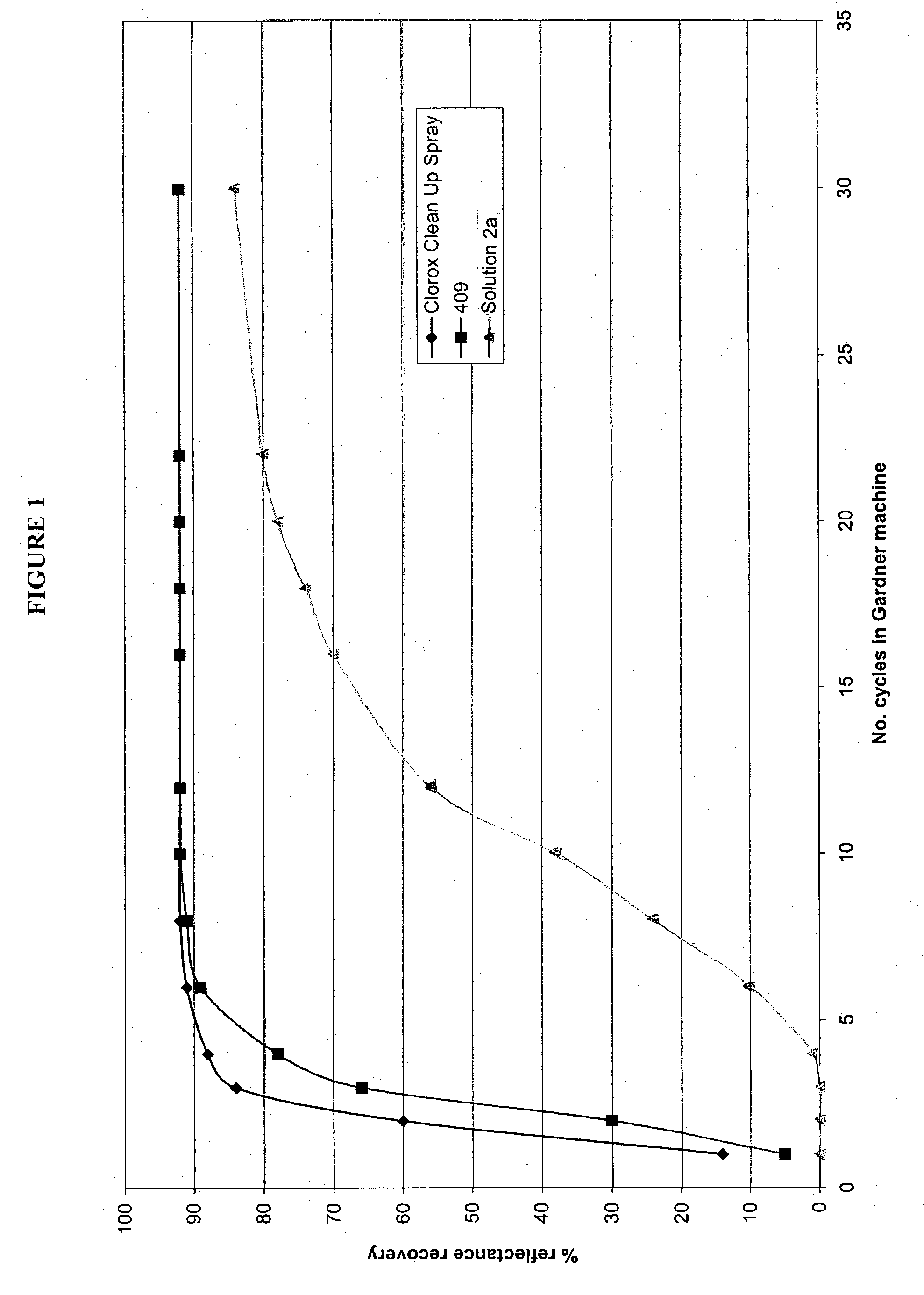
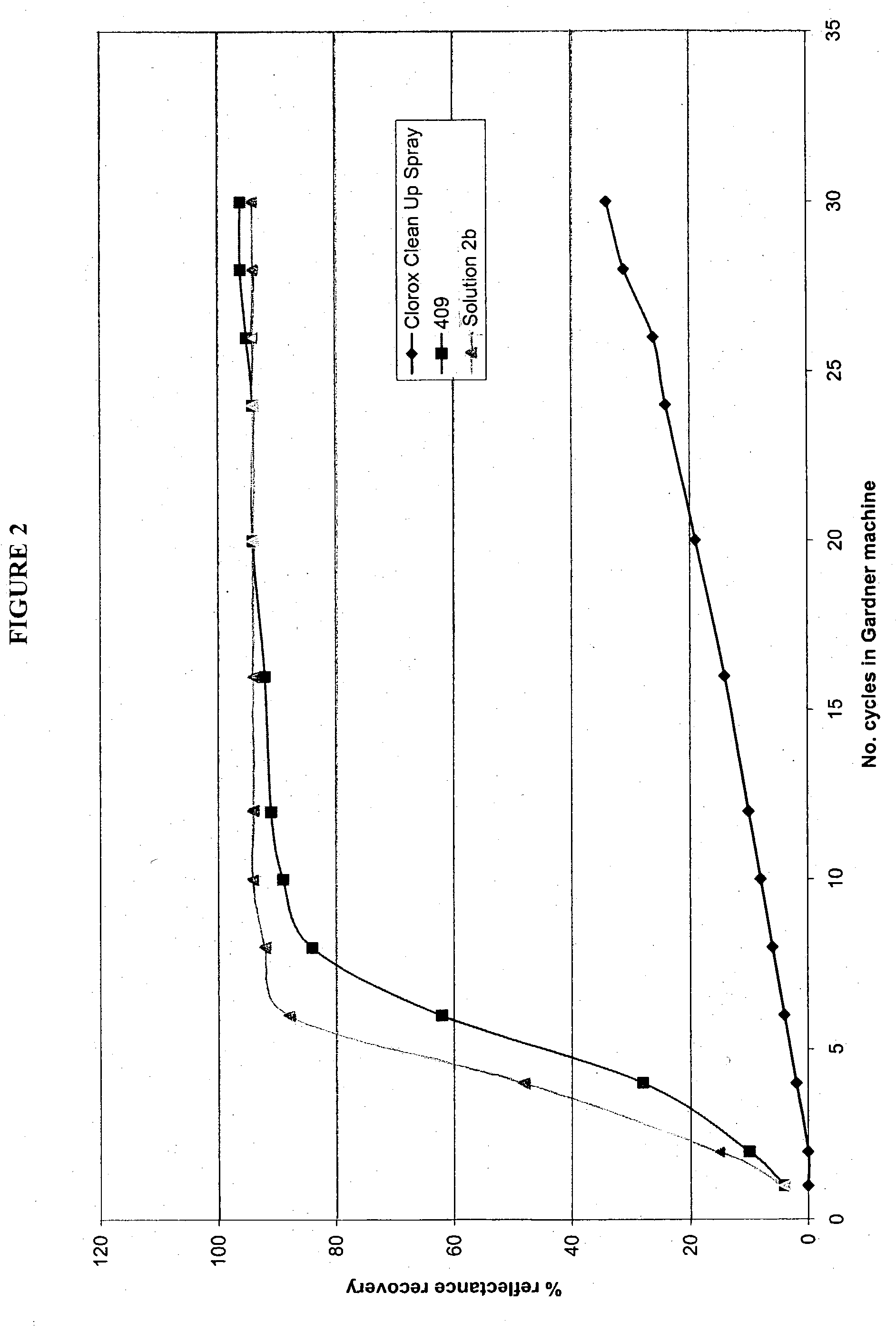
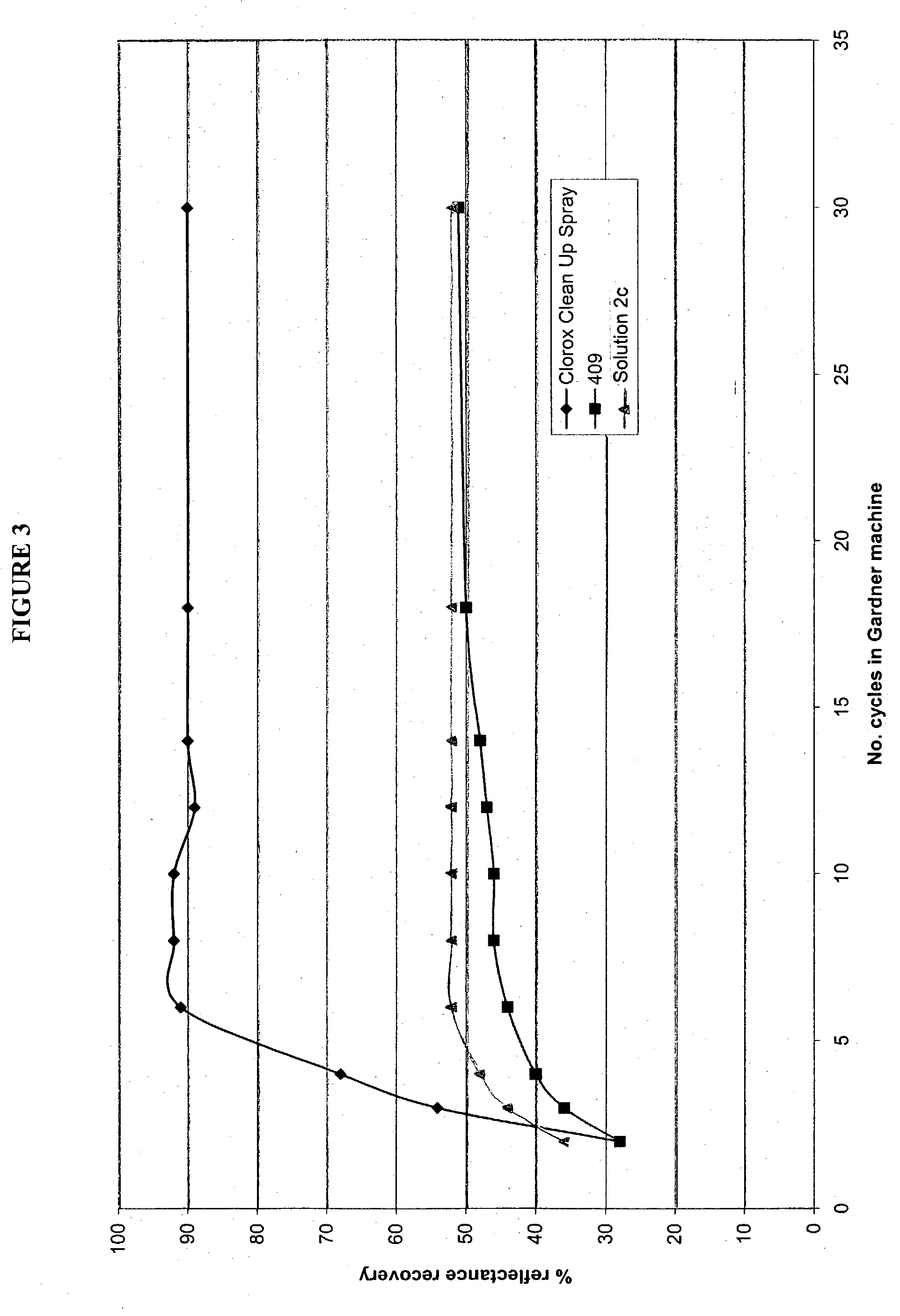
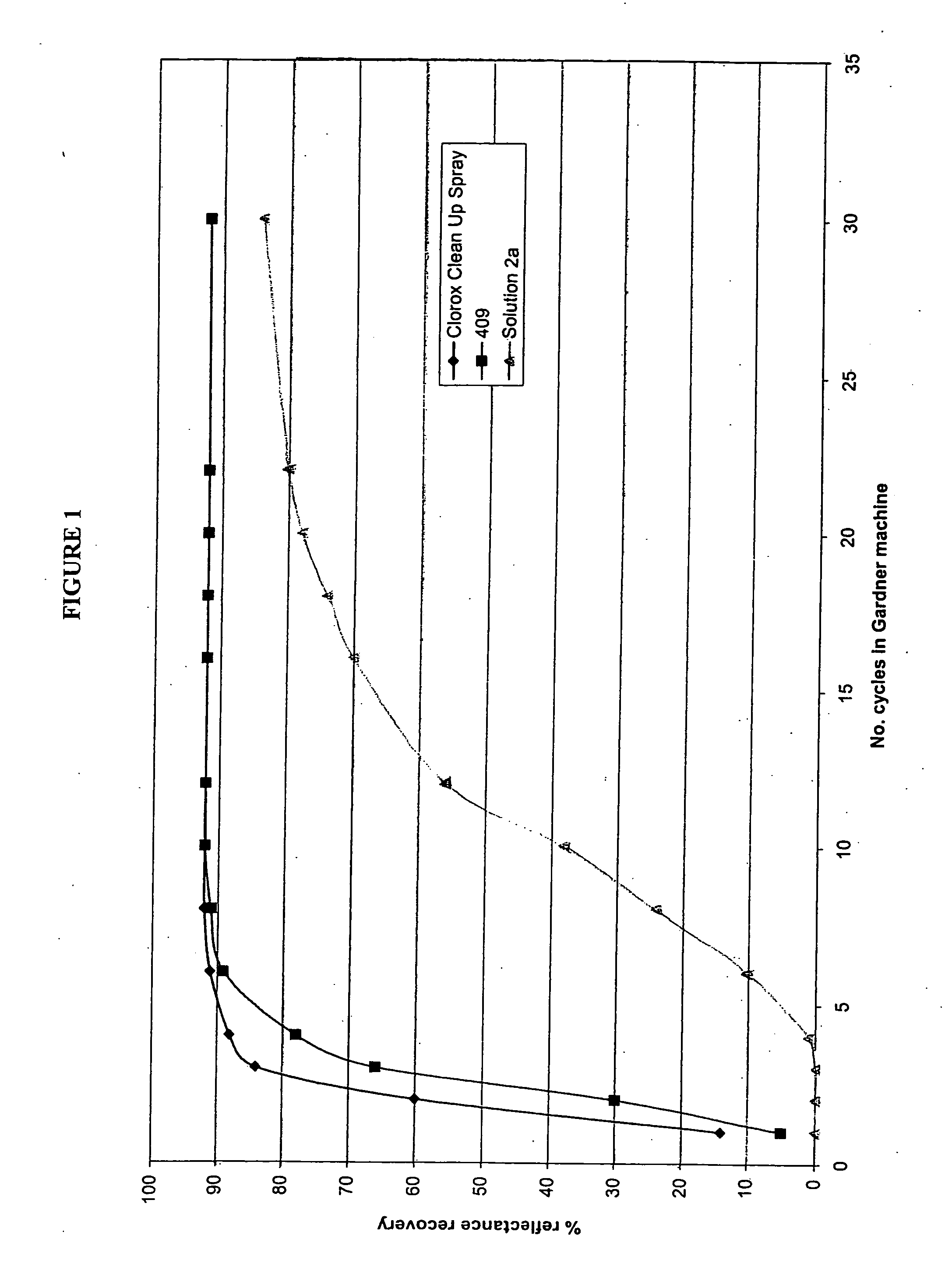
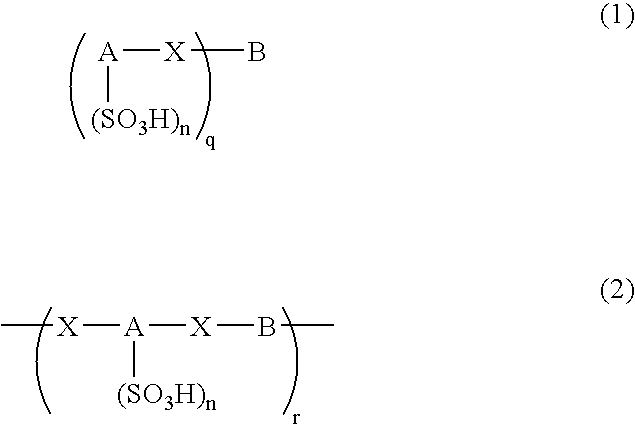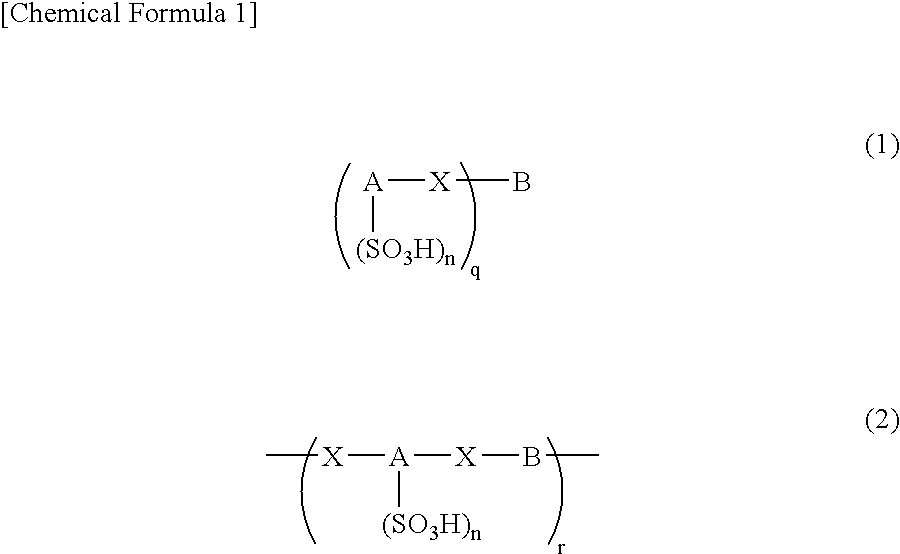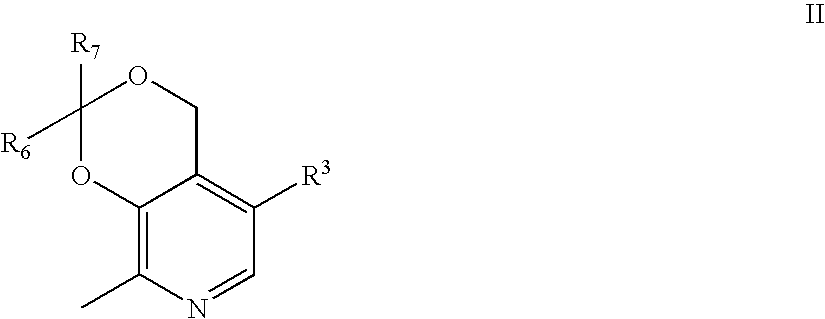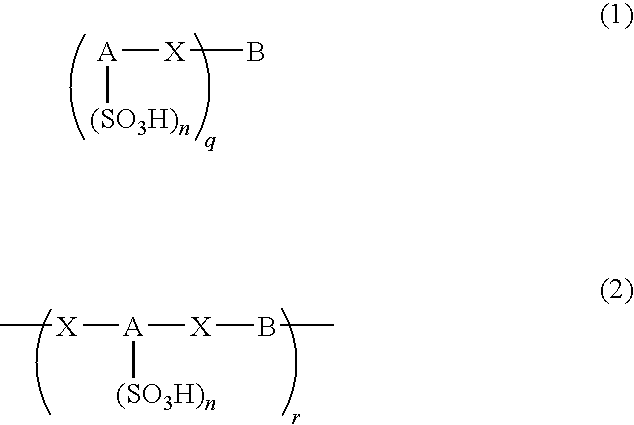Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Arylsulfonic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Organic sulfonic acid derivatives which contain an aromatic hydrocarbon radical.
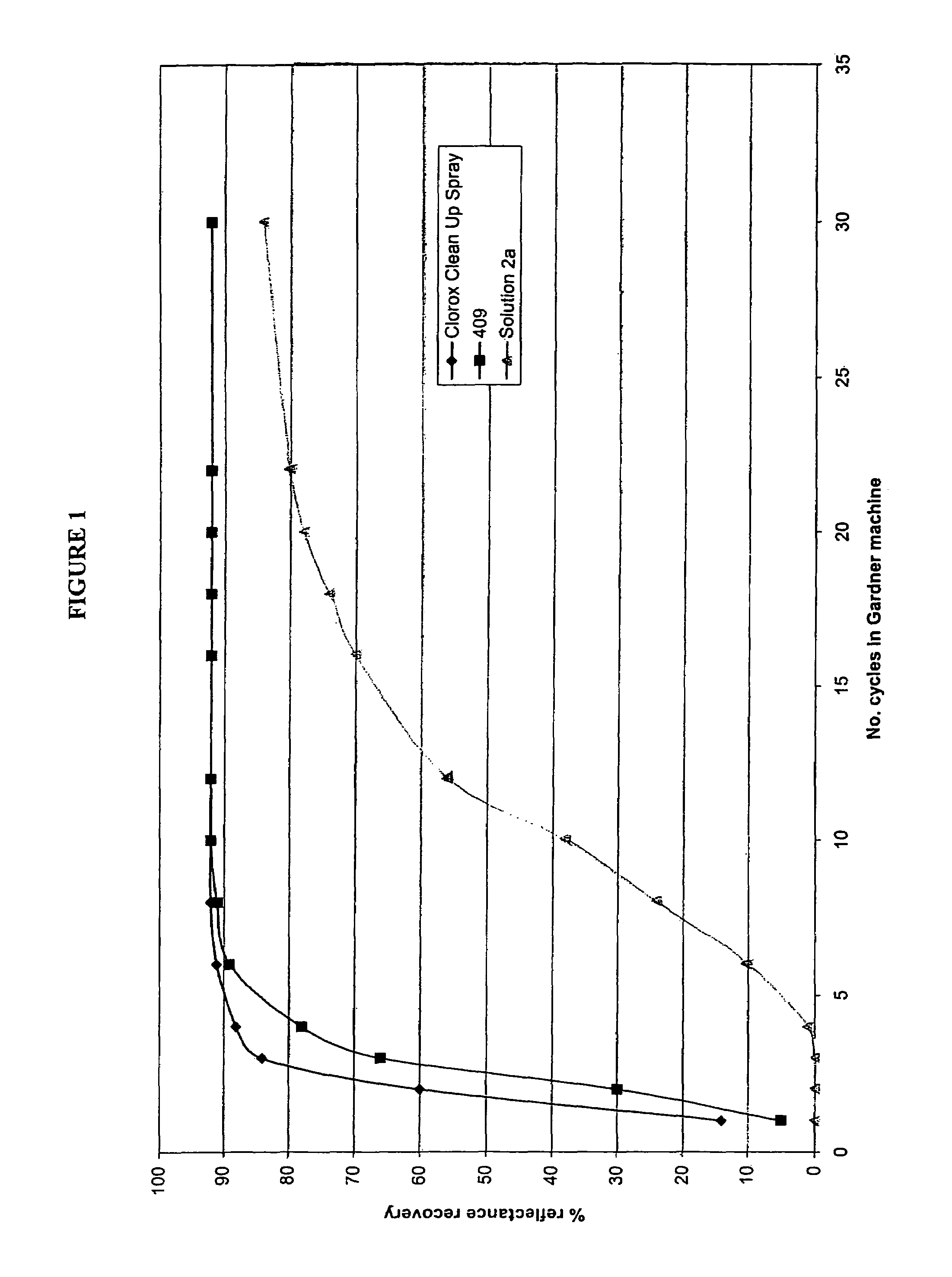
Hydrogen peroxide disinfectant with increased activity
InactiveUS6346279B1High activityReduced activityBiocideInorganic phosphorous active ingredientsDisinfectantPhosphoric acid
An acidic aqueous hydrogen peroxid solution is provided, with improved disinfectant activity. Concentrated solutions preferably contain up to about 8% and as-used concentrations contain about 0.5% peroxide. The solution also contains from 0.1 to 5.0% of at least one acid compound, e.g. phosphoric and / or a phosphonate with from 1 to 5 phosphonic acid groups, and from 0.02 to 5% of at least one anionic surfactant. The surfactant is selected from C8 to C16-alkyl aryl sulphonic acids, sulphonated C12 to C22 carboxylic acids, C8 to C22-alkyl diphenyl oxide sulphonic acids, naphthalene sulphonic acids, C8 to C22 alkyl sulphonic acids, and alkali metal and ammonium salts thereof, and alkali metal C8 to C18 alkyl sulphates, and mixtures thereof. Most preferably the solution has an emulsifier, e.g. a salt of an alkylated diphenyl oxide. The solution may also contain corrosion inhibitors and / or lower alcohols.
