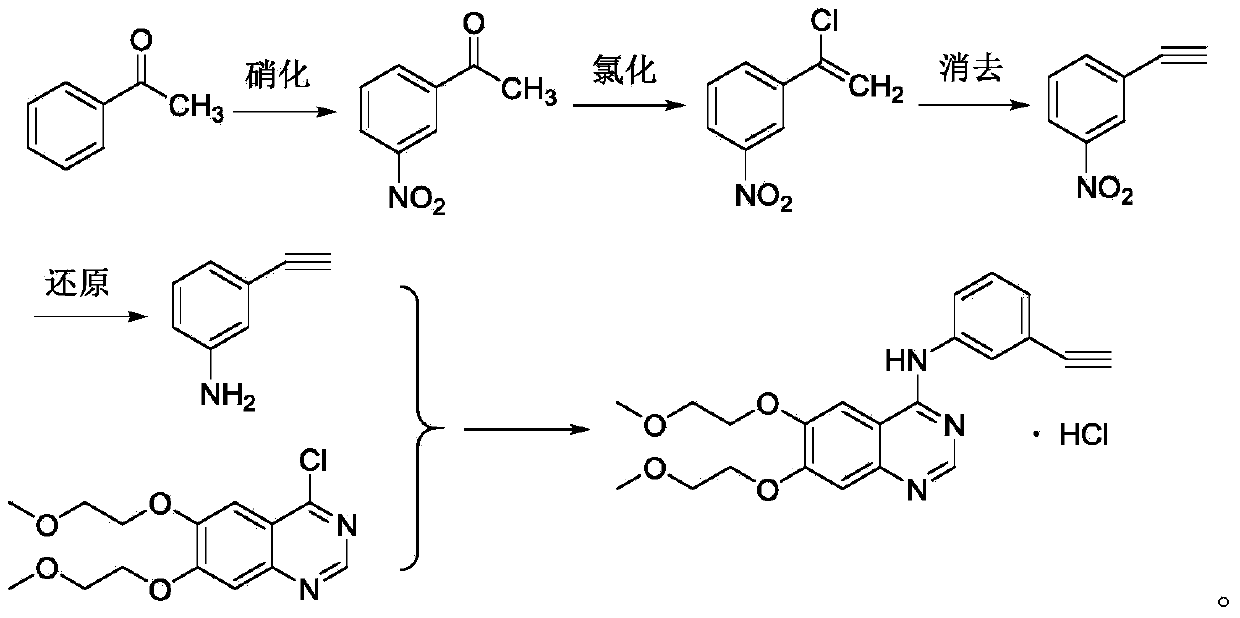
Method for synthesizing erlotinib hydrochloride
A technology of erlotinib hydrochloride and a synthetic method, which is applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, high production cost, and high cost, and achieve the effects of cheap raw materials, low production cost, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 m-nitroacetophenone
[0025]
[0026] Add 400ml of concentrated sulfuric acid to a 1000ml reaction bottle, add 180.0g (1.5mol) of acetophenone dropwise, mix 200ml of concentrated sulfuric acid with 150ml of concentrated nitric acid and add it dropwise into the system. During the whole process, the temperature of the system should not exceed -5°C. Afterwards, the reaction was continued for 20 min, and the reaction was detected by TLC. Slowly pour the system into a large amount of ice water while stirring, filter with suction, and wash the filter cake with saturated sodium carbonate to remove residual acid to obtain a crude product. The crude product was recrystallized from ethanol to obtain 220.1 g of light yellow needle-like crystals, with a yield of 88.9% and a content of ≥99%.
