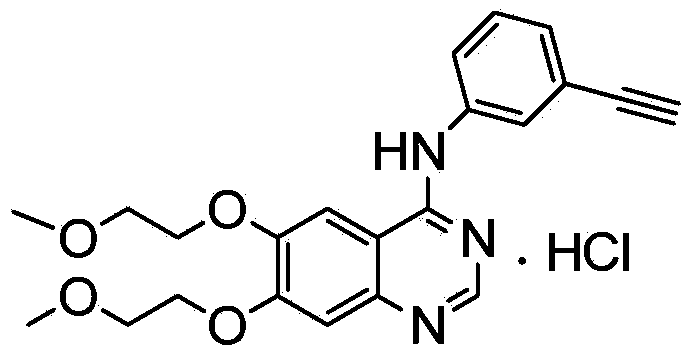
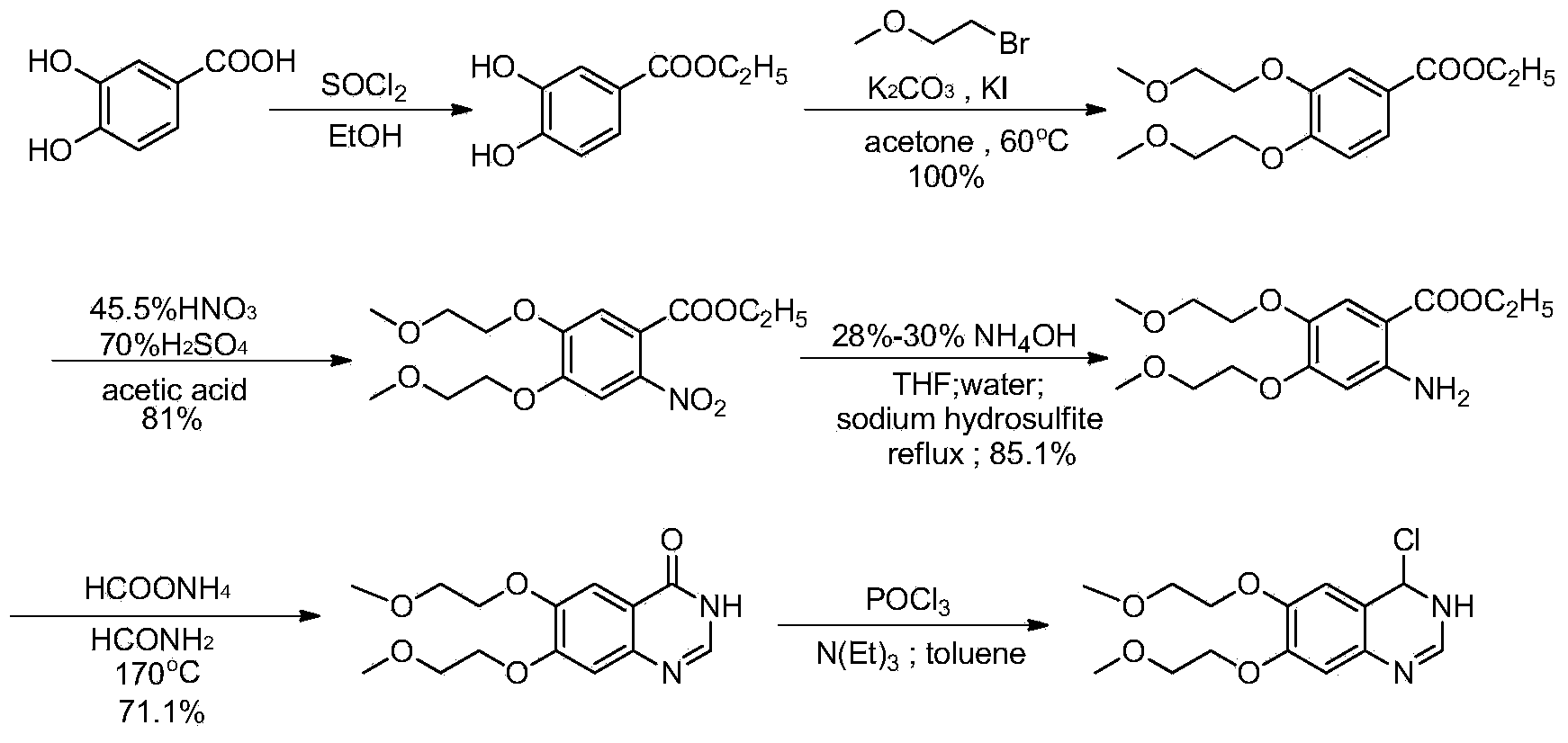
Preparation method of erlotinib hydrochloride key intermediate
A technology of erlotinib hydrochloride and an intermediate, which is applied in the field of pharmaceutical compound synthesis, can solve problems such as the recovery and application of unfavorable solvents, and achieve the effects of easy mass production, good environmental protection and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A. Synthesis of compound (i)
[0057] Add methanesulfonyl chloride (250g), ethylene glycol monomethyl ether (97.8g) and dichloromethane (587mL), cool down to 0-5°C, slowly add KOH (144g) in batches, raise the temperature to about 25°C for 3 hours , TLC shows that the reaction is complete, add 1360g ice water, stir to dissolve, separate layers, extract the water layer with dichloromethane (645mL×2), combine the dichloromethane layers, wash with 635g water, wash with 20.5g1mol / L hydrochloric acid (pH95%;
[0058] B. Synthesis of compound (iii)
[0059] Compound (i) (194.2g), compound (ii) (111g), potassium carbonate (252.3g), toluene (2220mL) and tetrabutylammonium bromide (5g) were mixed, heated to reflux, reacted for 6 hours, HPLC It shows that the reaction is complete, cool down to room temperature, filter with suction, rinse with 370mL of toluene, wash the filtrate with 1110mL of water, extract the water layer with toluene (555mL×2), combine the toluene layers, wash ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com