Owner:VIROX TECH
Enhanced activity hydrogen peroxide disinfectant
An enhanced activity aqueous disinfecting solution having a pH of from about 0.5 to about 6 and consisting essentially of (i) hydrogen peroxide in a concentration of from about 0.05 to about 8 w / w % of the solution; (ii) at least one anionic surfactant selected from the group consisting of C8 to C16 alkyl aryl sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, sulfonated C12 to C22 carboxylic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C6 to C22 alkyl diphenyl oxide sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, naphthalene sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C8 to C22 alkyl sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, alkali metal, ammonium, calcium and magnesium C8 to C18 alkyl sulfates, alkyl or alkenyl esters or diesters of sulfosuccinic acid in which the alkyl or alkenyl groups independently contain from six to eighteen carbon atoms and alkali metal, ammonium, calcium and magnesium salts thereof, and mixtures thereof, in a concentration range of from about 0.02 to about 8 w / w % of the solution. Optionally, the solution may contain (iii) at least one additional ingredient chosen from a monocarboxylic acid, a polycarboxylic acid, and mixtures thereof, in a concentration of from about 0.05 to about 4 w / w % of the solution; and (iv) at least one further additional ingredient chosen from benzyl alcohol, an alcohol comprising one to six carbon atoms, and mixtures thereof, in a concentration of from about 0.1 to about 10 w / w % of the solution.
Owner:TRIKON TECH LTD +1
Enhanced activity hydrogen peroxide disinfectant
InactiveUS20070059380A1Commercially acceptable stability and cleaning abilityWithout usingAntibacterial agentsBiocideMagnesium saltDisinfectant
An enhanced activity aqueous disinfecting solution having a pH of from about 0.5 to about 6 and consisting essentially of (i) hydrogen peroxide in a concentration of from about 0.05 to about 8 w / w % of the solution; (ii) at least one anionic surfactant selected from the group consisting of C8 to C16 alkyl aryl sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, sulfonated C12 to C22 carboxylic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C6 to C22 alkyl diphenyl oxide sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, naphthalene sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C8 to C22 alkyl sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, alkali metal, ammonium, calcium and magnesium C8 to C18 alkyl sulfates, alkyl or alkenyl esters or diesters of sulfosuccinic acid in which the alkyl or alkenyl groups independently contain from six to eighteen carbon atoms and alkali metal, ammonium, calcium and magnesium salts thereof, and mixtures thereof, in a concentration range of from about 0.02 to about 8 w / w % of the solution. Optionally, the solution may contain (iii) at least one additional ingredient chosen from a monocarboxylic acid, a polycarboxylic acid, and mixtures thereof, in a concentration of from about 0.05 to about 4 w / w % of the solution; and (iv) at least one further additional ingredient chosen from benzyl alcohol, an alcohol comprising one to six carbon atoms, and mixtures thereof, in a concentration of from about 0.1 to about 10 w / w % of the solution.
Owner:JOHNSONDIVERSEY INC
Enhanced activity hydrogen peroxide disinfectant
InactiveUS7632523B2Commercially acceptable stability and cleaning abilityWithout usingAntibacterial agentsBiocideDisinfectantMagnesium salt
An enhanced activity aqueous disinfecting solution having a pH of from about 0.5 to about 6 and consisting essentially of (i) hydrogen peroxide in a concentration of from about 0.05 to about 8 w / w % of the solution; (ii) at least one anionic surfactant selected from the group consisting of C8 to C16 alkyl aryl sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, sulfonated C12 to C22 carboxylic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C6 to C22 alkyl diphenyl oxide sulfonic acids and alkali metal, ammonium, ethanolamine, calcium and magnesium salts thereof, naphthalene sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, C8 to C22 alkyl sulfonic acids and alkali metal, ammonium, calcium and magnesium salts thereof, alkali metal, ammonium, calcium and magnesium C8 to C18 alkyl sulfates, alkyl or alkenyl esters or diesters of sulfosuccinic acid in which the alkyl or alkenyl groups independently contain from six to eighteen carbon atoms and alkali metal, ammonium, calcium and magnesium salts thereof, and mixtures thereof, in a concentration range of from about 0.02 to about 8 w / w % of the solution. Optionally, the solution may contain (iii) at least one additional ingredient chosen from a monocarboxylic acid, a polycarboxylic acid, and mixtures thereof, in a concentration of from about 0.05 to about 4 w / w % of the solution; and (iv) at least one further additional ingredient chosen from benzyl alcohol, an alcohol comprising one to six carbon atoms, and mixtures thereof, in a concentration of from about 0.1 to about 10 w / w % of the solution.
Owner:JOHNSONDIVERSEY INC
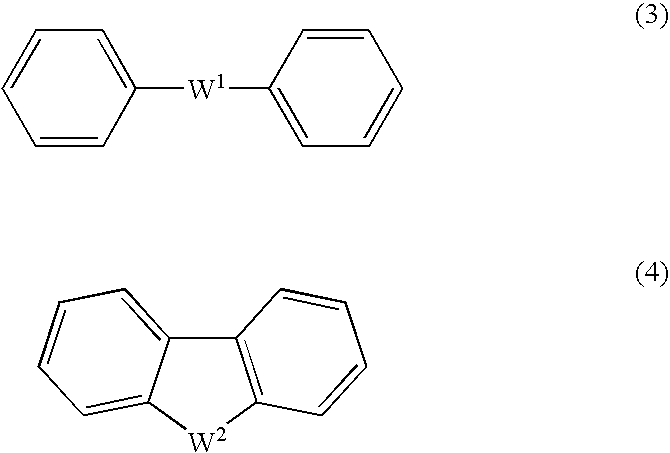
Arylsulfonic Acid Compound And Use Thereof As Electron -Acceptor Material
ActiveUS20080029742A1Improved initial characteristicExcellent EL device characteristicOrganic chemistryConductive materialElectronArylsulfonic Acids
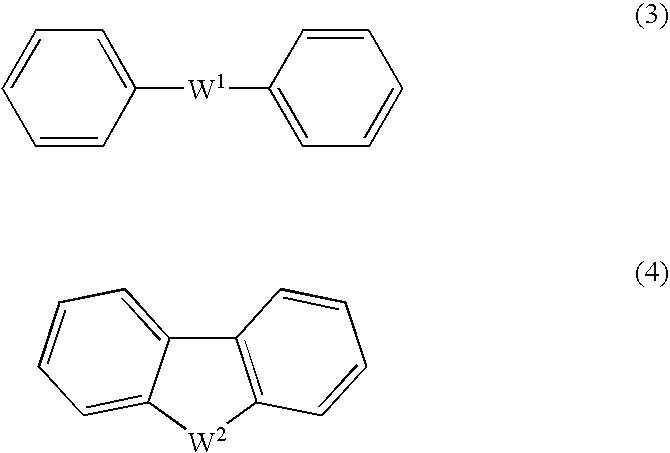
An excellent EL device having low driving voltage, high luminous efficiency and long life can be obtained by using a charge-transporting thin film composed of a charge-transporting varnish which contains an arylsulfonic acid compound represented by the formula (1) or (2) below as an electron-acceptor material especially in an OLED device or a PLED device. [In the formulae, X represents O, S or NH; A represents a naphthalene ring or anthracene ring having a substituent other than X and n SO3H groups; B represents a substituted or unsubstituted hydrocarbon group, 1,3,5-triazine group or a substituted of unsubstituted group represented by the following formula (3) or (4): (wherein W1 and W2 each independently represents O, S, an S(O) group, an S(O2) group, or a substituted or unsubstituted N, Si, P or P(O) group); n indicates the number of sulfonic acid groups bonded to A which is an integer satisfying 1≦n≦4; q indicates the number of B—X bonds which is an integer satisfying 1≦q; and r indicates the number of recurring units which is an integer satisfying 1≦r.]
Owner:NISSAN CHEM IND LTD
Aryl sulfonic pyridoxines as antiplatelet agents
Aryl sulfonic pyridoxine compounds with antiplatelet aggregation characteristics for the treatment of cardiovascular and cardiovascular related disease, are described. The methods are directed to administering pharmaceutical compositions comprising aryl sulfonic pyridoxines.
Owner:MEDICURE INT INC
Arylsulfonic acid compound and use thereof as electron-acceptor material
ActiveCN101039899AEasy to manufactureImprove solubilityOrganic chemistrySolid-state devicesAnthraceneAryl
An excellent EL device having low driving voltage, high luminous efficiency and long life can be obtained by using a charge-transporting thin film composed of a charge-transporting varnish which contains an arylsulfonic acid compound represented by the formula (1) or (2) below as an electron-acceptor material especially in an OLED device or a PLED device. [In the formulae, X represents O, S or NH; A represents a naphthalene ring or anthracene ring having a substituent other than X and n SO 3 H groups; B represents a substituted or unsubstituted hydrocarbon group, 1,3,5-triazine group or a substituted of unsubstituted group represented by the following formula (3) or (4): (wherein W 1 and W 2 each independently represents O, S, an S(O) group, an S(O 2 ) group, or a substituted or unsubstituted N, Si, P or P(O) group); n indicates the number of sulfonic acid groups bonded to A which is an integer satisfying 1<=n<=4; q indicates the number of B-X bonds which is an integer satisfying 1= C07C 309 / 43 H01L 51 / 50 0 23 2 2005 / 8 / 30 101039899 2007 / 9 / 19 000000000 Nissan Chemical Ind Ltd. Japan Yoshimoto Takuji Ono Go chencuan 11038 The Patent Agency of the Chinese Council for the Promotion of International Trade (CCPIT) No.1 Waidajie, Fuxingmen, Beijing 100086 Japan 2004 / 8 / 31 251774 / 2004 2007 / 4 / 11 PCT / JP2005 / 015689 2005 / 8 / 30 WO2006 / 025342 2006 / 3 / 9 Japanese
Owner:NISSAN CHEM CORP
Resist composition and patterning process
ActiveUS20160231652A1Increase contrastSatisfactory pattern profilePhotomechanical coating apparatusPhotomechanical exposure apparatusImage resolutionDissolution
Owner:SHIN ETSU CHEM IND CO LTD
Process for preparing thick oil hydrothermally catalytic cracking viscosity reducer containing amphiphilic structure
InactiveCN1944572AExert catalytic activityImprove qualityDrilling compositionLiquid viscosityIron powder
The present invention relates to preparation process of hydrothermally catalytic cracking viscosity reducer containing amphiphilic structure for thick oil exploitation. The preparation process includes the following steps: 1. preparing material arylsulfonic acid in 19-21 weigh portions, and iron oxide powder in 1.8 or 1.2 weigh portions; and 2. reaction between arylsulfonic acid and iron oxide powder at 80-160 deg.c for 1-3 hr, dissolving the resultant with acetone in 0.9-1.3 times arylsulfonic acid weight, filtering and stoving the filtrate in a stove at 50-80 deg.c to obtain the brown ropy liquid viscosity reducer. The present invention has the features of low cost, high universality and good low temperature viscosity reducing effect.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Alkyl salicylic acid synthesis method
ActiveCN103508881AStrong acidNot corrosiveOrganic compound preparationChemical recyclingCarbon numberAlkyl transfer
The present invention discloses an alkyl salicylic acid synthesis method, which comprises: mixing olefin with a carbon number of 4-50 and salicylic acid, and carrying out an alkylation reaction under a catalysis effect of aryl sulfonic acid at a temperature of 50-160 DEG C. According to the present invention, aryl sulfonic acid is adopted as a catalyst, a conversion rate can achieve 60-95%, the catalyst does not cause corrosion on the equipment, price is relatively low, the catalyst can be recycled, no three-waste emission problem exists, and environmental protection is easily achieved.
