Synthesis method of erlotinib hydrochloride
A technology of erlotinib hydrochloride and a synthesis method, applied in the field of drug synthesis, can solve the problems of being unsuitable for industrial production and high production cost of erlotinib hydrochloride, and achieve the effects of low production cost, easy operation and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The technical solution of this patent will be described in further detail below in conjunction with specific embodiments.
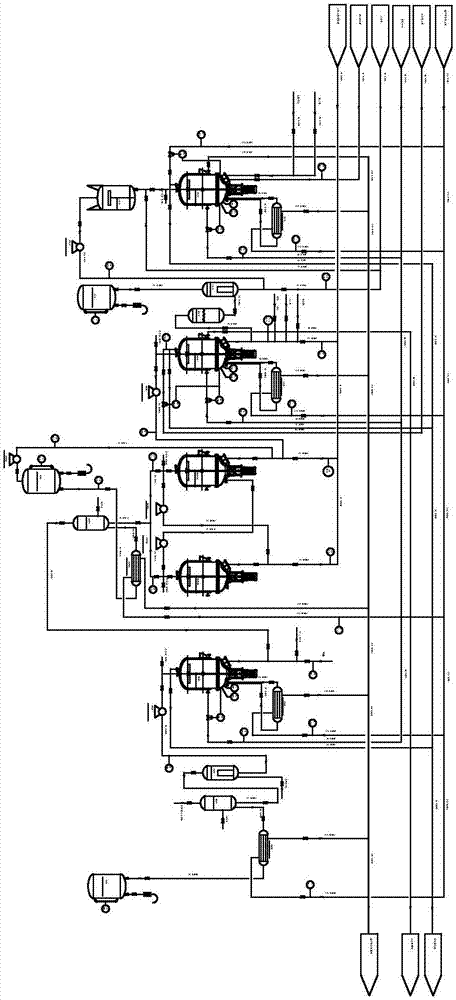
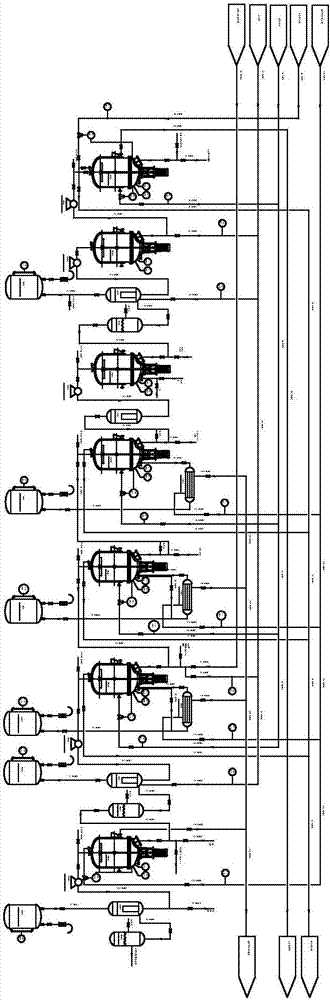
[0038] See Figure 1-2 , A synthetic method of erlotinib hydrochloride, comprising the following steps:
[0039] (1) The first stage: preparing 3-methoxy-4-hydroxybenzonitrile from vanillin and hydroxylamine hydrochloride
[0040] Add vanillin and hydroxylamine hydrochloride to the reactor R0101 respectively, add the solvent DMF to dissolve the raw materials under stirring, and pass high-pressure steam S601001 to heat the reactor R0101. The raw materials are stirred and refluxed in the reactor R0101 for 2h, and the detection port 01001 Check the material composition, the reaction kettle stops heating reaction; then pass the condensed water CWS01002 into the reaction kettle R0101, when the temperature of the reaction solution drops to about 85℃, transfer the reaction solution to the crystallization kettle C0101, and add industrial water to the crystalliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com