. Use of a special auxiliary material of sodium dodecyl sulfate in the composition is rejected, the problem that in vitro dissolution reaches a dissolution platform under special determination conditions is solved simultaneously, the erlotinib hydrochloride pharmaceutical composition is consistent with a reference preparation, in vivo bioavailability is not affected, and the safety of a product in clinical use is improved.
Owner:CHENGDU SINO STRONG PHARMA
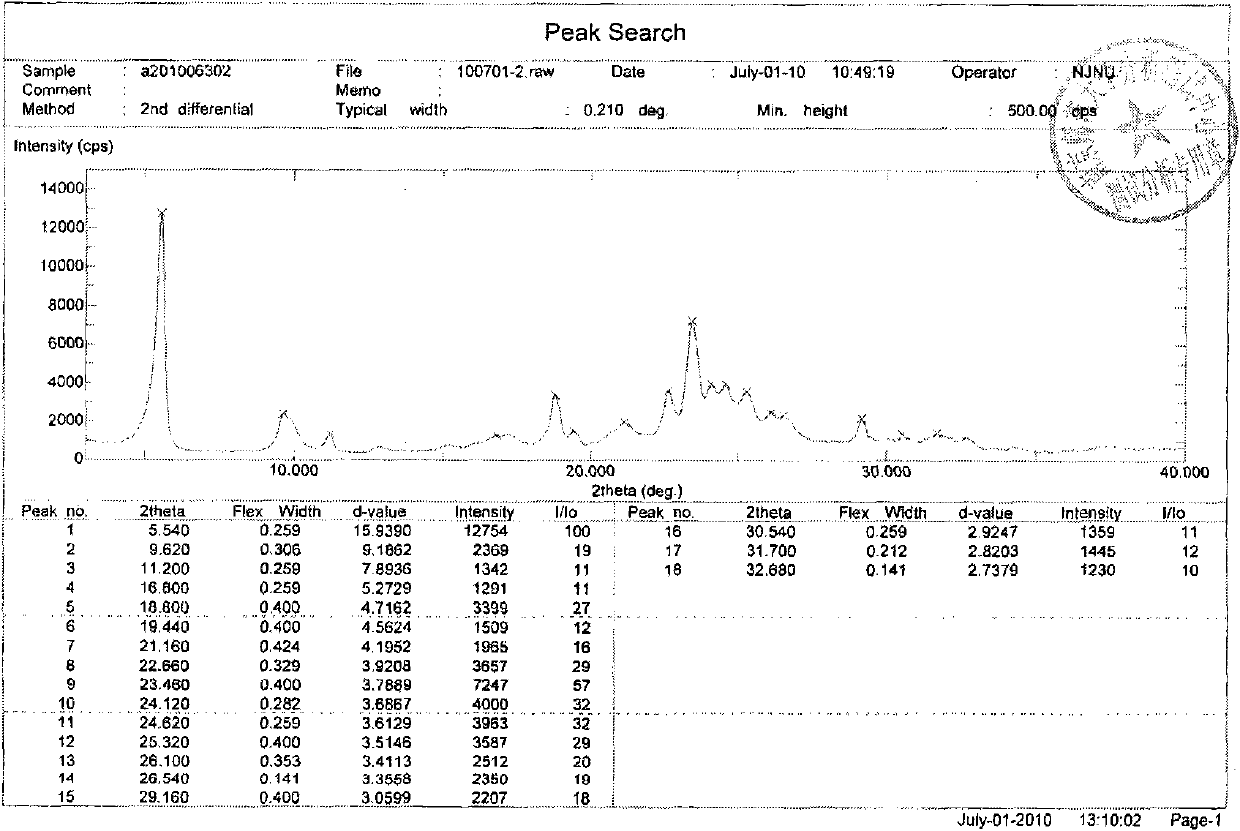
Preparation method of hydrochloric acid erlotinib crystal form F
The invention relates to a novel preparation method of hydrochloric acid erlotinib crystal form F. The method comprises steps that erlotinib free alkali in C4 alcohol is added into hydrogen chloride dissolved in an organic solvent, and the formed hydrochloric acid erlotinib is crystallized. The method has the advantages of suitability for mass production and good reproducibility. Furthermore, the invention also comprises a method of converting hydrochloric acid erlotinib crystal form F into other forms.