Owner:PETROCHINA CO LTD
Silsesquioxane resins
InactiveUS8304161B2Low temperature curabilityReasonable resistancePhotosensitive materialsPhotosensitive material processingArylSulfonate
A silsesquioxane resin comprised of the units (Ph(CH2)rSiO(3-x) / 2(OR′)x)m, (HSiO(3-x) / 2(OR′)x)n′(MeSiO(3-x) / 2(OR′)x)o′(RSiO(3-x) / 2(OR′)x)p, (R1SiO(3-x) / 2(OR′)x)q where Ph is a phenyl group, Me is a methyl group; R′ is hydrogen atom or a hydrocarbon group having from 1 to 4 carbon atoms; R is selected from an aryl sulfonate ester group; and R1 is selected from substituted phenyl groups, ester groups, polyether groups; mercapto groups, and reactive or curable organic functional groups; and r has a value of 0, 1, 2, 3, or 4; x has a value of 0, 1 or 2; wherein in the resin m has a value of 0 to 0.95; n has a value of 0.05 to 0.95; o has a value of 0.05 to 0.95; p has a value of 0.05 to 0.5; q has a value of 0 to 0.5; and m+n+o+p+q=1.
Owner:DOW CORNING CORP
Method for preparing halogen substituted dibenzyl alcohol, its product and application
Production of halogenated dibenzyl alcohols (I) comprises reacting the corresponding halo-substituted terephthalic acids with sodium boranate in an organic solvent at 0-150 degrees C and then with an alkylating agent or H2SO4 or an alkyl- or aryl-sulfonic acid. The H2SO4 or alkyl- or aryl-sulfonic acid containing a maximum of 5 volume% water. Production of halogenated dibenzyl alcohols of formula (I) comprises reacting the corresponding halo-substituted terephthalic acids of formula (II) with sodium boranate in an organic solvent at 0-150 degrees C, and then with an alkylating agent or H2SO4 or an alkyl- or aryl-sulfonic acid, the H2SO4 or alkyl- or aryl-sulfonic acid containing a maximum of 5 volume% water. X = H, F, Cl or Br, with at least one being F, Cl or Br An Independent claim is also included for compounds of formula (I) in which there is a maximum of 1% (especially zero) of (I) having asymmetric substituents in the 1 and 4 positions, i.e. (I) is especially completely free of e.g. halogenated 1-benzylalcohol-4-benzylamines, 1-benzaldehyde-4-benzylalcohols or 1-benzoicacid-4-benzylalcohols.
Owner:SALTIGO GMBH
Silsesquioxane Resins
InactiveUS20110003249A1Photosensitive materialsSemiconductor/solid-state device manufacturingPhenyl groupSiloxane
A silsesquioxane resin comprised of the units (Ph(CH2)rSiO(3-x) / 2(OR′)x)m, (HSiO(3-x) / 2(OR′)x)n′(MeSiO(3-x) / 2(OR′)x)o′(RSiO(3-x) / 2(OR′)x)p, (R1SiO(3-x) / 2(OR′)x)q where Ph is a phenyl group, Me is a methyl group; R′ is hydrogen atom or a hydrocarbon group having from 1 to 4 carbon atoms; R is selected from an aryl sulfonate ester group; and R1 is selected from substituted phenyl groups, ester groups, polyether groups; mercapto groups, and reactive or curable organic functional groups; and r has a value of 0, 1, 2, 3, or 4; x has a value of 0, 1 or 2; wherein in the resin m has a value of 0 to 0,95; n has a value of 0.05 to 0.95; o has a value of 0.05 to 0.95; p has a value of 0.05 to 0.5; q has a value of 0 to 0.5; and m+n+o+p+q=1.
Owner:DOW CORNING CORP
High solid content aqueous polyurethane and preparation method thereof
The invention discloses a highly solid aqueous polyurethane, which comprises the following steps: 45-50g toluene diisocyanate, 90-100g polycarbonate, 3-5g neopentyl glycol, 20-30g polyester polyol and 100-120g water. The making method comprises the following steps: adding polycarbonate, neopentyl glycol and toluene diisocyanate; heating to 70 deg. c for 1-1. 5h; heating to 85deg. c for 2-3h; cooling to 65deg. c; adding aryl thiophene sodium polyester polyol; reacting at 60 deg. c for 2-3h; measuring NCO value to reach the prepolymer; adding water in the emulsifying autoclave; stirring under low speed; adding the prepolymer in the emulsifying autoclave to emulsify 3-4h; obtaining the product.
Owner:GUANGDONG YINYANG ENVIRONMENT FRIENDLY NEW MATERIALS CO LTD
Deodorizer and method for using the same
