Erlotinib sustained-release preparation for treating non-small cell lung cancer
A slow-release preparation, the technology of erlotinib hydrochloride, is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, which can solve the problem of difficulty in maintaining effective therapeutic concentrations and large fluctuations in blood drug concentrations. , adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Prescription (makes 1000 tablets):
[0045] Erlotinib Hydrochloride 150mg
[0046] Lactose 75 mg
[0047] Microcrystalline Cellulose 75mg
[0048] Sodium Lauryl Sulfate 3.75mg
[0049] Hypromellose 81.25mg
[0050] Magnesium stearate (internal addition) 1.87mg
[0051] Magnesium stearate (extra) 3.75mg
[0052] Opadry 9.38mg
[0053] Appropriate amount of purified water
[0054] Preparation:
[0055] Erlotinib hydrochloride, lactose, microcrystalline cellulose, hypromellose, and magnesium stearate of the prescribed amount were pulverized respectively and passed through an 80-mesh sieve, mixed evenly, and prepared with an aqueous solution of sodium lauryl sulfate of the prescribed amount. Made of soft material with binder, granulated, dried in a fluidized bed at 60°C, added with prescribed amount of magnesium stearate, mixed evenly, compressed into tablets, coated, ready to be obtained.
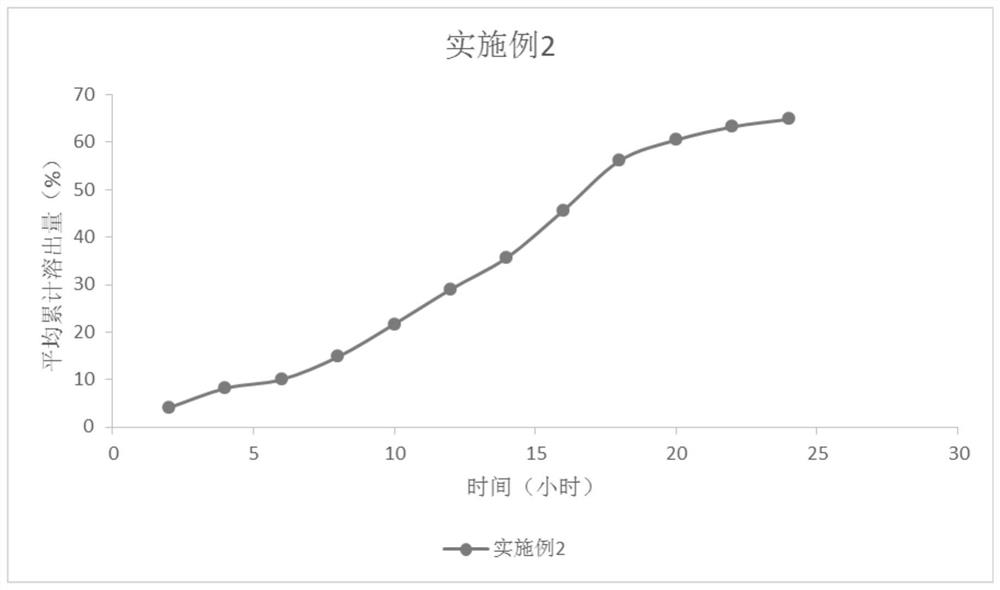
Embodiment 2
[0057] Erlotinib Hydrochloride 150mg
[0058] Lactose 75mg
[0059] Microcrystalline Cellulose 75mg
[0060] Sodium Lauryl Sulfate 3.75mg
[0061] Sodium Alginate 81.25mg
[0062] Magnesium stearate (internal addition) 1.87mg
[0063] Magnesium stearate (extra) 3.75mg
[0064] Opadry 9.38mg
[0065] Appropriate amount of purified water
[0066] Preparation:
[0067] The prescribed amount of erlotinib hydrochloride, lactose, microcrystalline cellulose, sodium alginate, and magnesium stearate were respectively crushed and passed through an 80-mesh sieve, mixed evenly, and the aqueous solution of the prescribed amount of sodium lauryl sulfate was used as a binder Preparation of soft material, granulation, drying at 60°C, adding the prescribed amount of magnesium stearate, mixing evenly, pressing into tablets, coating, to obtain.
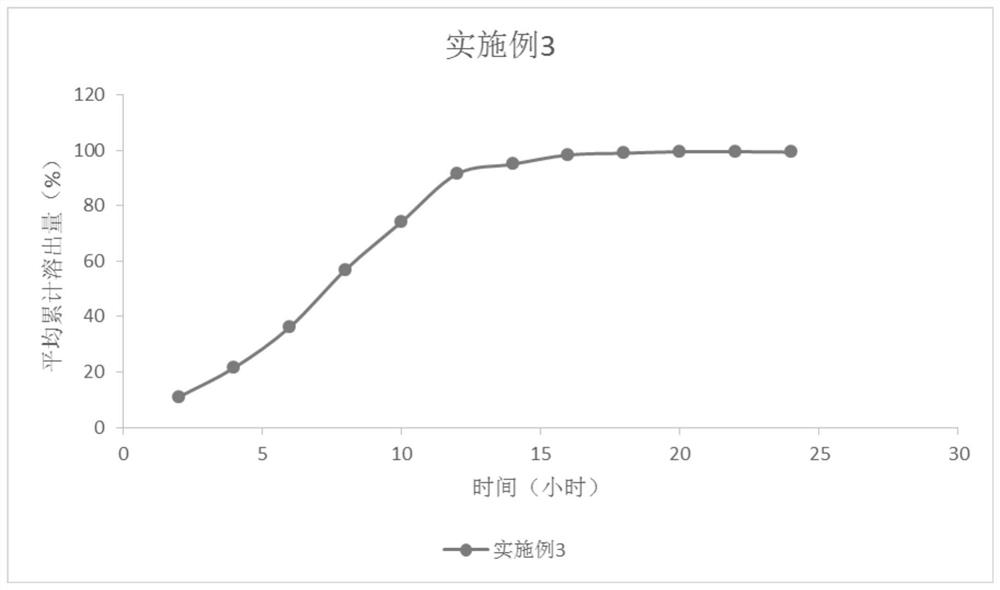
Embodiment 3
[0069] Erlotinib Hydrochloride 150mg
[0070] Lactose 75mg
[0071] Microcrystalline Cellulose 75mg
[0072] Sodium Lauryl Sulfate 3.75mg
[0073] Polyvinylpyrrolidone 81.25mg
[0074] Magnesium stearate (internal addition) 1.87mg
[0075] Magnesium stearate (extra) 3.75mg
[0076] Opadry 9.38mg
[0077] Appropriate amount of purified water
[0078] Preparation:
[0079] Grind the prescribed amount of erlotinib hydrochloride, lactose, microcrystalline cellulose, polyvinylpyrrolidone, and magnesium stearate respectively and pass through an 80-mesh sieve, mix well, and use an aqueous solution of prescribed amount of sodium lauryl sulfate as a binder Preparation of soft material, granulation, drying at 60°C, adding the prescribed amount of magnesium stearate, mixing evenly, pressing into tablets, coating, to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com