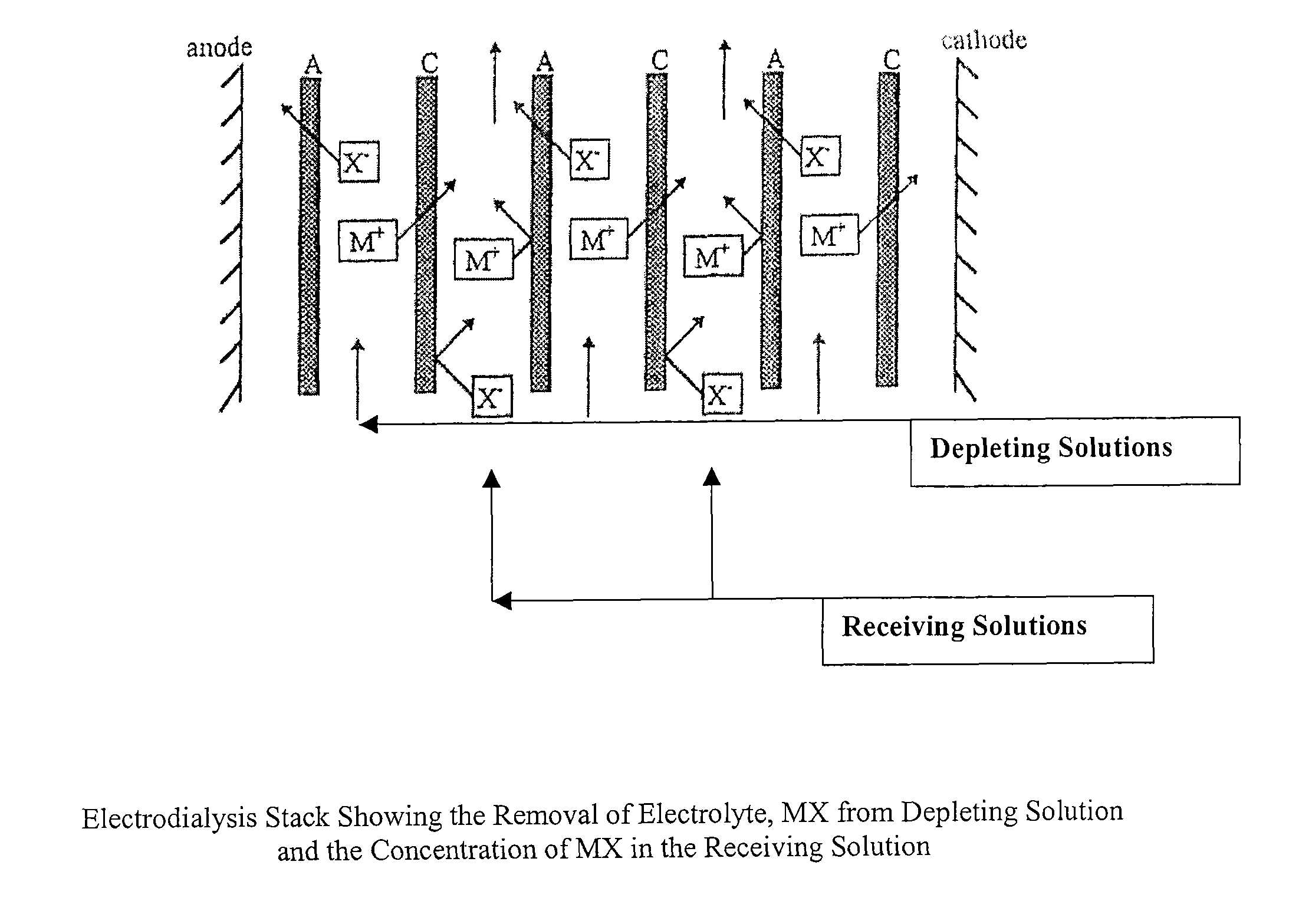
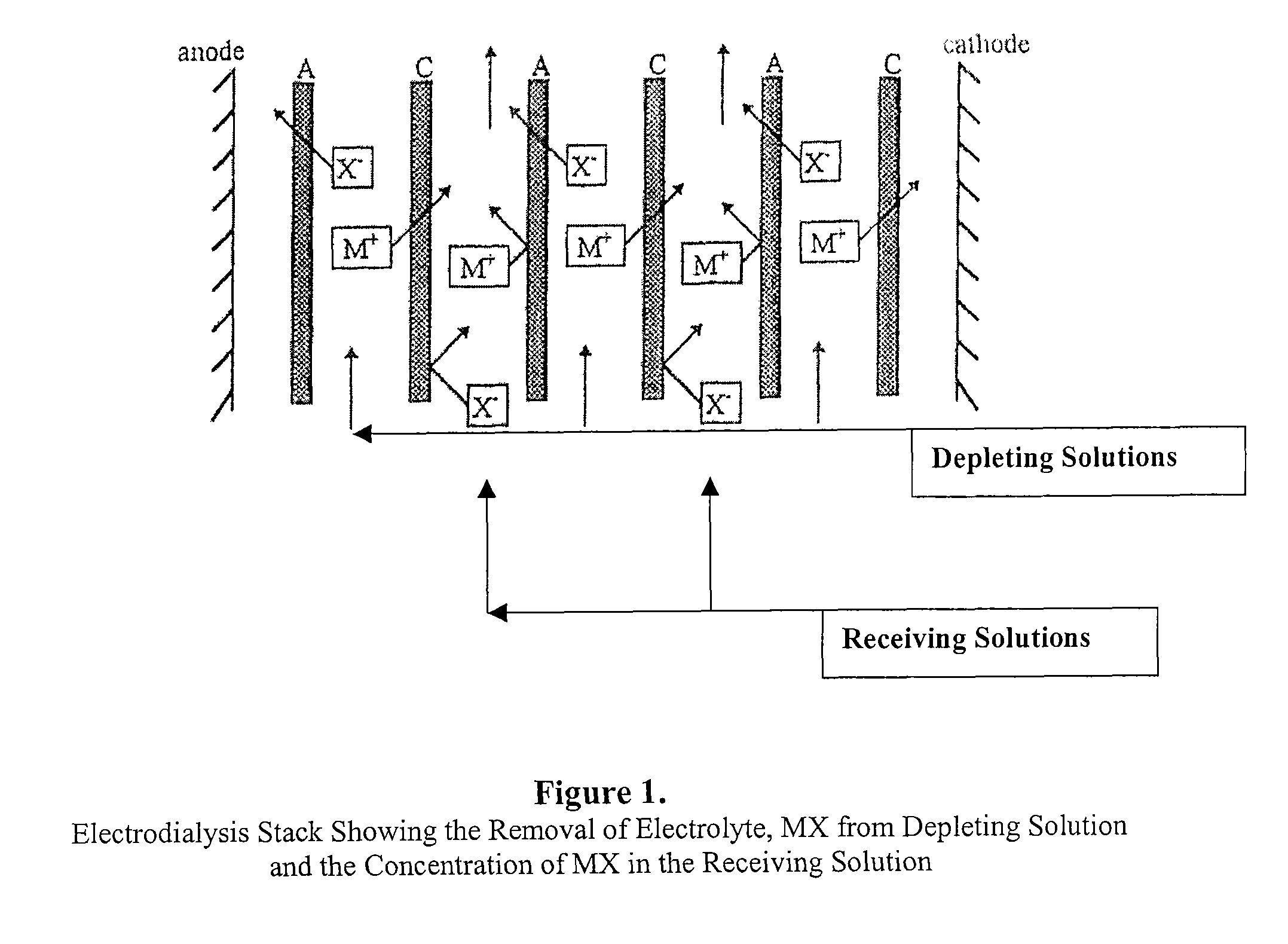
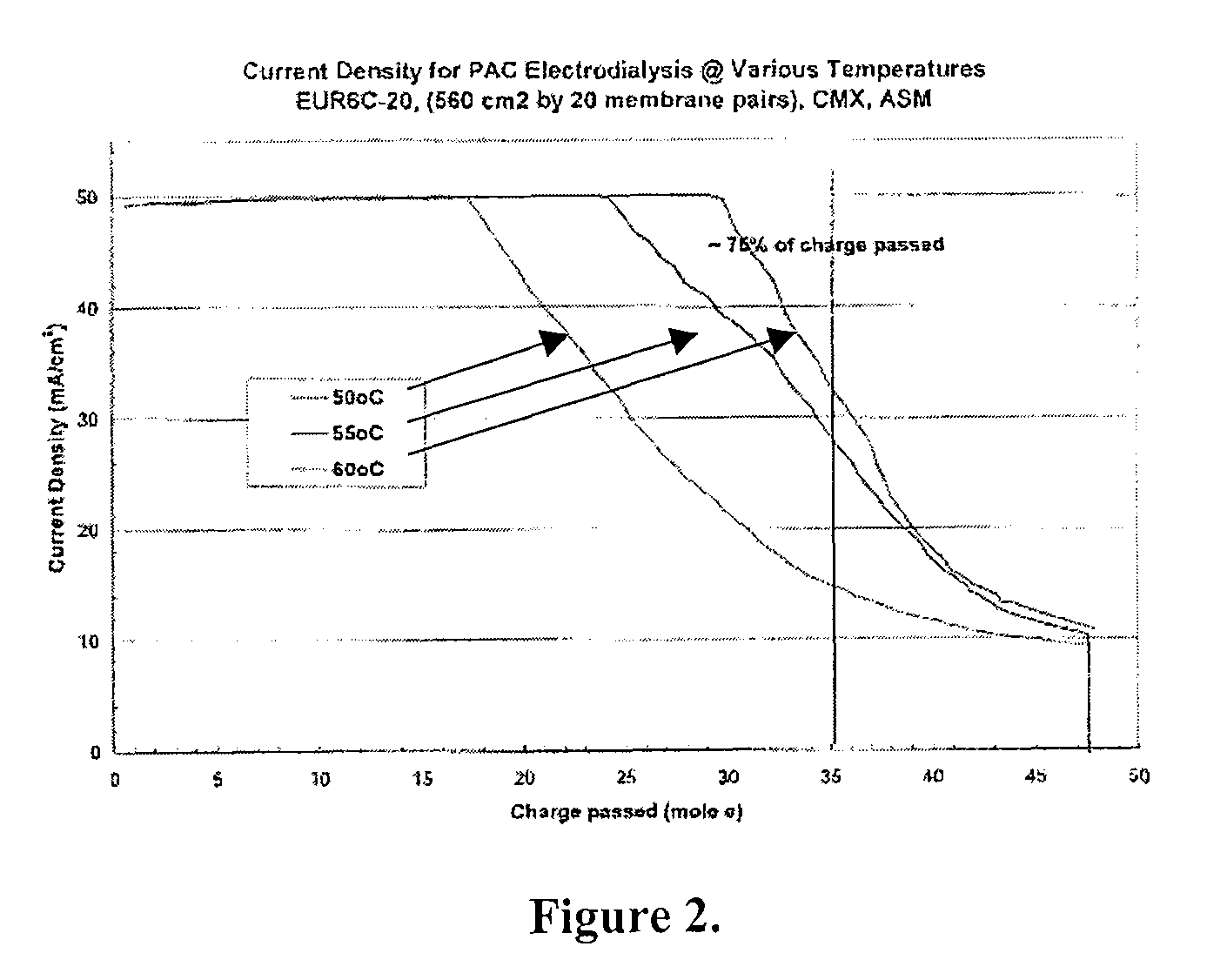
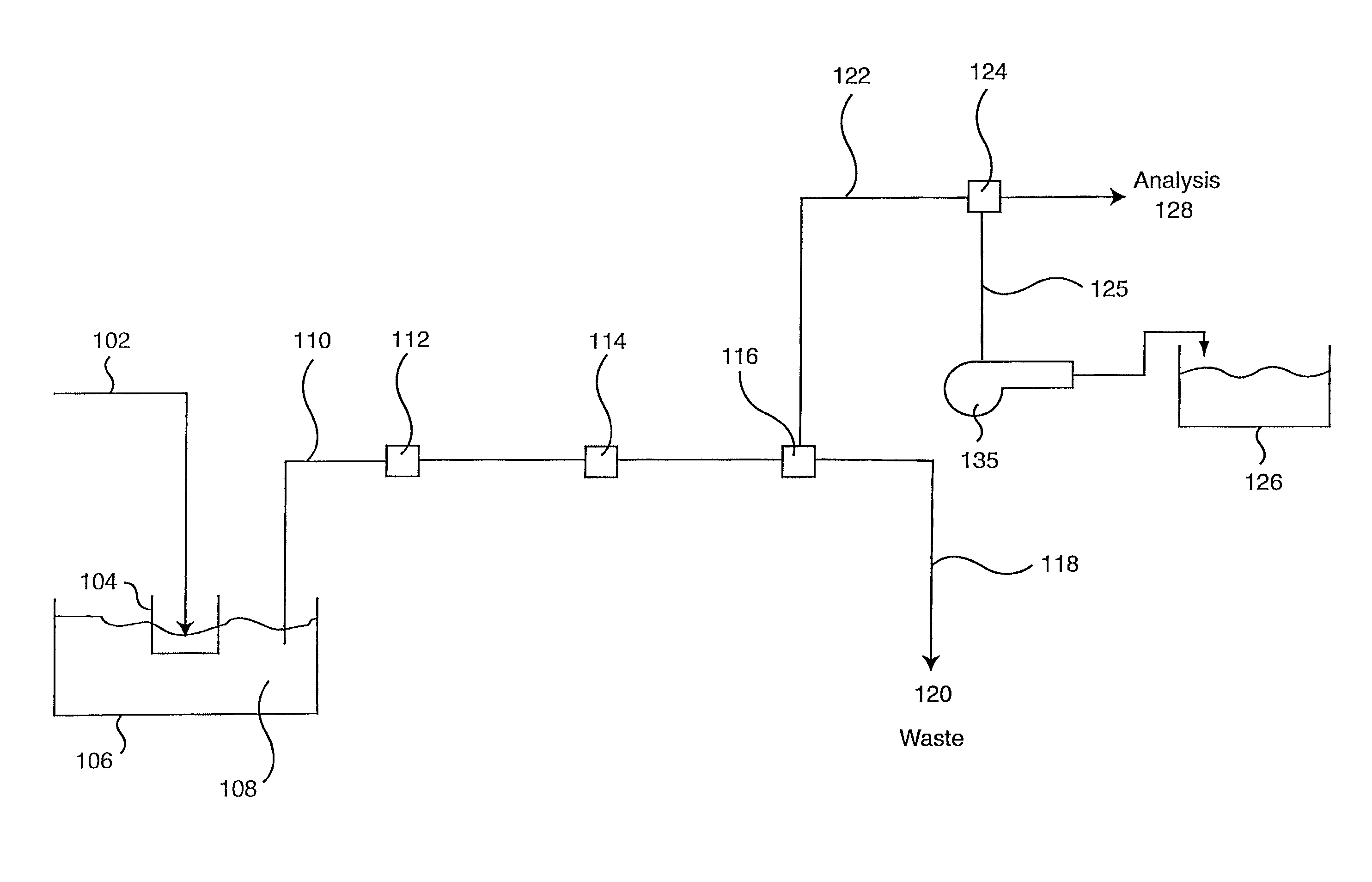
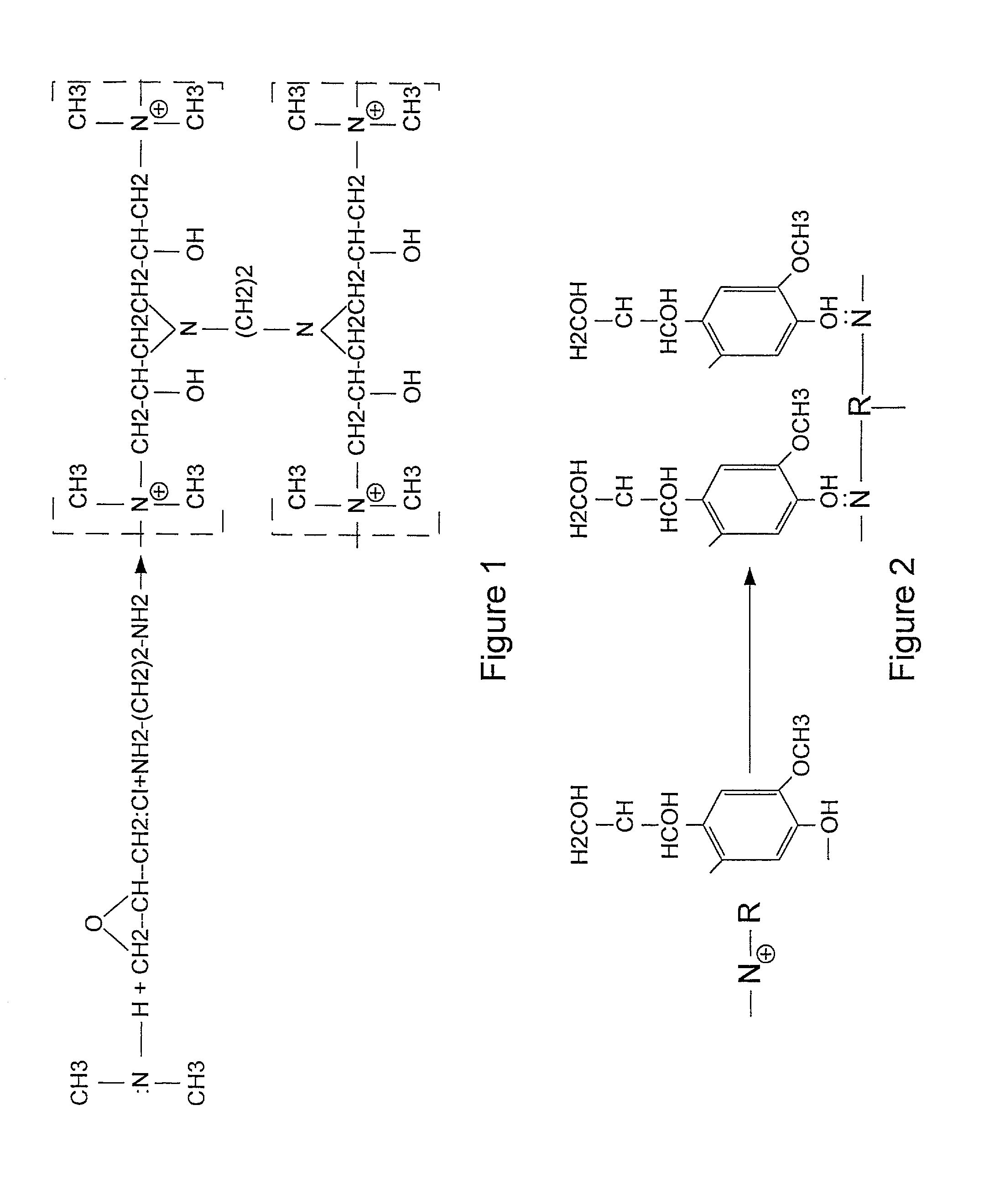