A deodorizer is disclosed which is superior both in odor elimination effect eliminating an already existing odor and in odor prevention effect preventing odor occurrence, without leaving a colored trace on an application site. The deodorizer of the present invention comprises a salt of copper (II) ion and / or zinc ion; at least one acid component selected from the group consisting of alkylsulfonic acids having 1 to 6 carbon atoms, arylsulfonic acids having 5 to 14 carbon atoms, amidosulfuric acids, and derivatives thereof; and water being a solvent. The acid component may be substituted by at least one selected from the group consisting of amine groups, imine groups, and hydroxyl group. The deodorizer comprises the salt in an amount of more than 0.001 mM and less than 10 mM.
Owner:TOTO LTD
Method for preparing diaryl ketone from aryl sulfonate
InactiveCN106146271AHigh yieldImprove toleranceCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventDiaryl ketone
The invention discloses a method for preparing diaryl ketone from aryl sulfonate. The method includes following steps: in the presence of a catalyst and carbon monoxide, allowing aryl sulfonate and aryl boronic acid to react in an organic solvent; after reaction is finished, performing aftertreatment to obtain diaryl ketone. Aryl sufonate is adopted as an electrophilic reagent for Suzuki cross carbonylation coupling reaction, diaryl ketone is directly synthesized through carbon monoxide, aryl sulfonate and aryl boronic acid, reaction conditions are milk, functional groups are high in tolerance, a substrate is cheap and easy to get, and diaryl ketone can be prepared with high yield.
Owner:SHAOXING UNIVERSITY
Near infrared waveband reaction type biological thiol two-photon fluorescent probe as well as preparation method and application thereof
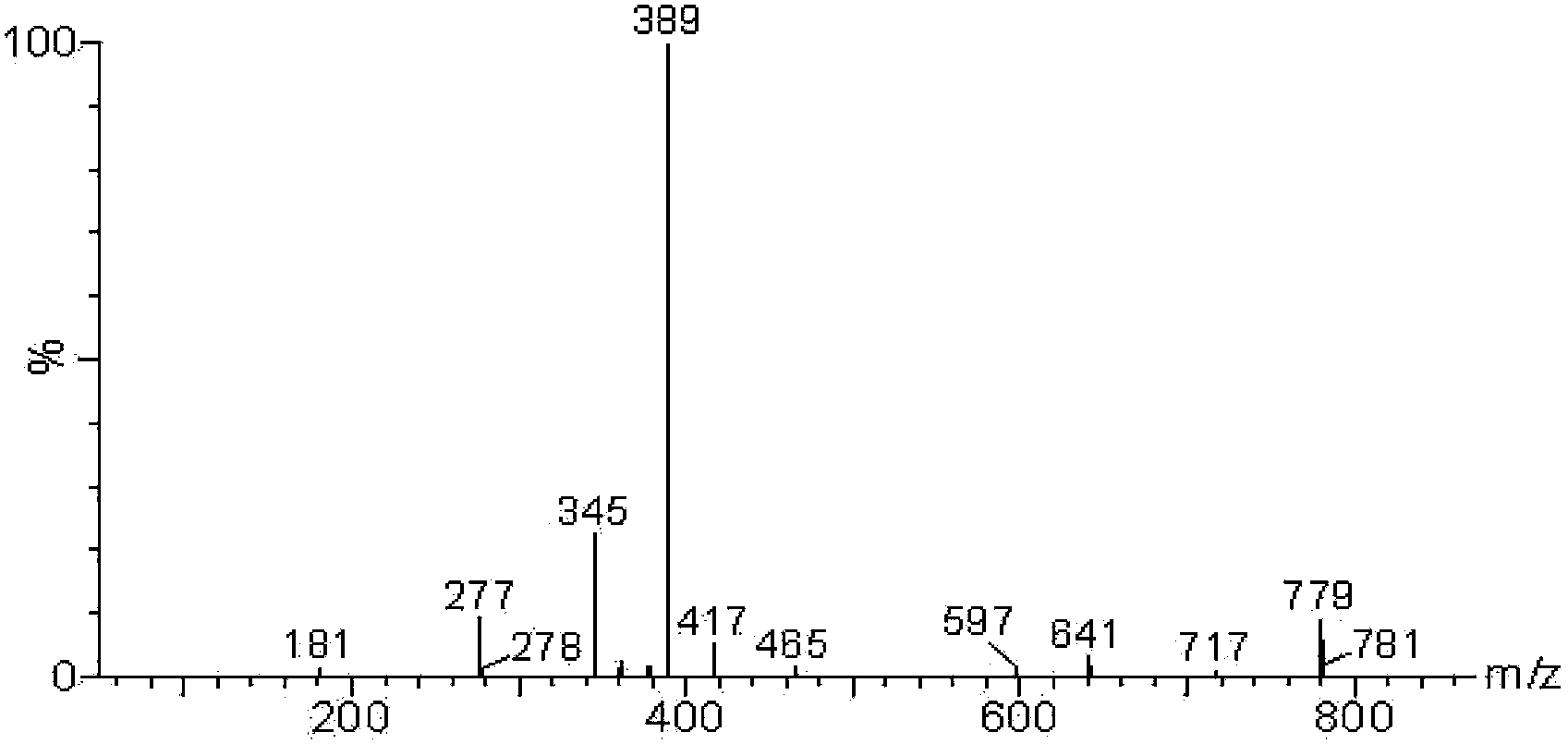
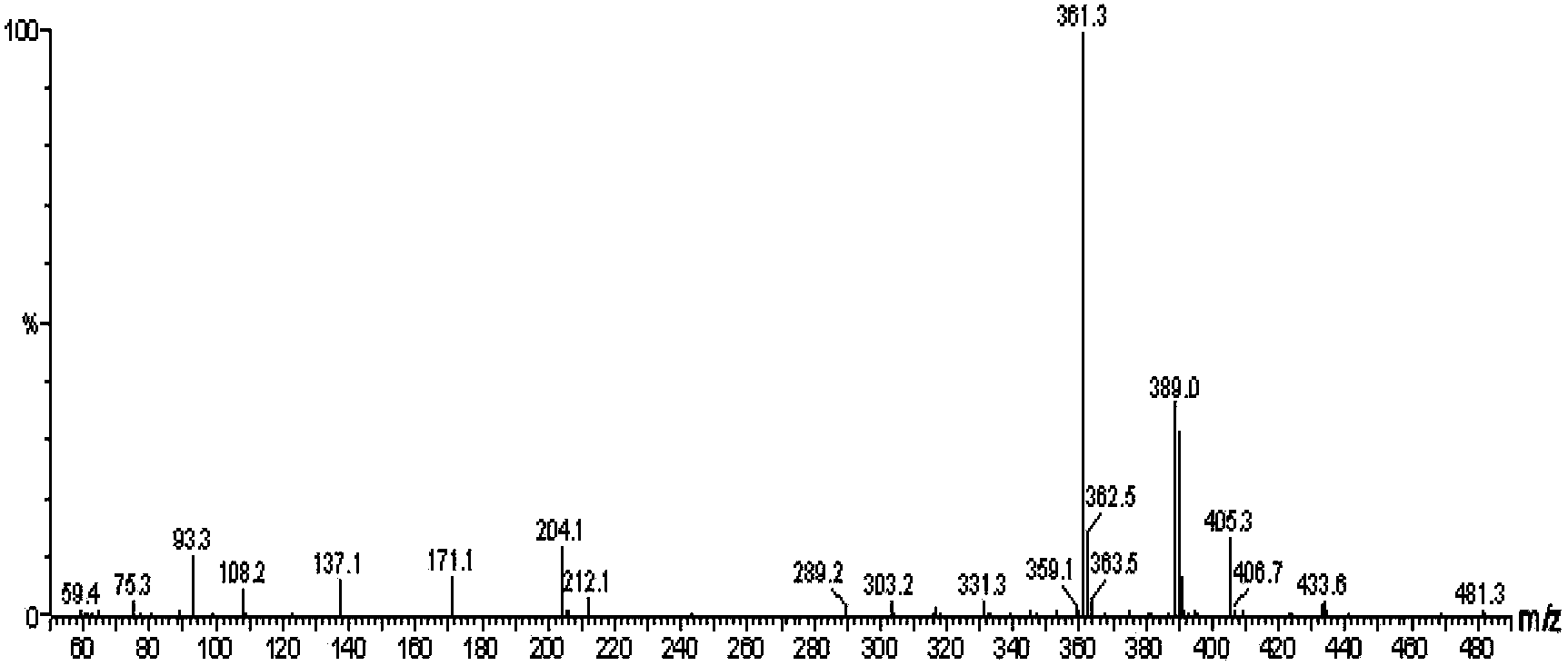
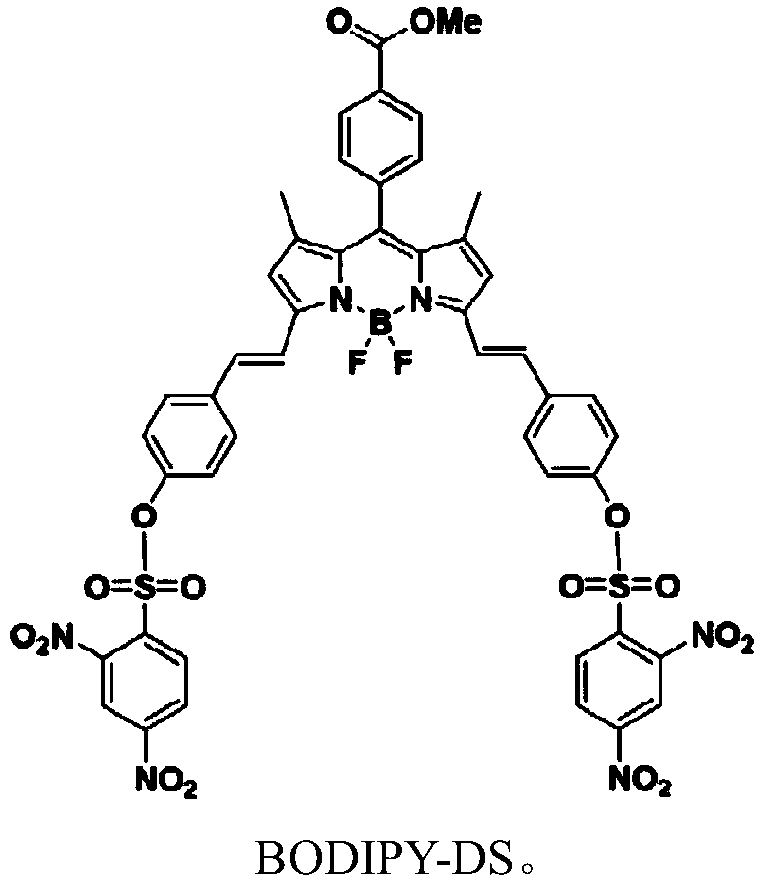
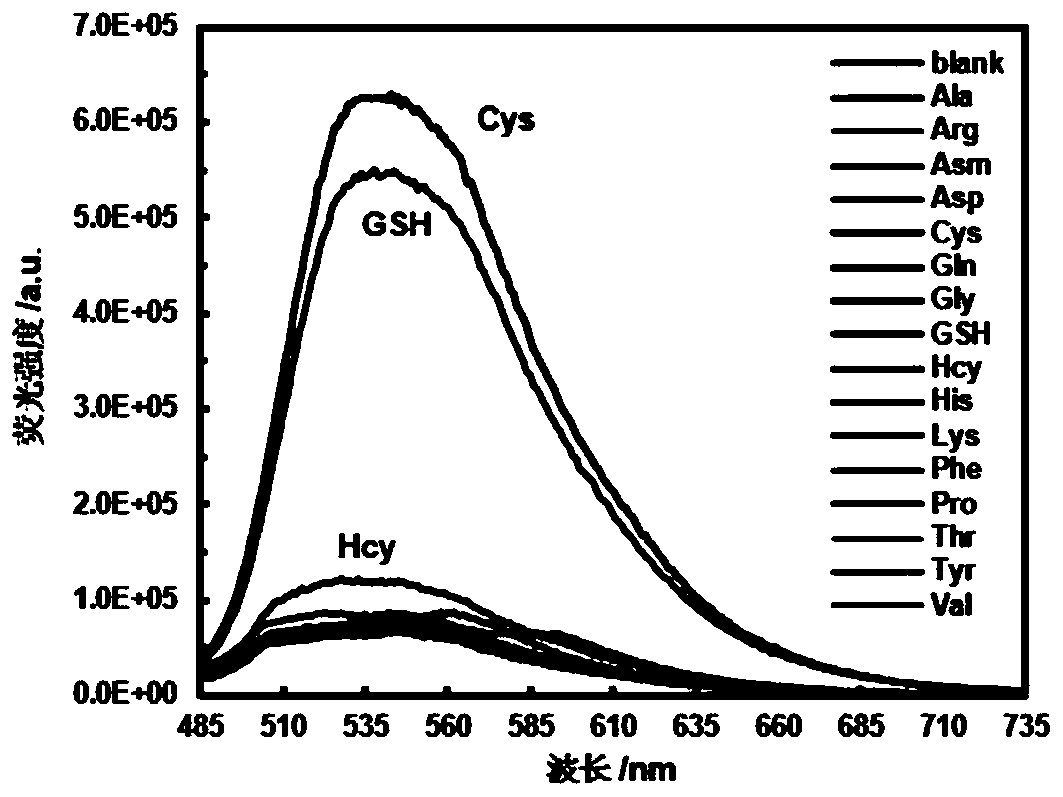
ActiveCN108191899AHigh sensitivityHigh selectivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceSulfonyl chlorideFluoProbes
The invention provides a fluorescent probe molecule BODIPY-DS, which has a diaryl sulfonate structure of conjugate fluoroboron dipyrrole. The invention also provides a preparation method and application of the fluorescent probe molecule BODIPY-DS. According to a near infrared waveband reaction type biological thiol two-photon fluorescent probe, fluorescent molecules are prepared through reaction between fluorboron dipyrrole conjugate arylphenol and 2,4-dinitrobenzene sulfonyl chloride; through biological thiol induced aromatic nucleophilic substitution reaction, the diaryl sulfonate structureis removed; the biphoton fluorescence emission performance of the probe molecule can be sensitively regulated and controlled; the sensitivity is high; the selectivity is high; the biocompatibility ishigh; the fluorescent probe can be used as the near infrared waveband reaction type biological thiol two-photon fluorescent probe with excellent performance, and can be widely applied to the aspects of near infrared wave band fluorescent imaging, fluorescent sensing, biological fluorescence analysis, fluorescent marking and endogenic and ectogenic biological thiol detection.
Owner:SOUTHEAST UNIV
Fluorescent probe molecule ML-FP and preparation method and applications thereof
ActiveCN110041311AEasy accessLarge displacementOrganic chemistryFluorescence/phosphorescenceLysosome localizationMorpholine
A fluorescent probe molecule ML-FP disclosed by the invention comprises a green fluorescent protein-sulfonate structure and a morpholine lysosome orientating group. The invention also discloses a preparation method and applications of the fluorescent probe molecule ML-FP. The fluorescent molecule can be used as a lysosome orientating active mercaptan fluorescent probe. The fluorescent probe is a fluorescent molecule prepared by reacting a morpholine-indole fluorescent protein derivative with 2,4-dinitrobenzenesulfonyl chloride. An aryl sulfonate structure is removed by an aromatic nucleophilicsubstitution reaction selectively induced by biological mercaptan so as to switch a fluorescence switch to realize selective recognition of cysteine and glutathione. The fluorescent probe molecule has the advantages of strong anti-background interference ability, high sensitivity, low cytotoxicity and good biocompatibility, can quickly enter lysosome, can be used as the lysosome orientating active mercaptan fluorescent probe, and has wide applications in fluorescence imaging, bioluminescence analysis, medicine research and development, fluorescence labeling and endogenous or exogenous activemercaptan detection.
