Medicine composition containing erlotinib hydrochloride and preparation method of medicine composition
A technology of erlotinib hydrochloride and composition, which is applied in the field of pharmaceutical composition and preparation containing erlotinib hydrochloride, can solve problems such as flying dust, environmental pollution, and small bulk density, and achieve high process stability and dissolution Good performance and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
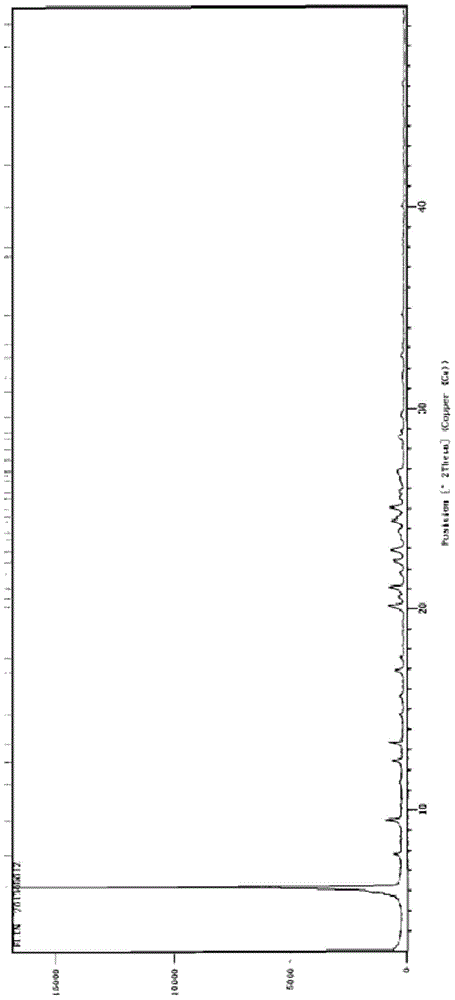
Image
Examples
Embodiment 1
[0054] type Quality (mg / tablet) parts by mass Erlotinib hydrochloride (calculated as Erlotinib) 100 1 lactose 70 0.7 microcrystalline cellulose 90 0.9 Sodium carboxymethyl starch 25 0.25 silica 10 0.1 Sodium dodecyl sulfate 3 0.03 Magnesium stearate 3 0.03
[0055] 1. Erlotinib hydrochloride and sodium lauryl sulfate are sieved for subsequent use;
[0056] 2. Mix the prescribed amount of raw materials, sodium carboxymethyl starch, and sodium lauryl sulfate for 5 minutes, add silicon dioxide and mix, and pass through a 40-mesh sieve to obtain mixed powder A;
[0057] 3. Then add the prescribed amount of lactose and microcrystalline cellulose and mix for 10 minutes to obtain the mixed powder B;
[0058] 4. Add the mixed powder B to the dry granulator for granulation, control the distance between the pressing wheels to 0.5mm, and the speed of the pressing wheels to 3r / min, to obtain a density of 1.03g / cm 3 The fl...
Embodiment 2
[0061] type Quality (mg / tablet) parts by mass Erlotinib hydrochloride (calculated as Erlotinib) 100 1 lactose 70 0.7 microcrystalline cellulose 100 1.0 Sodium carboxymethyl starch 27 0.27 silica 10 0.1 Sodium dodecyl sulfate 3 0.03 Magnesium stearate 2 0.02
[0062] 1. Erlotinib hydrochloride and sodium lauryl sulfate are sieved for subsequent use;
[0063] 2. Mix the prescribed amount of raw materials, sodium carboxymethyl starch, and sodium lauryl sulfate for 5 minutes, add silicon dioxide and mix, and pass through a 40-mesh sieve to obtain mixed powder A;
[0064] 3. Then add the prescribed amount of lactose and microcrystalline cellulose and mix for 10 minutes to obtain the mixed powder B;
[0065] 4. Add the mixed powder B to the dry granulator for granulation, control the distance between the pressing wheels to 0.5mm, and the speed of the pressing wheels to 2r / min, to obtain a density of 1.06g / cm 3 The f...
Embodiment 3
[0068] type Quality (mg / tablet) parts by mass Erlotinib hydrochloride (calculated as Erlotinib) 100 1 lactose 70 0.7 microcrystalline cellulose 100 1.0 Sodium carboxymethyl starch 25 0.25 silica 10 0.1 Sodium dodecyl sulfate 3 0.03 Magnesium stearate 2 0.02
[0069] 1. Erlotinib hydrochloride and sodium lauryl sulfate are sieved for subsequent use;
[0070] 2. Mix the prescribed amount of raw materials, sodium carboxymethyl starch, and sodium lauryl sulfate for 5 minutes, add silicon dioxide and mix, and pass through a 20-mesh sieve to obtain mixed powder A;
[0071] 3. Then add the prescribed amount of lactose and microcrystalline cellulose and mix for 10 minutes to obtain the mixed powder B;
[0072] 4. Add the mixed powder B to the dry granulator for granulation, control the spacing between the pressing wheels to 0.4mm, and the speed of the pressing wheels to 3r / min, to obtain a density of 1.18g / cm 3 The fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com