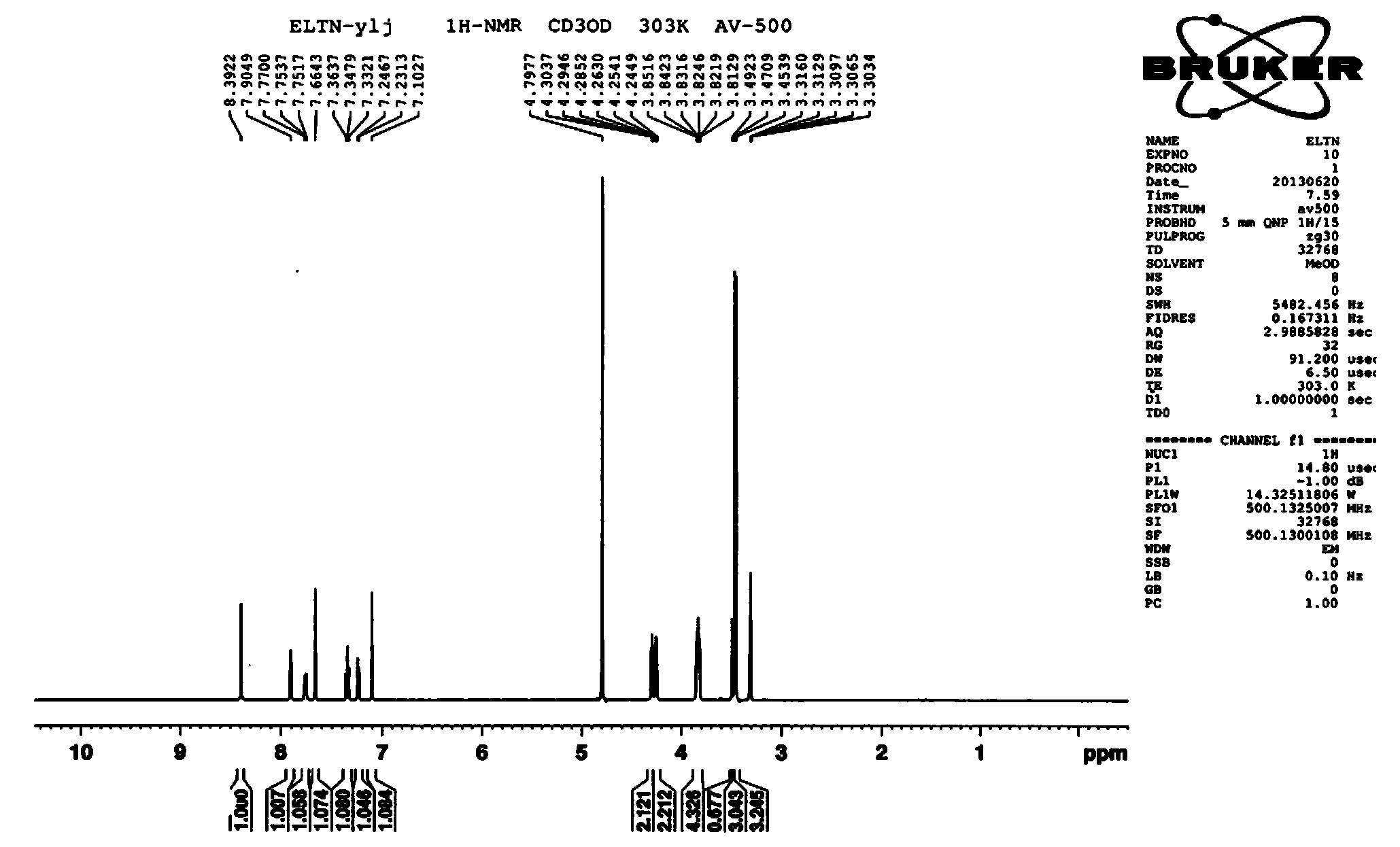
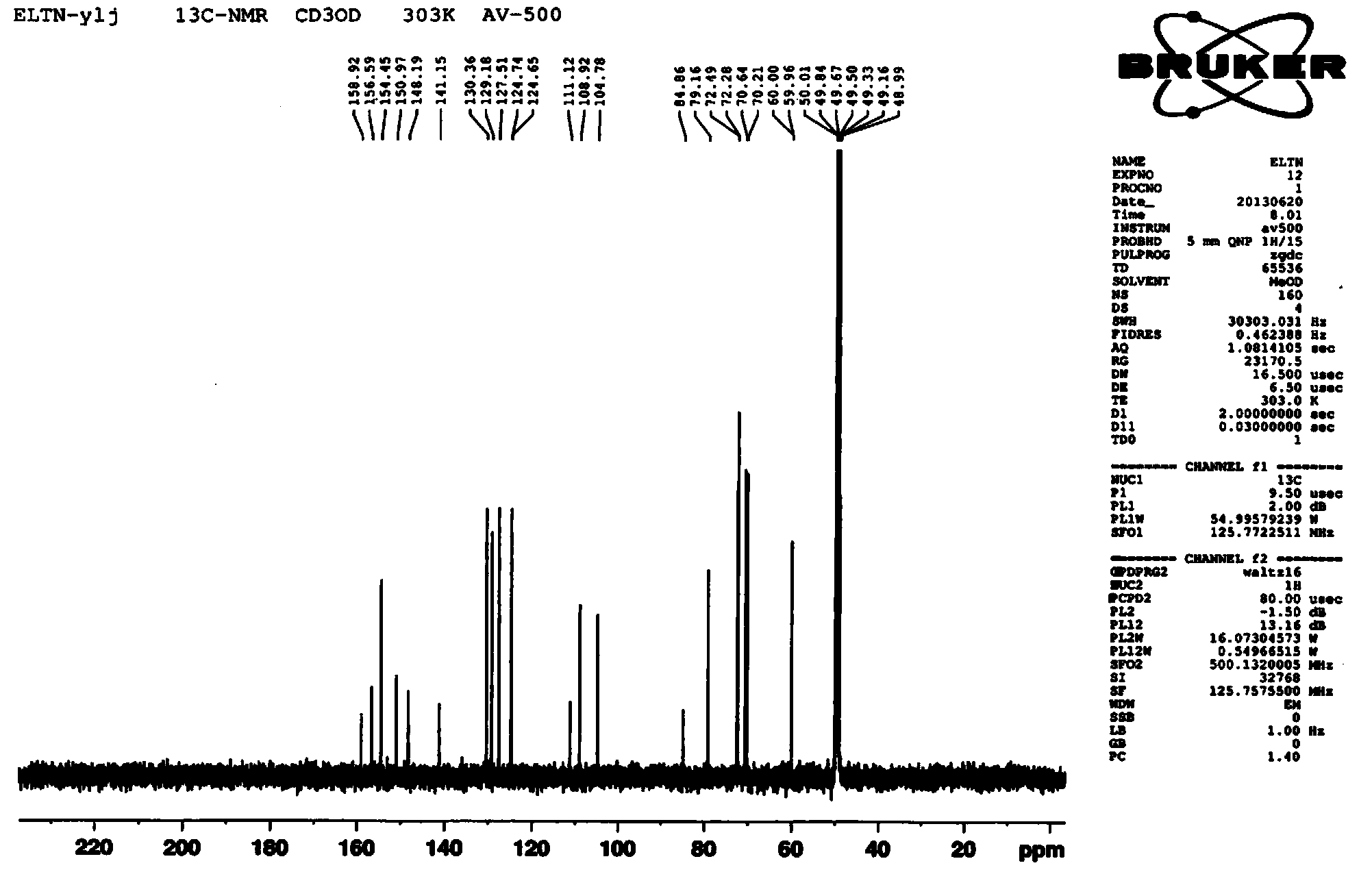
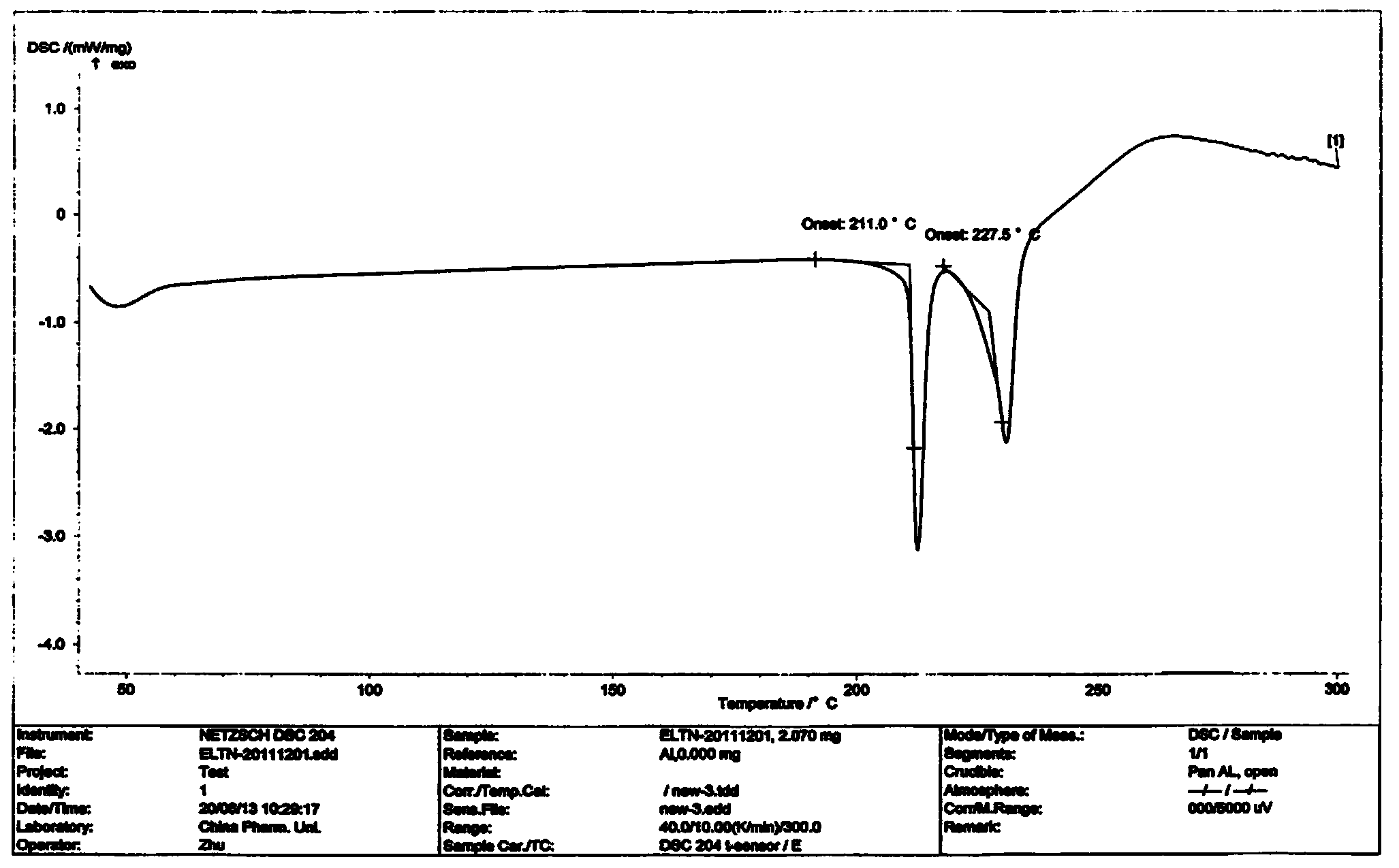
Preparation method of erlotinib hydrochloride crystal form A
A technology of erlotinib hydrochloride and crystal form, which is applied in the field of preparation of erlotinib hydrochloride crystal form A, can solve problems such as easy transformation, achieve cumbersome steps, improve experimental efficiency, and be beneficial to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 2-Amino-4,5-bis(2-methoxyethoxy)benzonitrile (compound of formula II, 1Kg, 3.76mol), toluene 5L, acetic acid 20mL and DMF-DMA (492g, 4.13mol, is the formula 1.1 times the molar amount of compound II) mixed and stirred, refluxed in an oil bath, kept at 80-90°C for 3 hours, and then concentrated under reduced pressure at 60-65°C to obtain about 1.3Kg of the intermediate Schiff