Owner:SOUTHEAST UNIV
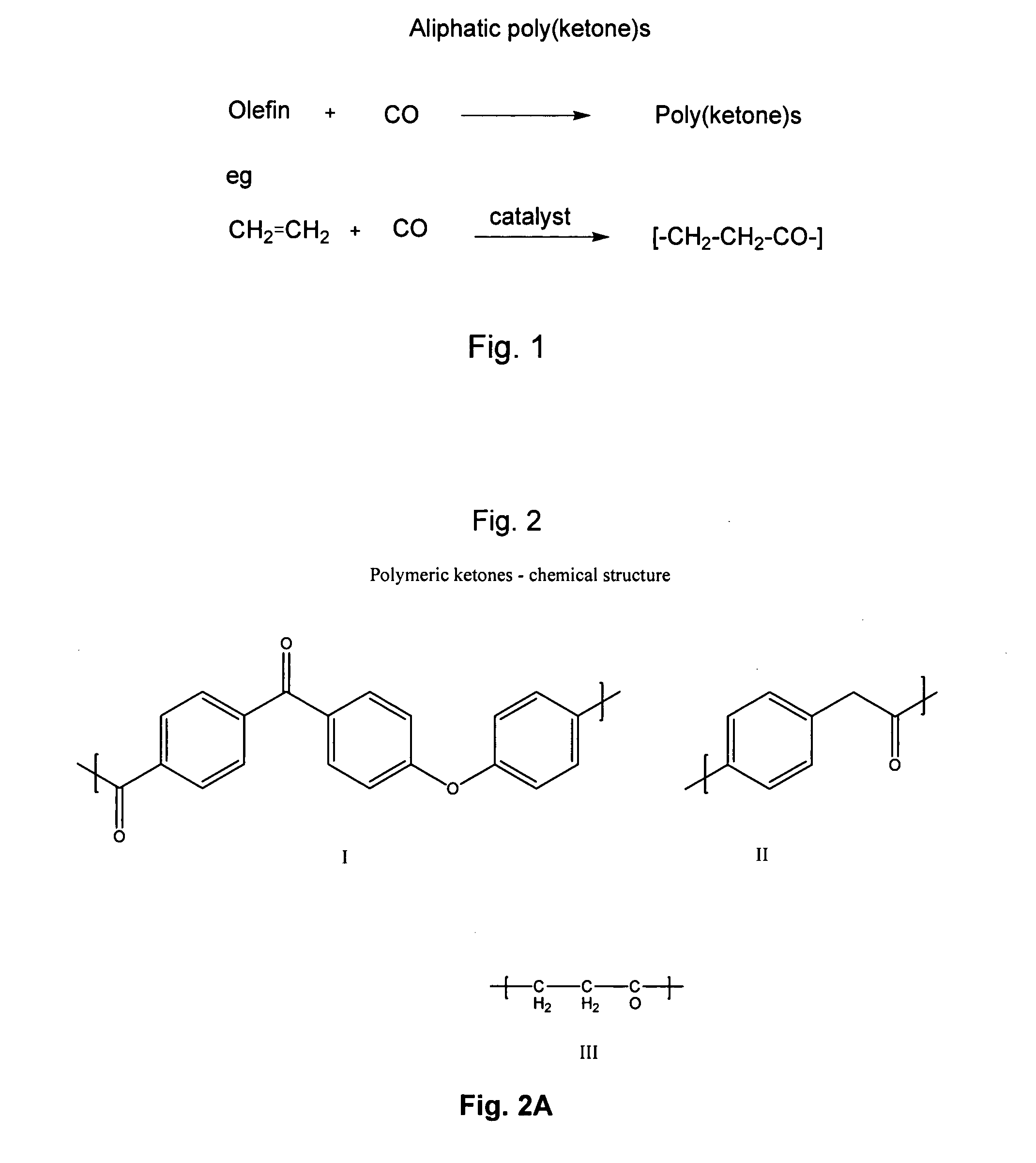
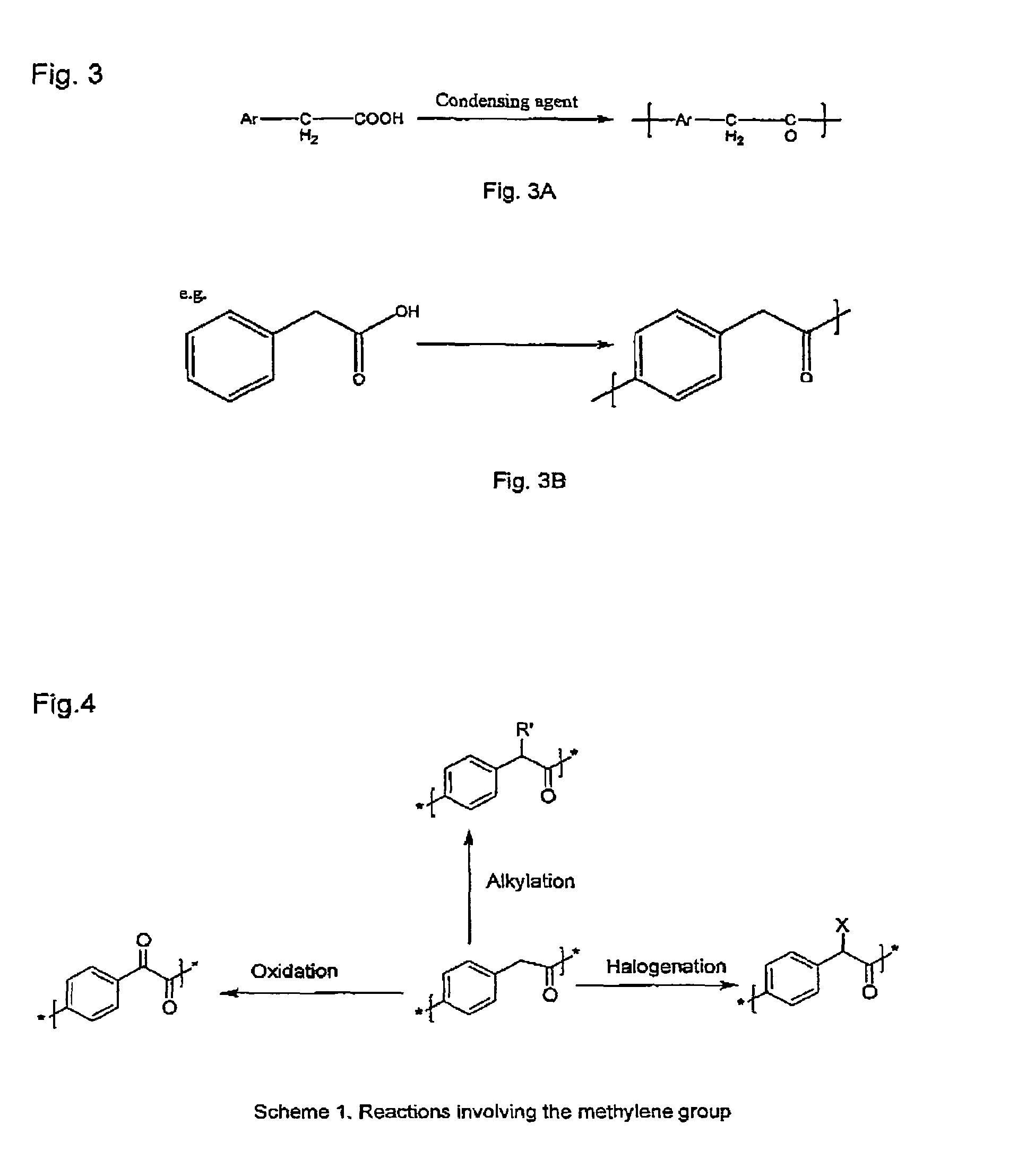
Poly(aralkyl ketone)s and methods of preparing the same
Disclosed are processes for preparing modifiable poly(aralkyl ketone)s having active methylene group(s) in the main chain comprising reacting an aralkanoic acid or mixtures thereof in the presence of one alkane or aryl sulfonic acid and a condensing agent. Also disclosed are novel modifiable poly(aralkyl ketone)s obtainable by the processes of the inventions.
Owner:AGENCY FOR SCI TECH & RES
Arylsulfonic acid compound and use thereof as electron-acceptor material
ActiveUS7862747B2Improve featuresImprove deteriorationOrganic chemistryConductive materialAnthracene1,3,5-Triazine
Owner:NISSAN CHEM IND LTD
Method for catalytically dehydrogenating and oxidizing aryl secondary alcohol into ketone
ActiveCN110483261AGreat application potentialReduce usageOrganic compound preparationPreparation by dehydrogenationKetoneDiethyl ether
The invention discloses a method for catalytically dehydrogenating and oxidizing aryl secondary alcohol into ketone. The method comprises the following steps: dissolving the aryl secondary alcohol inan organic solvent, and reacting at 40-120 DEG C in the presence of an acid catalyst and a cocatalyst to obtain substituted aryl ketone, wherein the acid is aryl sulfonic acid (ArSO3H), alkyl sulfonicacid (RSO3H) or boron trifluoride diethyl ether, and the cocatalyst is tertiary halogenated hydrocarbon or benzyl halogenated hydrocarbon. According to the method, a metal catalyst and an oxidizing agent are not used, the adopted catalyst is cheap and non-toxic and can tolerate water and air, the reaction condition is mild, and the method is simple to operate and high in atom economy, and can beused for large scale production.
Owner:XI'AN PETROLEUM UNIVERSITY
Preparing method of aromatic nitrile or alkenyl nitrile compound
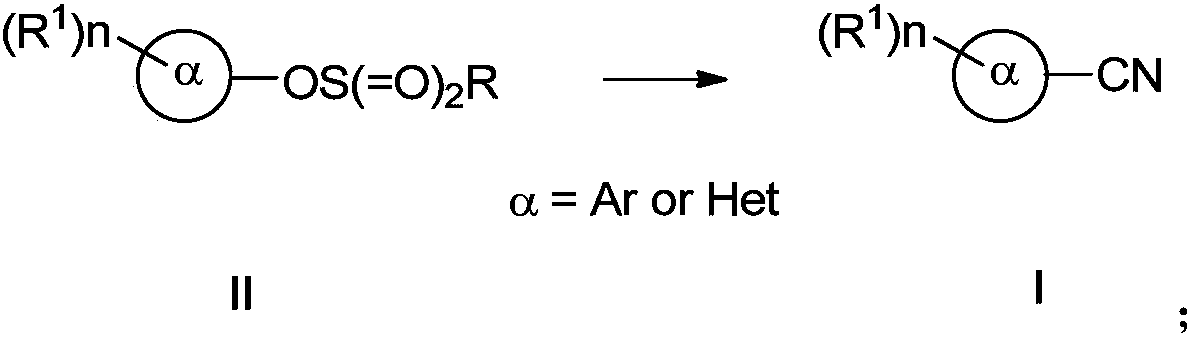
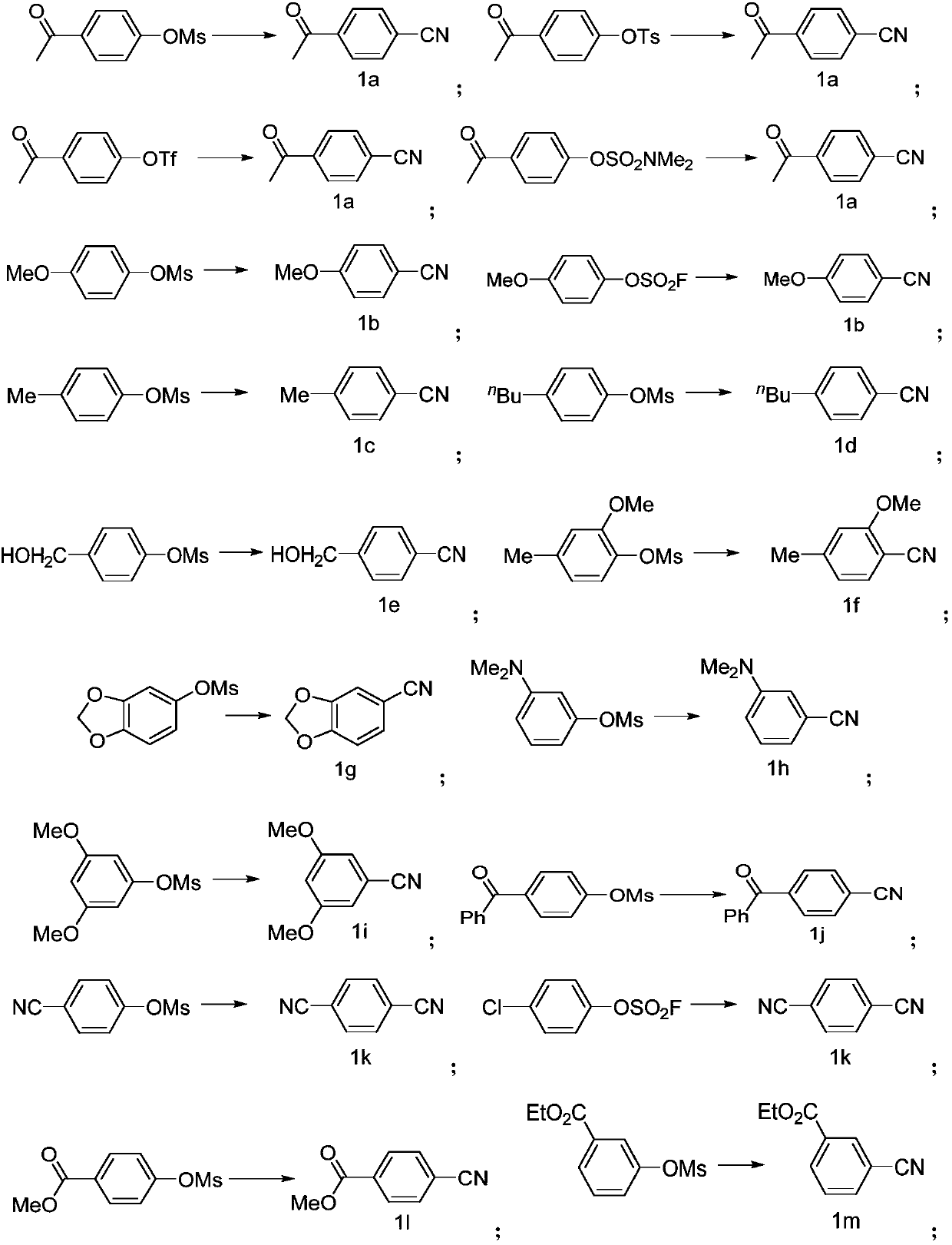
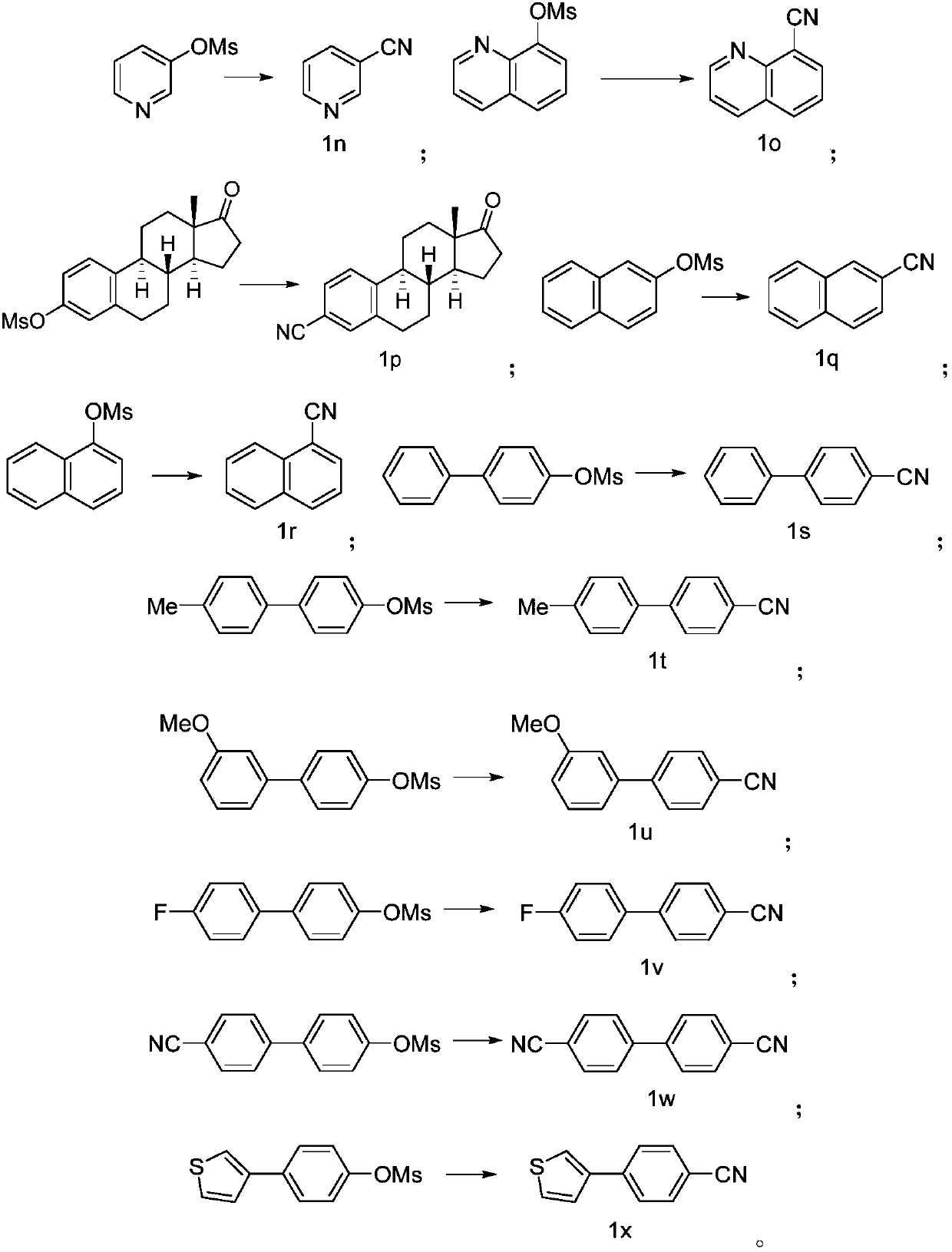
PendingCN110117237AImprove compatibilityImprove universalityPreparation by cyanide reactionSteroidsArylSolvent
The invention discloses a preparing method of an aromatic nitrile or alkenyl nitrile compound. The preparing method comprises the following step that under protection of inert gas, an aryl or heteroaryl sulphonate compound shown in a formula II or an alkenyl sulphonate compound shown in a formula IV and a cyanation reagent are subjected to a cross-coupling reaction as is shown below in a solvent under the condition of the presence of a nickel complex, metal zinc and an additive to obtain the aromatic nitrile or alkenyl nitrile compound, wherein 4-dimethylamiopryidine (DMAP) is adopted as the additive, and zinc cyanide is adopted as the cyanation reagent. By means of the preparing method, cyanation of aryl sulphonate, heteroaryl sulphonate or alkenyl sulphonate can be simply and efficientlyachieved with a cheap catalysis system; moreover, the functional group compatibility and substrate universality are good, and a better application prospect and higher using value are provided for achieving industrial synthesis of the aromatic nitrile or alkenyl nitrile compound.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
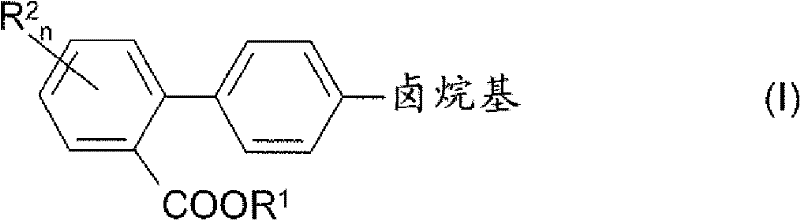
Process for preparing 4'-halogenalkyl-biphenyl-2-carboxylic acids
InactiveCN102190581APromote conversionOrganic compound preparationCarboxylic acid esters preparationArylHalogen