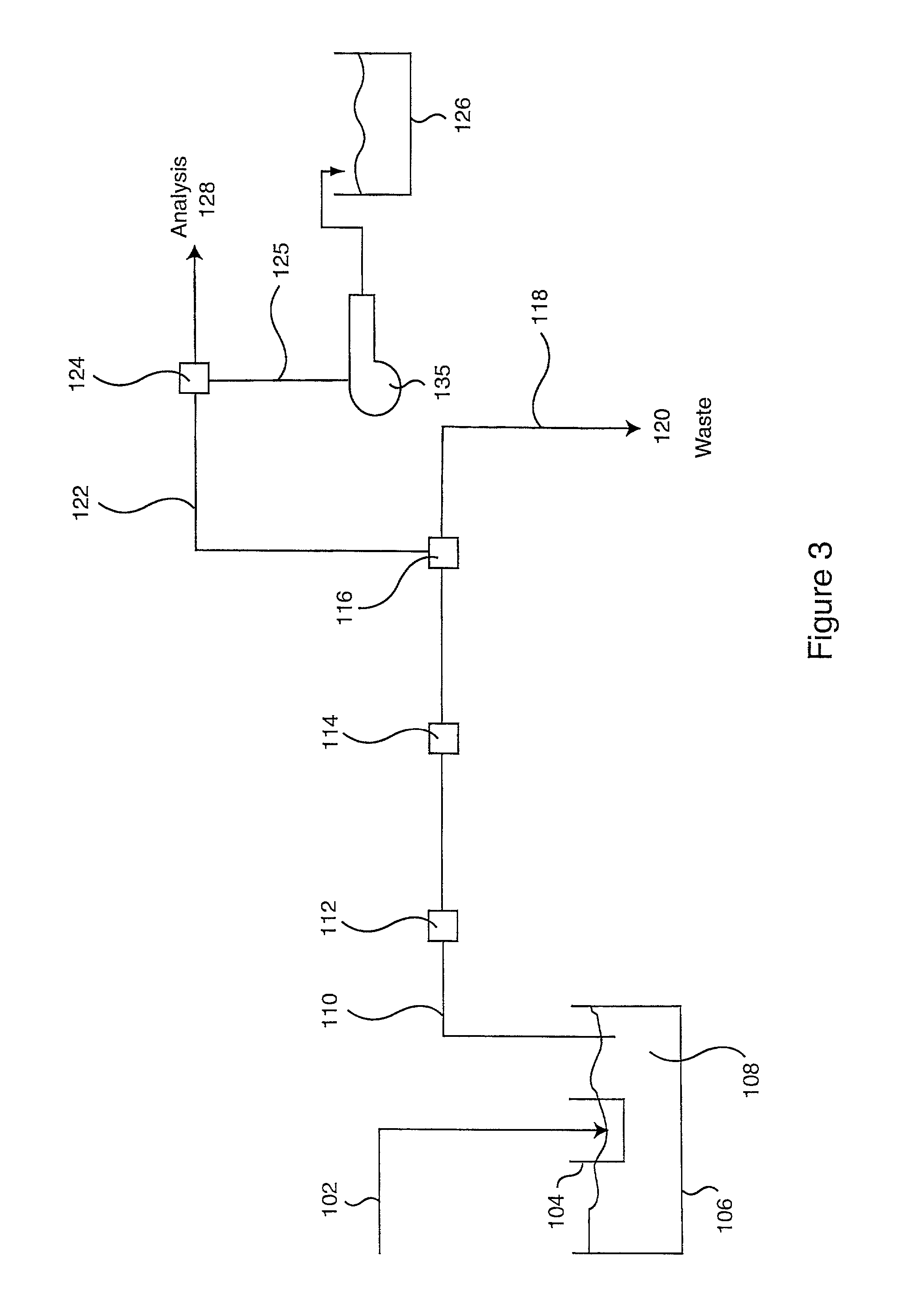
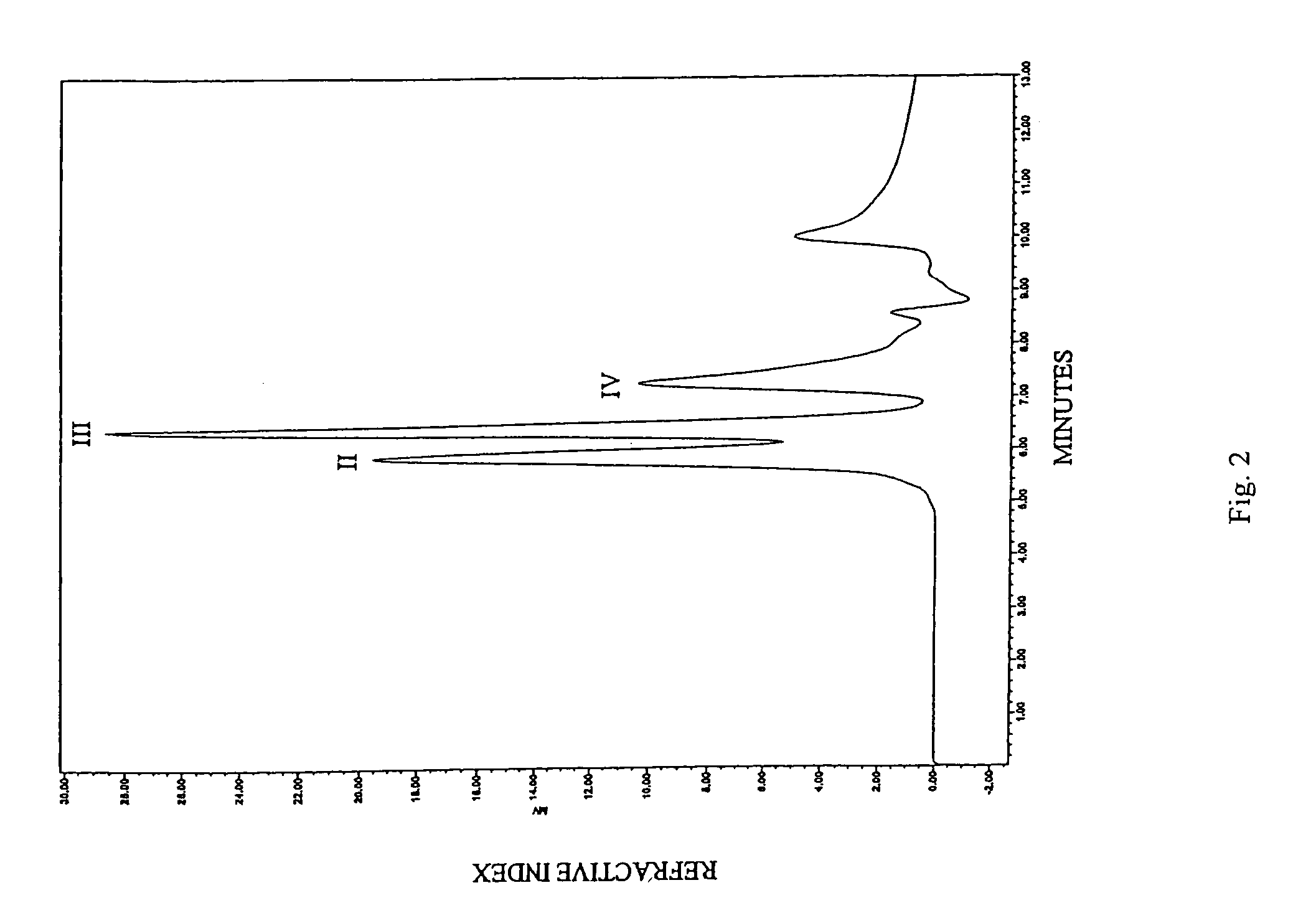
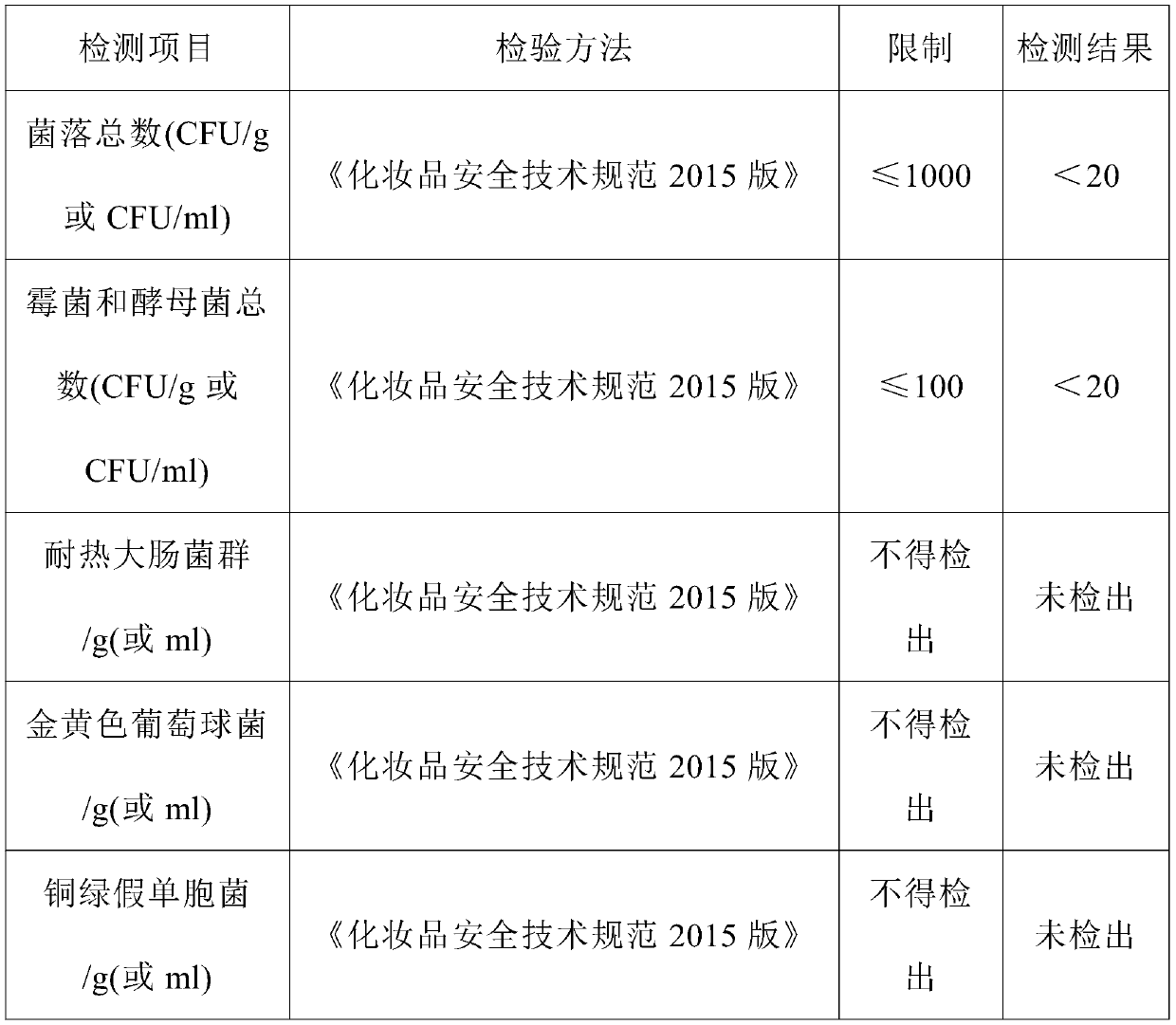
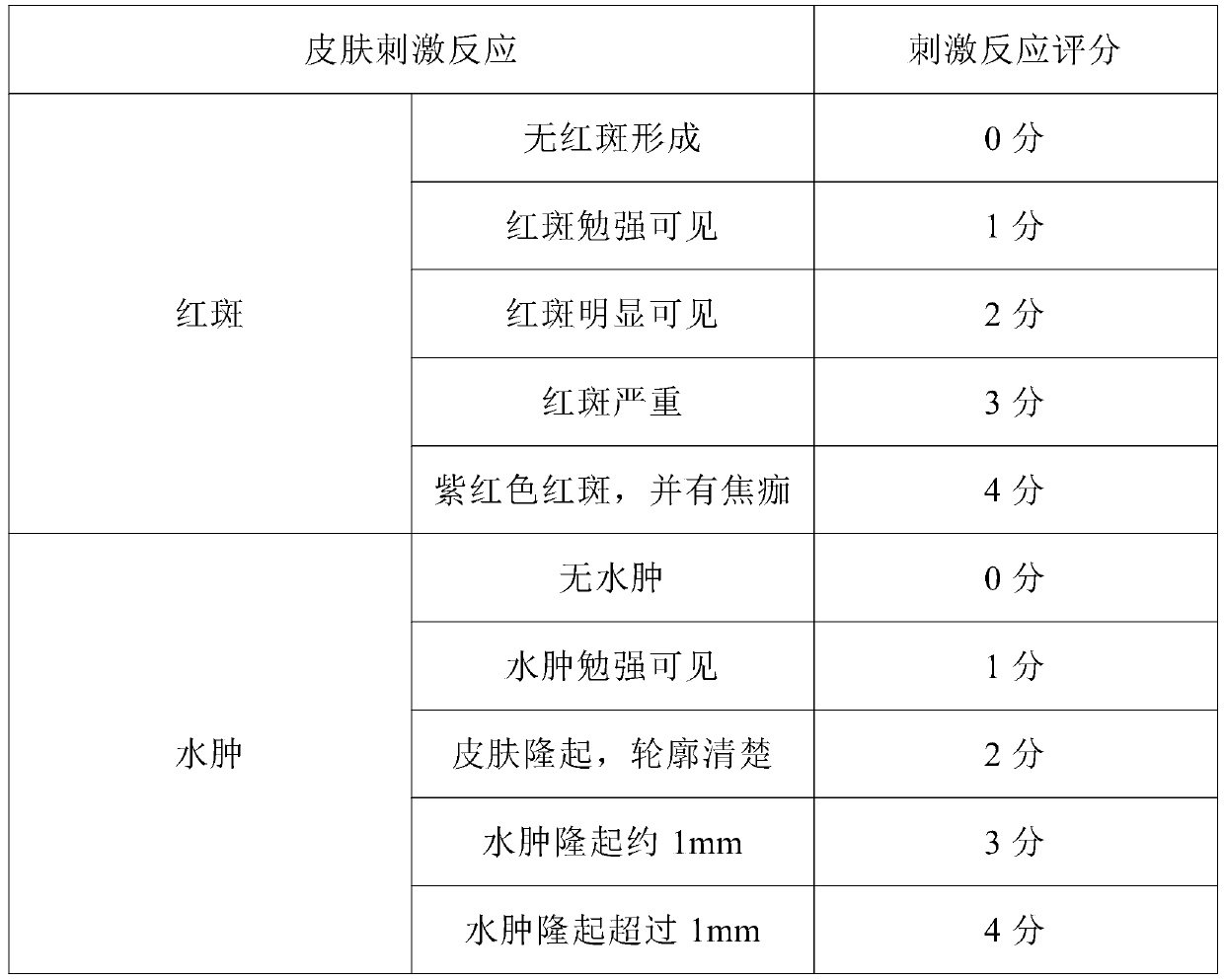
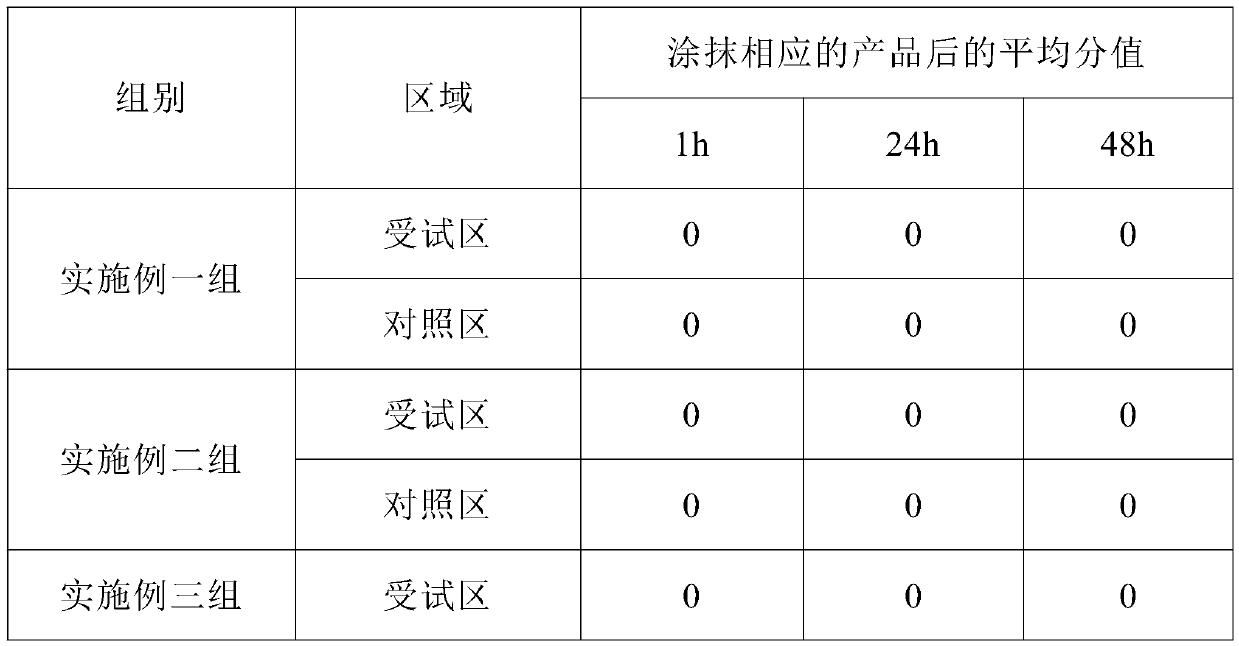
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "ALUMINUM CHLOROHYDRATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Textile ink composition
A textile ink composition and a method of manufacturing a textile ink composition are disclosed. The textile ink composition is formed from a cationically charged metal oxide and a dye, and is capable of durably dyeing different types of fabric. The cationically charged metal oxide is typically an oligomer, such as an alumina oligomer. A suitable alumina oligomer is aluminum chlorohydrate. Typical dyes have anthraquinone, catechol, hydroxyazo or salicylic acid groups or are mordant dyes, such as Carminic Acid, Alizarin Red, Acid Blue 45, Acid Green 41, Hematoxylin, Chromoxane Cyanine R, Calconcarboxylic Acid, Plasmocorinth B, Pyrocatechol, Acid Alizarin Violet N, Alizarin Yellow GG, Mordant Yellow 12 and Mordant Blue 9.