Owner:ESTEVE HUAYI PHARMA
Erlotinib hydrochloride composition tablet and preparation method thereof
InactiveCN105748429AThe preparation process route is stableEasy to operateOrganic active ingredientsOrganic chemistry methodsCelluloseCyclodextrin
The invention discloses an erlotinib hydrochloride composition tablet and a preparation method thereof. The erlotinib hydrochloride composition tablet comprises the following ingredients in percentage by weight: 20-38% of erlotinib hydrochloride crystals, 30-50% of pregelatinized starch, 25-45% of calcium sulfate, 1-5% of ethyl cellulose, 6-12% of beta-cyclodextrin, 0.2-3% of Tween, 5-10% of crosslinked sodium carboxymethyl starch, 0.5-1.5% of sodium stearyl fumarate and 0.2-2% of micropowder silica gel. A preparation process route of the erlotinib hydrochloride composition tablet is stable, simple and easy to operate, set technological parameters can effectively control each reaction, and sample detection results show that an erlotinib hydrochloride crude drug is stable in crystal form and relatively good in purity and quality requirements of a final product can be met. A synthesis and production technology of the erlotinib hydrochloride composition tablet is simple, stable and feasible and is applicable to mass production; and quality is controllable, and stability is relatively good.
Owner:DEYANG HUAKANG PHARMA
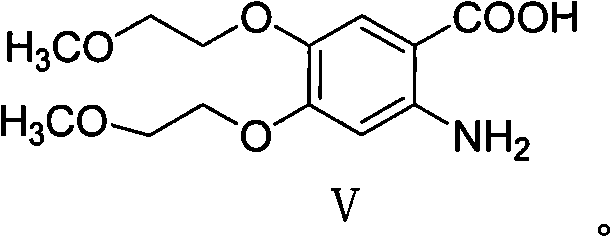
Preparation method of erlotinib hydrochloride impurities
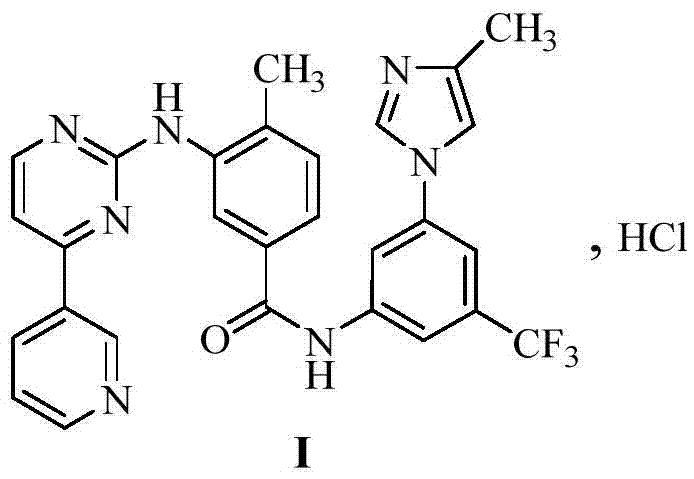
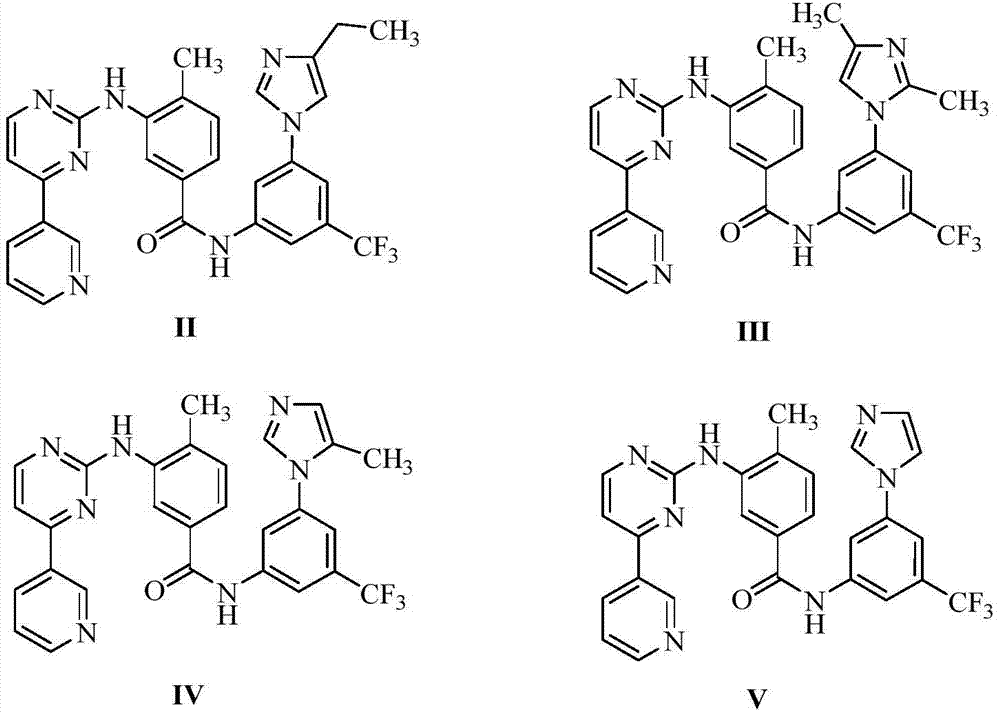
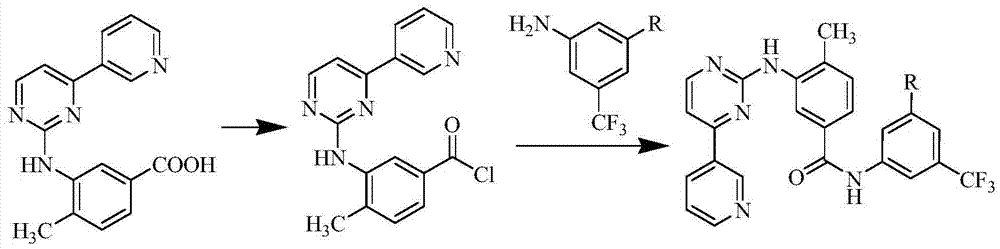
InactiveCN106905298AEasy to operateThe reaction conditions are mild and controllableOrganic chemistryMedicinal chemistryImpurity
The invention relates to a preparation method of important impurities in an erlotinib hydrochloride raw material or preparation technology. Compounds with formulas II to V are erlotinib hydrochloride impurities, and can be used as impurity reference substances to control the purity of theerlotinib hydrochloride raw material or the preparation.
