Near infrared waveband reaction type biological thiol two-photon fluorescent probe as well as preparation method and application thereof
A fluorescent probe and near-infrared technology, applied in the field of fluorescent probes, can solve problems such as limiting the scope of application, and achieve the effects of high sensitivity, good biocompatibility and strong selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
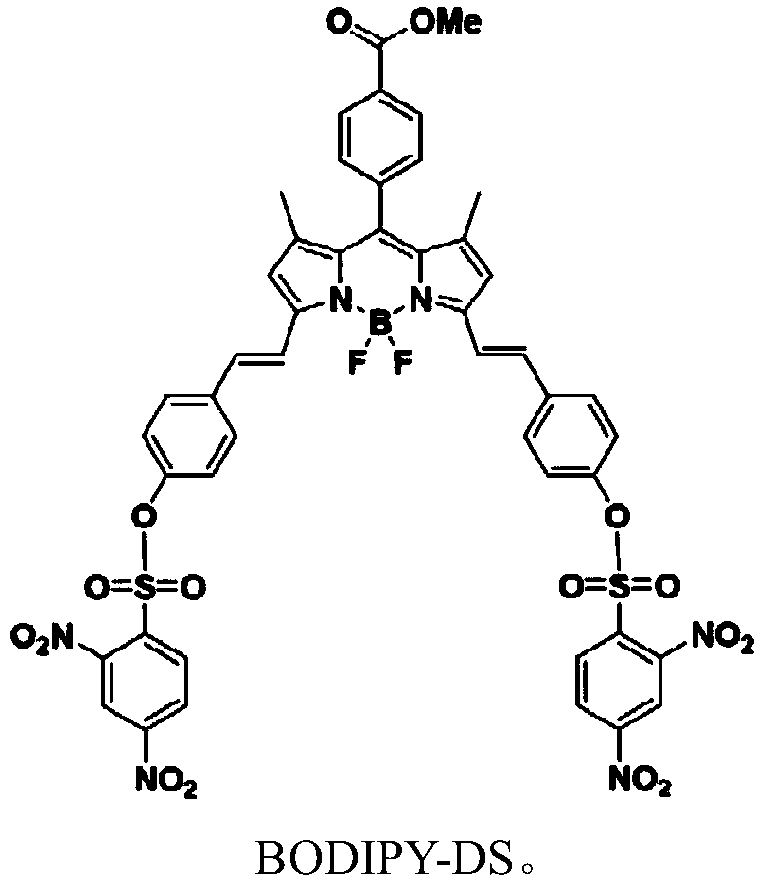
[0019] (1) The preparation method of fluorescent probe molecule BODIPY-DS is:
[0020] Under the protection of an inert gas and at 0°C, fluoroboron dipyrrole conjugated arylphenol BODIPY-OH (0.59g, 1.00mmol) was dissolved in 10mL of dry dichloromethane, and 2,4-dinitrobenzenesulfonyl chloride ( 0.58g, 2.18mmol), then add 10mL of dry dichloromethane and triethylamine (2.18mmol), stir and react for 10h, after the reaction, extract, dry, concentrate, and purify by column chromatography to obtain the fluorescent probe molecule BODIPY -DS, yield 45%.
[0021] The synthetic route of compound BODIPY-DS is as follows:
[0022]
[0023] Among them, fluoroboron dipyrrole conjugated arylphenol BODIPY-OH was prepared according to the literature method (Bao-xingShen, YingQian. A novel triphenylamine-BODIPY dendron: click synthesis, near-infraredemission and a multi-channelchemosimeter for Hg 2+ and Fe 3+ . Journal of Materials Chemistry B, 2016, 4, 7549-7559).
[0024] (2) Structura...
Embodiment 2
[0030] The preparation method of fluorescent probe molecule BODIPY-DS is basically the same as in Example 1, the difference is:
[0031] Under the protection of an inert gas and at 0°C, fluoroboron dipyrrole conjugated arylphenol BODIPY-OH (0.59g, 1.00mmol) was dissolved in 10mL of dry dichloromethane, and 2,4-dinitrobenzenesulfonyl chloride ( 0.64g, 2.40mmol), then add 10mL of dry dichloromethane and triethylamine (2.40mmol), and stir the reaction for 12h. After the reaction, the fluorescent probe molecule BODIPY-DS was obtained through extraction, drying, concentration and column chromatography purification, with a yield of 48%.
Embodiment 3
[0033] The preparation method embodiment 1 of fluorescent probe molecule BODIPY-DS is basically the same, the difference is:
[0034] Under the protection of an inert gas and at 0°C, fluoroboron dipyrrole conjugated arylphenol BODIPY-OH (0.59g, 1.00mmol) was dissolved in 10mL of dry dichloromethane, and 2,4-dinitrobenzenesulfonyl chloride ( 0.66g, 2.48mmol), then added 10mL of dry dichloromethane and triethylamine (2.48mmol), and stirred for 12h. After the reaction is completed, the fluorescent probe molecule BODIPY-DS is obtained through extraction, drying, concentration and column chromatography purification, with a yield of 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com