The invention relates to a process for preparing a 4'-halogenalkyl-biphenyl-2-carboxylic acid. The invention relates to a process for preparing the optionally substituted 4'-halogenalkyl-biphenyl-2-carboxylic ester and to a process for preparing the corresponding optionally substituted 4'-halogenalkyl-biphenyl-2-carboxylic acid. A process for preparing a compound with the following chemical formula (I), wherein R1 is an alkyl group with C1-C6, an aryl group with C6-C24 or an aralkyl group with C7-C15, and R2 can be independent of each other or same, n=1, 2, 3 or 4; a compound with the following chemical formula (II) is reacted with a compound with the following chemical formula (III) in the presence of at least one copper source, at least one cyclic chelating amine, at least one phosphine ligand and at least one palladium source to generate the compound with the chemical formula (I), wherein cat is an inorganic or organic single-charge cation, and X is a halogen element which is equal to Cl, Br, I, pseudo halogen, aryl sulphonate ester or alkylsulfonic acid ester.
Owner:SALTIGO GMBH
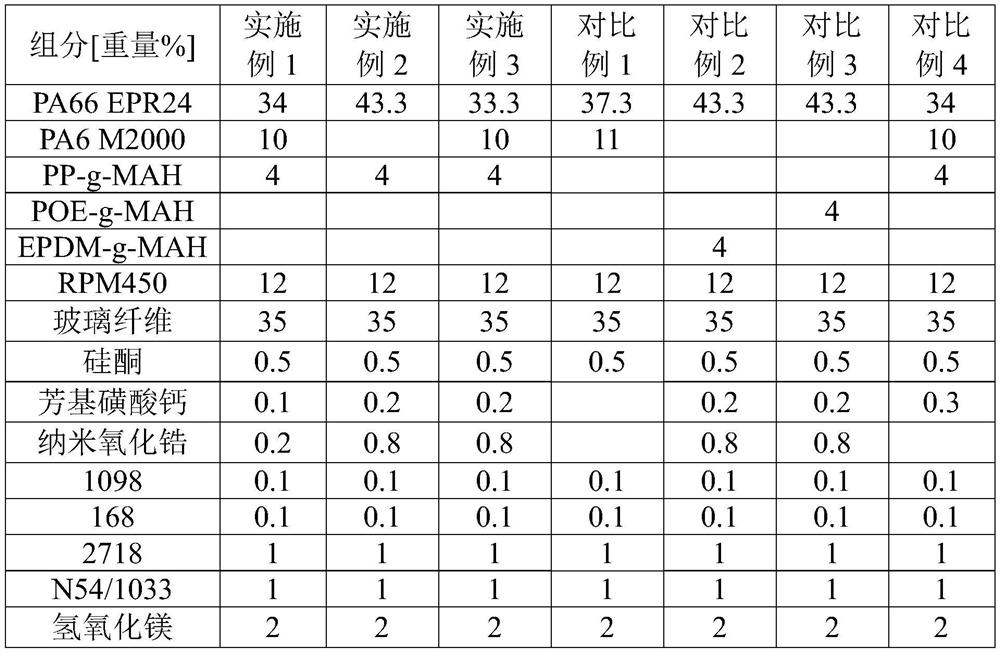
Polyamide material with excellent appearance, low water absorption and self-tapping slipping resistance, preparation method and application thereof
The invention discloses a polyamide material with excellent appearance, low water absorption and self-tapping slipping resistance, a preparation method and application of the polyamide material to preparation of an electric kettle temperature controller. The polyamide material is prepared from the following raw materials by weight percentage: 25-35% of PA66, 0-15% of PA6, 30-40% of a reinforcing component, 5-15% of microencapsulated red phosphorus flame-retardant master batch, 3-8% of a compatilizer, 0.2-2% of a self-tapping slipping resistant aid and 3-8% of other aids; wherein the compatilizer is at least one of polypropylene grafted maleic anhydride and polyethylene grafted maleic anhydride, and the melt index of the compatilizer is 35-150g / 10min under the conditions of 190DEG C and 1.2kg; and the self-tapping slipping resistant aid is prepared from calcium aryl sulfonate and nano zirconium oxide. The preparation method comprises the following steps of: uniformly blending the raw material components except the reinforcing component according to a ratio to obtain a premix, adding the premix into a double-screw extruder from a feeding port, adding the reinforcing component from afirst exhaust port of the double-screw extruder, and carrying out melt extrusion, cooling, drying and pelletizing to obtain the polyamide material.
Owner:中广核俊尔(浙江)新材料有限公司 +1
Process for preparing alkyl aryl sulphonic acids and alkyl aryl sulphonates
InactiveUS20080139840A1Efficient and more environmentally friendlyMolecular sieve catalystOrganic compound preparationArylAlkane
A process for preparing an alkyl aryl sulphonic acid comprising the steps of:(a) contacting an alkyl aromatic hydrocarbon with a gaseous sulphonating agent to produce (i) a first liquid reaction product comprising an alkyl aryl sulphonic acid and (ii) a gaseous effluent stream;(b) separating the first liquid reaction product from the gaseous effluent stream;(c) purifying the gaseous effluent stream to provide a cleaned gaseous stream and a second liquid reaction product;(d) recycling the second liquid reaction product to the first liquid reaction product produced after separation step (b) to produce a third liquid reaction product comprising alkyl aryl sulphonic acid;wherein the alkyl aromatic hydrocarbon is obtained by contacting an aromatic hydrocarbon with an olefin under alkylating conditions, and wherein said olefin is obtained by dehydrogenation of a Fischer-Tropsch derived paraffinic feedstock.
Owner:SHELL OIL CO
Organic acid systems
ActiveUS11319479B2Less smokeReduce riskDrilling compositionBorehole/well accessoriesOrganic acidAryl
An organic acid composition for use in oil industry activities, said composition comprising an arylsulfonic acid in water as well as the use of said composition to perform various operations in the oil industry. There is also disclosed a solid acid composition comprising a corrosion inhibitor ready for dissolution and use.