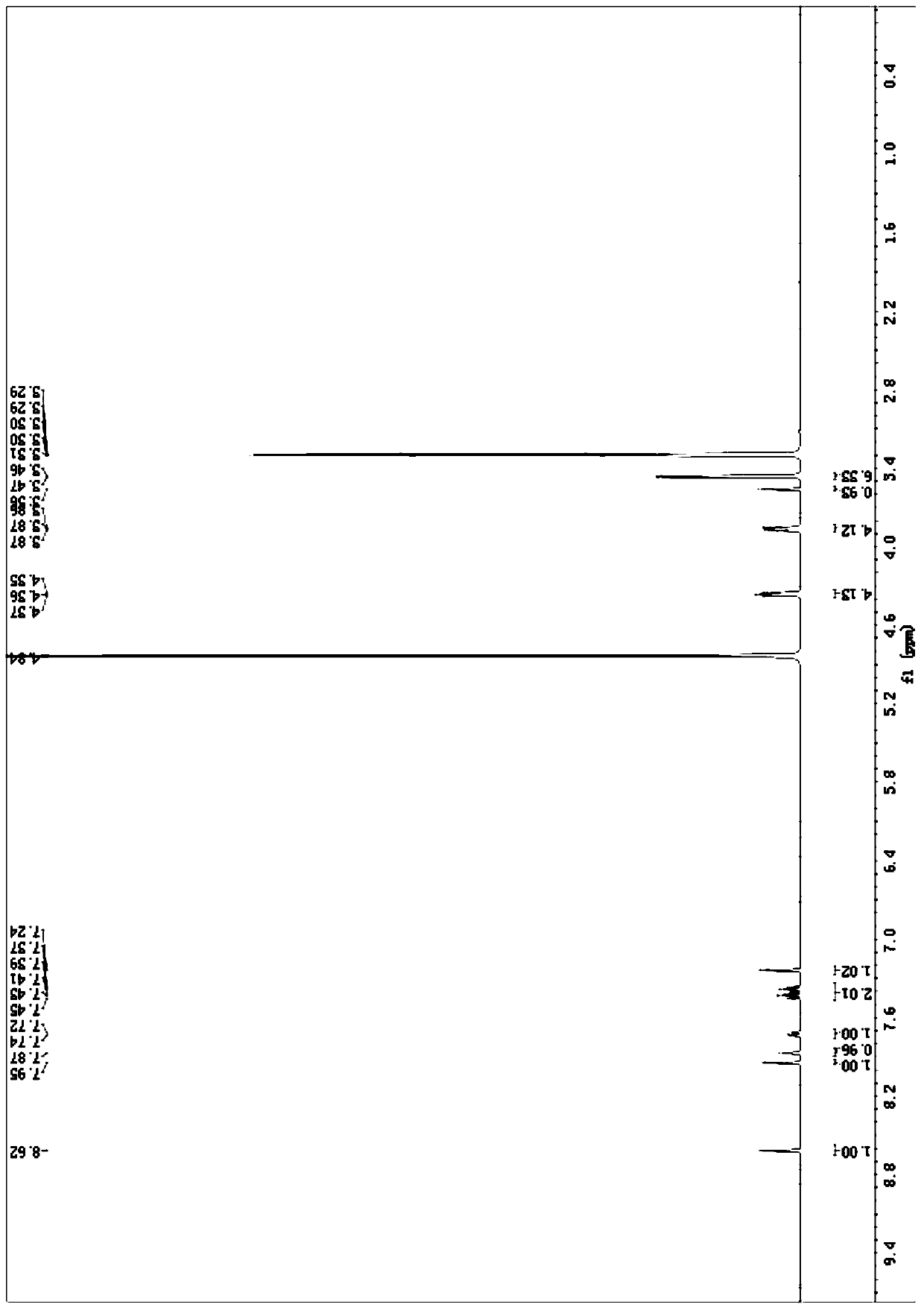
[0027] NMR data: 1 H NMR (400MHz, CDCl 3 )δ8.78(s,1H),8.47–8.40(m,1H),8.30(d,J=7.8Hz,1H),7.69(t,J=8.0Hz,1H),2.70(s,3H)
Embodiment 2
[0028] The synthesis of embodiment 2 m-nitroacetophenone
[0029] Add 400ml of concentrated sulfuric acid to a 1000ml reaction bottle, add 180.0g (1.5mol) of acetophenone dropwise, mix 120ml of concentrated sulfuric acid with 80ml of fuming nitric acid and add it dropwise into the system. After completion, the reaction was continued for 10 min, and the reaction was detected by TLC. Slowly pour the system into a large amount of ice water while stirring, filter with suction, and wash the filter cake with saturated sodium carbonate to remove residual acid to obtain a crude product. The crude product was recrystallized from ethanol to obtain 223.5 g of light yellow needle-like crystals, with a yield of 90.3% and a content of ≥99%.
Embodiment 3
[0030] Example 3 Synthesis of 1-chloro-1-(3-nitrophenyl)ethene
[0031]
[0032] Add 132.0g (0.8mol) of m-nitroacetophenone, 450ml of toluene, and 31.6g (0.4mol) of pyridine into a 1000ml reaction flask, stir to dissolve the raw materials, and slowly add 146.9g (0.96mol) of phosphorus oxychloride dropwise at room temperature , after the dropwise addition was completed, the temperature was raised to 115°C for reaction, and the reaction progress was detected by TLC. After the reaction was finished, it was cooled, and 100ml of water was added to the system. The organic layer was washed with saturated sodium carbonate and saturated brine, dried and concentrated to obtain a crude product, which was distilled under reduced pressure to obtain 133.2 g of a light yellow product with a yield of 91.0% and a content of ≥97%.
[0033] NMR data: 1 H NMR (400MHz, CDCl 3 )δ8.48(s, 1H), 8.21(d, J=8.4Hz, 1H), 7.95(d, J=7.8Hz, 1H), 7.56(t, J=8.0Hz, 1H), 5.93(d, J=2.1Hz, 1H), 5.70(d, J=2.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com