Preparation method of erlotinib hydrochloride crystal form A
A technology of erlotinib hydrochloride and crystal form, which is applied in the field of chemical pharmaceuticals, and can solve problems such as not being suitable for industrial production, difficult to complete the reaction, and cumbersome preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
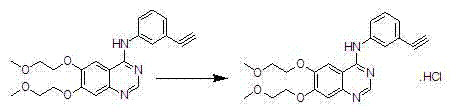
[0035] Add 1 g of erlotinib free base monomer and 10 ml of acetone into a clean 50 ml reaction bottle, start stirring, cool to an internal temperature of -20°C to -15°C, and slowly add 1.4 ml of 2mol / L hydrogen chloride acetone solution dropwise, To pH = 5, the dropwise addition was completed in 1 hour, kept stirring for 1 hour, filtered, drained, and dried in a vacuum oven at 20°C to 30°C to obtain 1.07g of dry crystal form, yield 99.1%, purity (HPLC: 99.7%) .
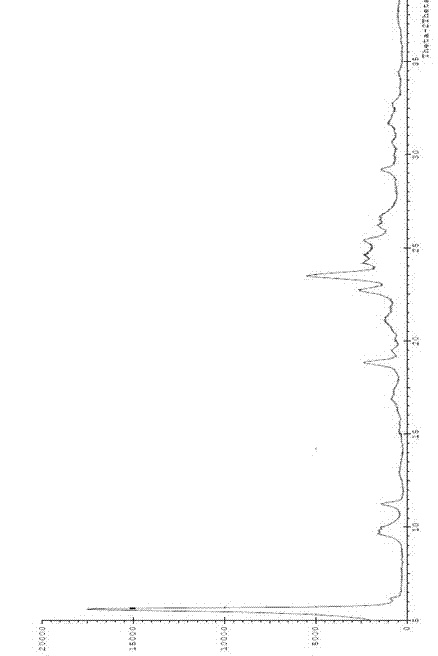
[0036] The obtained crystal form was tested by X powder diffractometer, CuKα source (α = 1.5406?) of Shimadzu XRD-6000 X-ray diffractometer, at 40kv, 30mA, scanning range 5 ~ 40 (deg), scanning speed, 4deg / min, get as figure 1 The diffraction pattern (XRPD) shows that the crystal form is erlotinib hydrochloride crystal form A.
Embodiment 2
[0038] Add 1 g of erlotinib free base monomer and 12 ml of acetone into a clean 50 ml reaction bottle, start stirring, cool to the inner temperature of -20 ° C ~ -15 ° C, and start slowly adding 2 mol / L of hydrogen chloride ethyl acetate solution 1.4 ml, until pH=5, the dropwise addition was completed in 0.5 hours, kept stirring for 1 hour, filtered, drained, and dried in a vacuum oven at 20-30°C to obtain 1.08 g of dry product, with a yield of 99.7%. After testing its XRPD, it was shown to be erlotinib hydrochloride crystal form A with a purity (HPLC: 99.6%).
Embodiment 3
[0040] Add 1 g of erlotinib free base monomer and 10 ml of acetone into a clean 50 ml reaction bottle, start stirring, cool to an internal temperature of -20°C to -15°C, and slowly add 1.4 ml of 2mol / L hydrogen chloride ethanol solution dropwise, To pH = 1, the dropwise addition was completed in 2 hours, kept stirring for 6 hours, filtered, pumped dry, and dried in a vacuum oven at 20-30°C to obtain 1.07 g of dry product, with a yield of 99.1%. After testing its XRPD, it was shown to be erlotinib hydrochloride crystal form A with a purity (HPLC: 99.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com