Owner:HAINAN SIMCERE PHARMA CO LTD +1
Industrialized production method for erlotinib hydrochloride B type crystal
An industrialized production method for erlotinib hydrochloride B type crystal comprises: reacting a raw material erlotinib with hydrogen chloride, and after the reaction cooling and precipitating a crystal to obtain the finished product erlotinib hydrochloride B type crystal, wherein the reaction solvent is acetone or a mixture of acetone and water. The usable raw materials of the method comprise erlotinib amorphous solid or crystal, wherein the crystal form of the crystal is unlimited and can be a singular crystal form or a mixed crystal form. The method is simple, has no crystal form conversion step, is applicable to industrialized production, and has no toxic and harmful solvents needed and no pollution to environment; and product purity reaches 99% or more and product yield reaches 95% or more.
Owner:CHONGQING HUAPONT PHARMA
Preparation method of erlotinib base monohydrate crystal form I
The invention belongs to the technical field of pharmaceutical chemicals, and in particular relates to a preparation method of an erlotinib base monohydrate crystal form I. The preparation method comprises the following steps: stirring and mixing erlotinib hydrochloride, base and an organic solvent, or stirring and mixing erlotinib base and an organic solvent; dropwise adding a solution system after an reaction or after being classified to water under a stirring condition; while stirring, decreasing temperature to 0-5 DEG; and filtering and drying in a vacuum environment to obtain erlotinib base monohydrate crystal form I. The erlotinib base monohydrate crystal form I is prepared by crystallization at room temperature through an organic solvent which is cheap, easy to prepare, green and environment-friendly; and the preparation method is safe and environment-friendly in process route, simple to operate, low in cost, good in repeatability, stable, applicable to industrial production and quite high in economic value. The prepared erlotinib base disclosed by the invention has high purity, and can be used for preparing erlotinib hydrochloride high in purity.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Erlotinib sustained-release preparation for treating non-small cell lung cancer
ActiveCN111617048AReduce releaseStable blood concentrationOrganic active ingredientsPharmaceutical non-active ingredientsCancer cellBlood drug concentration
The invention relates to an erlotinib (Erlotinib Hydrochloride) sustained-release preparation for treating the non-small cell lung cancer. The erlotinib sustained-release preparation is prepared fromthe following raw and auxiliary materials in percentage: 30% to 50% of erlotinib, 10% to 40% of sustained-release material, 30% to 50% of filler, 1% to 10% of solubilizer and 1% to 4% of lubricant. The invention further provides a preparation method of the sustained-release tablets. The preparation method comprises the steps of: firstly, placing the erlotinib, the sustained-release material and the filler into a high-efficiency wet granulator to uniformly mix; then adding the solubilizer into purified water to prepare solution; adding a proper volume of solution into a pot to carry out wet granulation; after particles are dried, adding a proper volume of lubricant; and after uniformly mixing, carrying out tabletting to form the tablets. Compared to a common drug, the erlotinib sustained-release preparation has the advantages of reducing the "peak valley" effect of in-vivo release of the drug, reducing a probability that cancer cells generate drug resistance, reducing adverse reactionson a patient, which are generated due to excessively high blood concentration of the drug, enabling the blood concentration to be continuously and stably kept to optimal treatment concentration, improving bioavailability of the drug, reinforcing the curative effect and improving administration safety.
Owner:苏州特瑞药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com