Owner:DORF KETAL CHEM FZE
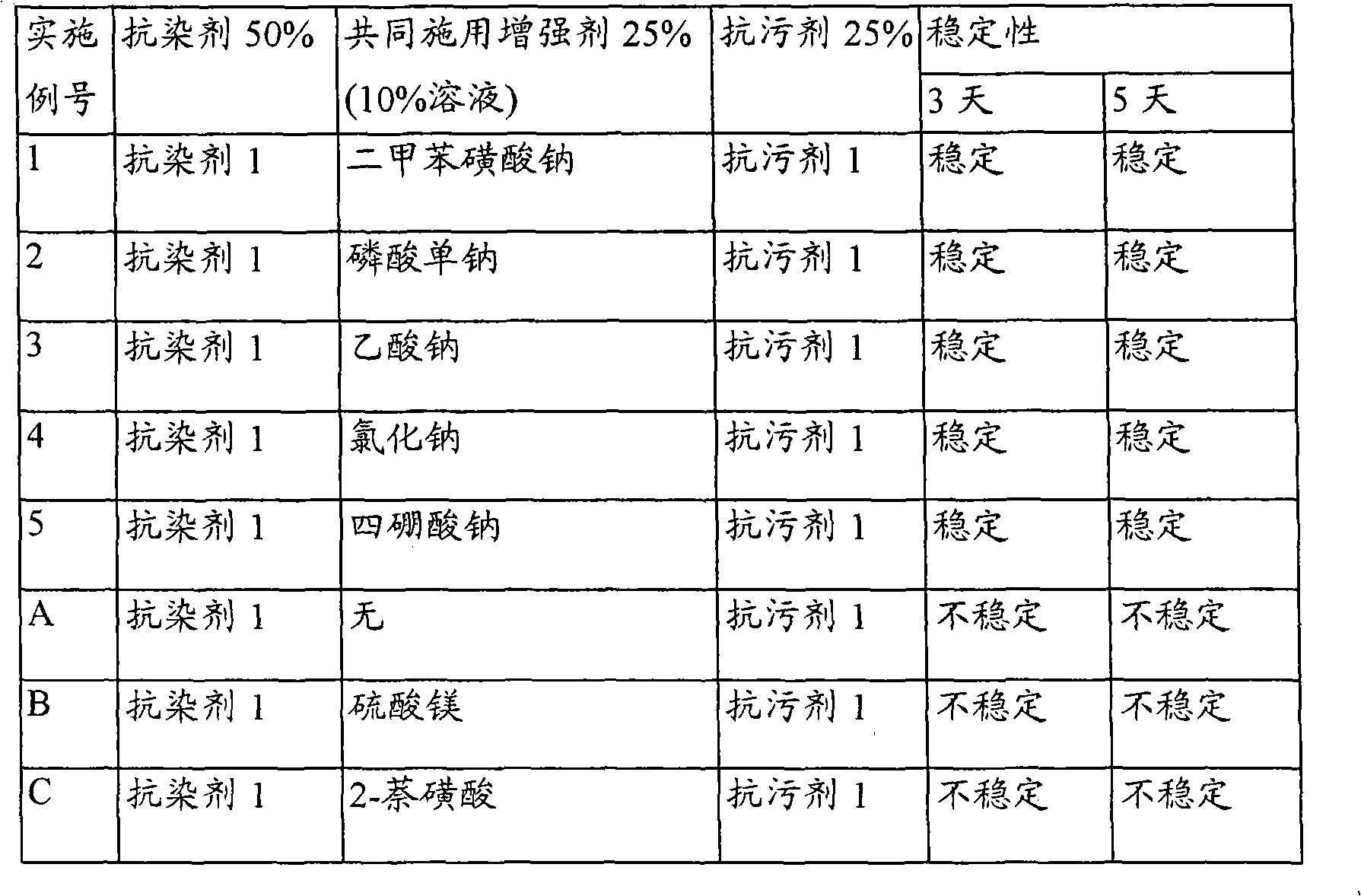
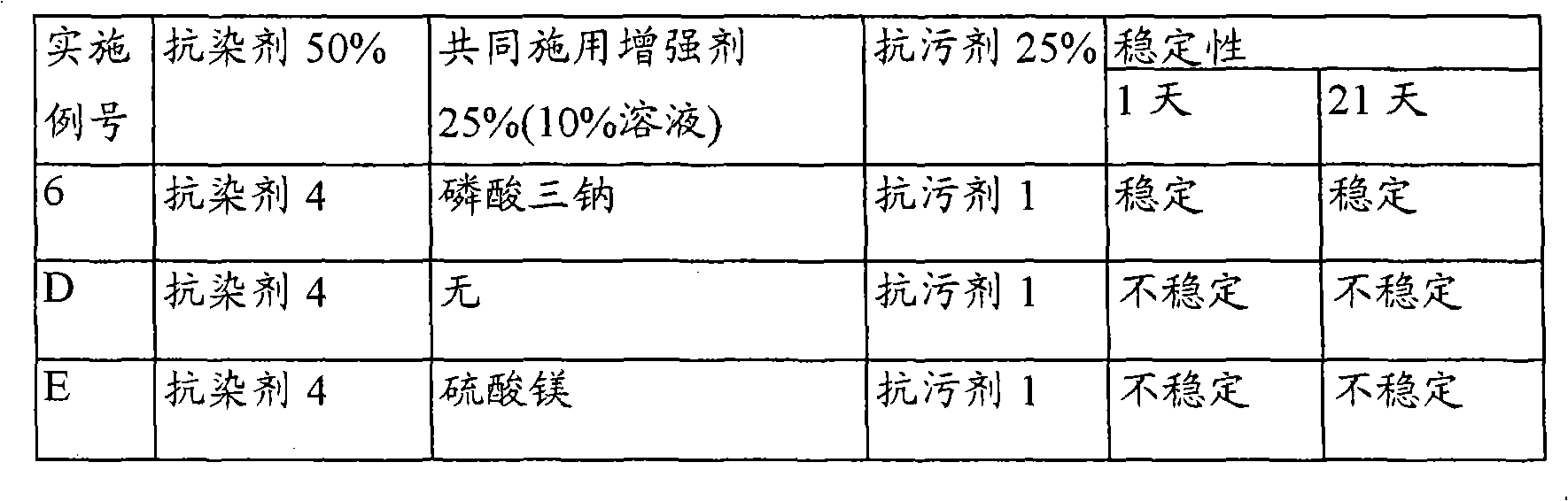
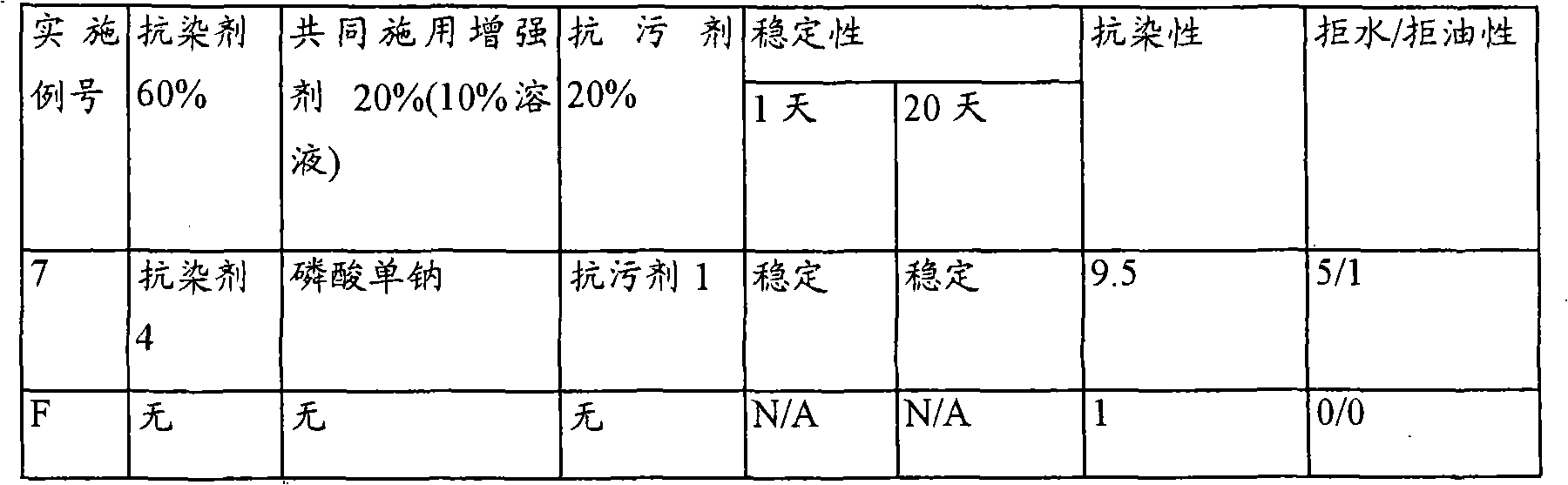
Reduction of turmeric and iodine staining
InactiveUS20070050912A1Reducing turmeric stainFibre treatmentDetergent compounding agentsArylCarbamate
A composition for reduction of turmeric and iodine complex stains comprising at least one stain resist agent and at least one stain resist enhancer, the enhancer comprising at least one of an alkali metal salt; alkali metal aryl salt; aryl sulfonic acid; urea; alkyl carbamate; and amide, alkylamide, dialkylamide, or cyclic amide of formic acid, of C1 to C6 alkanoic acids, or of C1 to C6 alkandioic acids.
Owner:EI DU PONT DE NEMOURS & CO
Alkylation method for salicylic acid
ActiveCN105777537AImprove conversion rateLow polymer contentOrganic compound preparationCarboxylic compound preparationSalicylic acidAlkylation
The invention discloses an alkylation method for salicylic acid. The method comprises the following steps: with long-chain olefin and excess salicylic acid as raw materials, adding a certain amount of a polymerization inhibitor into the raw materials and carrying out an alkylation reaction under the catalysis of aryl sulfonic acid so as to prepare long-chain alkyl salicylic acid; and after completion of the alkylation reaction, adding active alkene and carrying out a secondary alkylation reaction to allow salicylic acid which has not participated in the primary alkylation reaction to be completely converted into the target product, i.e., alkyl salicylic acid. The method provided by the invention can effectively reduce the contents of by-products like free salicylic acid, bialkyl products and olefin oligomer in products obtained in the prior art, increases the purity of the target product, and is simple to operate and beneficial for industrial production.
Owner:PETROCHINA CO LTD
Improved stability for coapplication
A composition comprising a stable mixture of at least one stain resist agent, at least one soil resist agent, and at least one coapplication enhancer, the enhancer comprising at least one of an alkali metal salt; alkali metal aryl salt; ammonium salt; ammonium aryl salt; aryl sulfonic acid; urea; amide; alkylamide; dialkylamide; amide of C1 to C6 alkanoic acids or of C2 to C6 alkandioic acids; diamides of C2 to C6 alkandioic acids; cyclic imide of C2 to C6 alkandioic acids; C3 to C6 lactams, or combinations thereof, is disclosed.
Owner:EI DU PONT DE NEMOURS & CO
Process for preparing thick oil hydrothermally catalytic cracking viscosity reducer containing amphiphilic structure
InactiveCN100443562CExert catalytic activityImprove qualityDrilling compositionLiquid viscosityIron oxide
The present invention relates to preparation process of hydrothermally catalytic cracking viscosity reducer containing amphiphilic structure for thick oil exploitation. The preparation process includes the following steps: 1. preparing material arylsulfonic acid in 19-21 weigh portions, and iron oxide powder in 1.8 or 1.2 weigh portions; and 2. reaction between arylsulfonic acid and iron oxide powder at 80-160 deg.c for 1-3 hr, dissolving the resultant with acetone in 0.9-1.3 times arylsulfonic acid weight, filtering and stoving the filtrate in a stove at 50-80 deg.c to obtain the brown ropy liquid viscosity reducer. The present invention has the features of low cost, high universality and good low temperature viscosity reducing effect.
Owner:CHINA UNIV OF GEOSCIENCES (WUHAN)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com