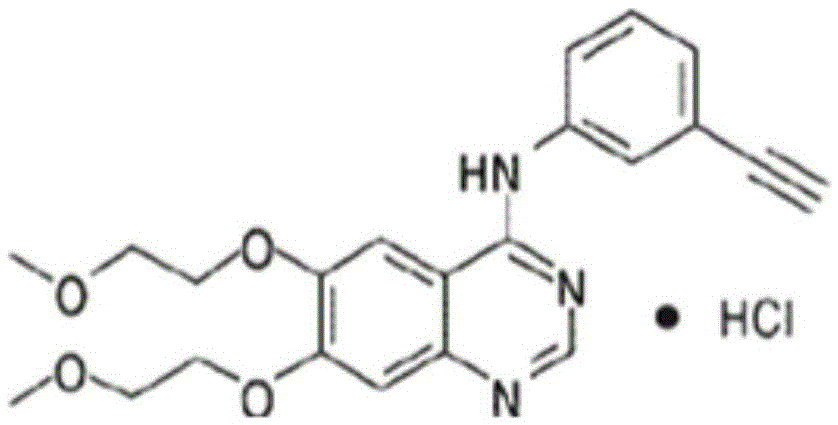
Preparation method of erlotinib hydrochloride
A technology of erlotinib hydrochloride and hydroxylamine hydrochloride, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome synthetic routes, high production costs, and environmental hazards, and achieve the effects of reducing process steps, simplifying operations, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
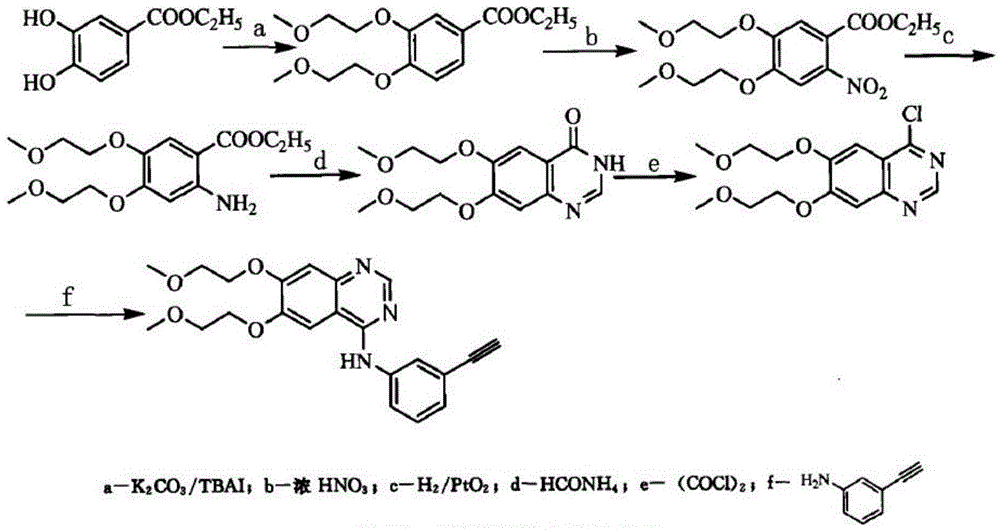
[0044] Embodiment 1: the synthesis of compound I
[0045] Add 55.2g (0.4mol) of 3,4-dihydroxybenzaldehyde, 54.4g (0.8mol) of sodium formate, and 250mL of formic acid into a three-necked flask, stir and heat up to 80°C, add 80.1g (0.5 mol), TLC tracking reaction complete (about 3h). Cool to room temperature, pour the reaction solution into 300mL cold saturated saline, stir, a large amount of white solid precipitates out, filter to obtain the crude product of compound Ⅰ, recrystallize with ethyl acetate, and obtain 49.2g of refined product after vacuum drying, the HPLC purity is 99.3% , yield 90.5%.
Embodiment 2
[0046] Embodiment 2: the synthesis of compound II
[0047] 40.8g (0.3mol) of compound I prepared in Example 1, 75.6g (0.8mol) of 2-chloroethyl methyl ether, 124.4g (0.9mol) of potassium carbonate, 22.2g (0.06 mol) and 300ml of DMSO were added to a four-neck flask, heated to reflux, and after the reaction was complete as detected by TLC, cooled, DMSO was distilled off under reduced pressure, poured into 600ml of ice water, extracted with dichloromethane, washed with brine, dried, and concentrated to obtain compound II70 .0g, HPLC purity 99.5%, yield 92.3%.
Embodiment 3
[0048] Embodiment 3: the synthesis of compound III
[0049] Compound II (62.8g, 0.25mol) was dissolved in 300ml of acetic acid, added dropwise to nitric acid (300ml) cooled to 0°C, and the temperature was controlled not to exceed 10°C. After stirring for 30 minutes, centrifuged, washed with water, dried, and recrystallized from methanol to obtain 71.0 g of compound III, the HPLC purity was 99.7%, and the yield was 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com