base oil. Dissolve the oily substance with isopropyl ether / tetrahydrofuran (20:1) (about 10.5L, 8 times the volume of the oily substance) at 35-40°C for clarification, slowly cool down to 10-15°C, and crystallize to obtain 1.09Kg of a white solid , the yield of Schiff's base was 91%, and the HPLC purity was 99.5%.
[0038] Mix the compound of formula III (1.09Kg, 3.39mol), m-aminophenylacetylene (438g, 3.73mol, 1.1 times the molar weight of the compound of formula III), 5L of acetic acid, start heating, control the internal temperature at 95-100°C, and react 2.5-3h, develop with thin-layer chromat...
Embodiment 2
[0042] 2-Amino-4,5-bis(2-methoxyethoxy)benzonitrile (compound of formula II, 1.3Kg, 4.88mol), toluene 6.5L, acetic acid 26mL and DMF-DMA (629g, 5.37mol) Mix and stir, reflux reaction in an oil bath, keep warm at 80-90°C for 3 hours, and then concentrate under reduced pressure at 60-65°C to obtain about 1.6Kg of the intermediate Schiff base oil. Dissolve and clarify the oil with methyl tert-butyl ether / ethyl acetate (25:1) (about 16 L, 10 times the volume of the oil) at 35-40°C, slowly cool down to 10-15°C, and crystallize to obtain White solid 1.39Kg, Schiff base yield 89%, HPLC purity 99.4%.
[0043] Mix the compound of formula III (1.39K, 4.33mol), m-aminophenylacetylene (558g, 4.76mol), and 7L of acetic acid, mix and stir, start heating, control the internal temperature at 95-100°C, react for 2.5-3h, and use thin-layer chromatography Expand the plate (DCM:MeOH=15:1), the reaction is complete, slowly cool down to an internal temperature of 60°C, add 8.4L of water and tetra...
Embodiment 3
[0047] 2-Amino-4,5-bis(2-methoxyethoxy)benzonitrile (compound of formula II, 1.5Kg, 5.64mol), toluene 7.5L, acetic acid 30mL and DMF-DMA (730g, 6.3mol) Mix and stir, reflux in an oil bath for reaction, keep warm at 80-90°C for 3 hours, and then concentrate under reduced pressure at 60-65°C to obtain about 1.9Kg of the intermediate Schiff base oil. The oil was dissolved and clarified with isopropyl ether / tetrahydrofuran (20:1) (about 17 L, 9 times the volume of the oil) at 35-40°C, slowly cooled to 10-15°C, and crystallized to obtain 1.58Kg of a white solid. The yield of Schiff's base was 88%, and the HPLC purity was 99.5%.
[0048] Mix the compound of formula III (1.58Kg, 4.92mol), 7.9L of m-aminophenylacetylene (633g, 5.41mol) acetic acid, mix and stir, start heating, control the internal temperature at 95-100°C, react for 2.5-3h, and use thin-layer chromatography Expand the plate (DCM:MeOH=15:1), the reaction is complete, slowly cool down to an internal temperature of 60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com