Owner:KIMBERLY-CLARK WORLDWIDE INC
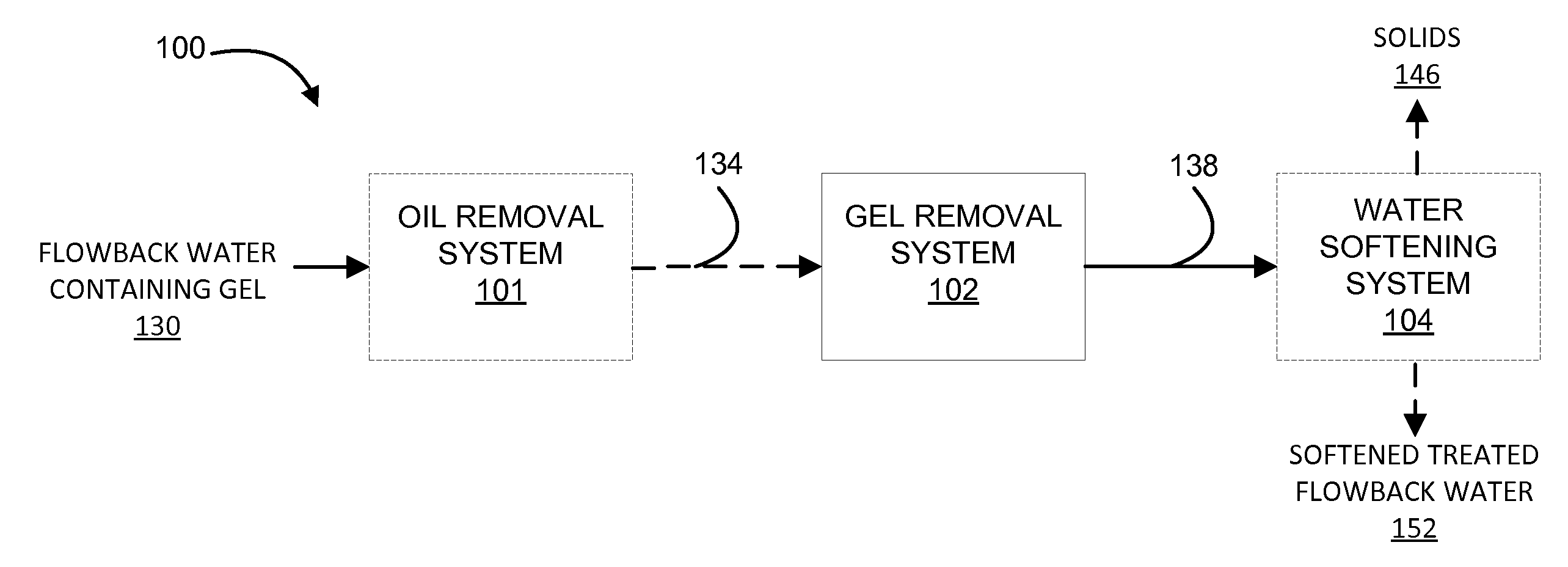
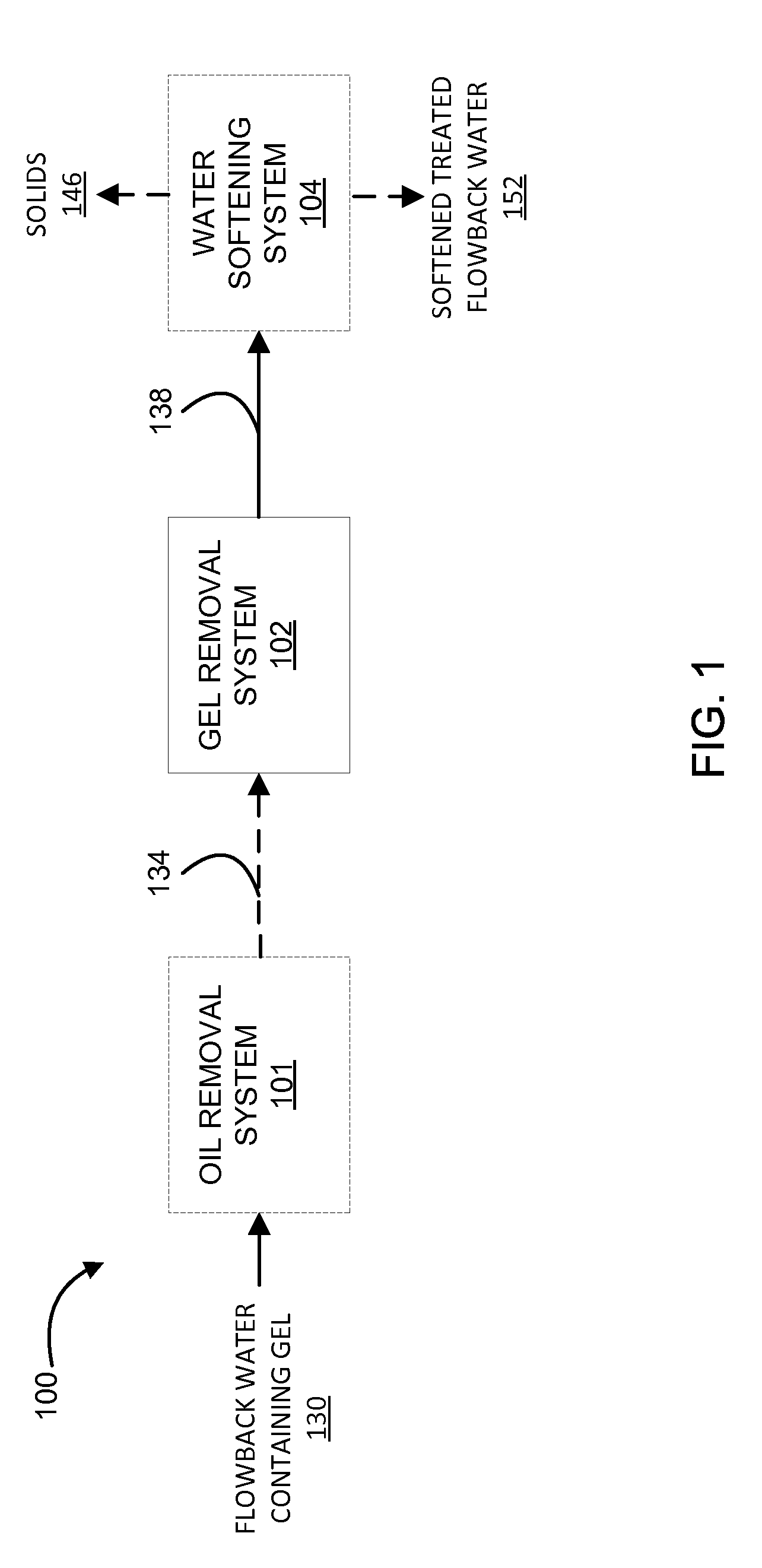
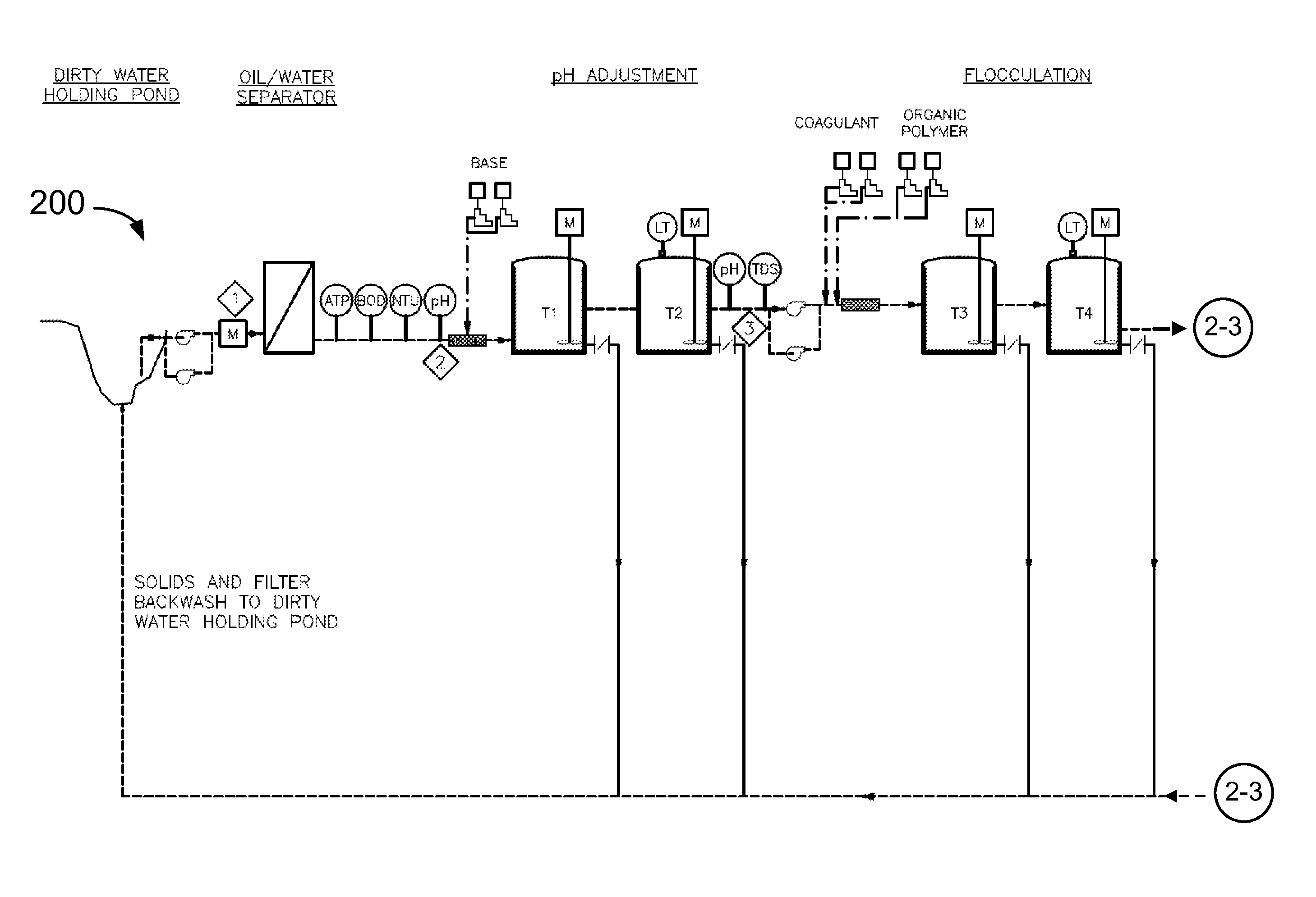
System and method for treatment of produced waters containing gel
This disclosure describes novel systems and methods for removing gel from flowback water. The methods and systems include treating acidified flowback water with aluminum chlorohydrate.
Owner:HIGH SIERRA ENERGY LP
Treatment of contaminated water from gas wells
ActiveUS20110042320A1Increase in sizeWaste water treatment from quariesWater treatment parameter controlParticulatesDistillation
Systems and methods for treating contaminated water from gas wells by adding an inorganic coagulant and a low molecular weight polymer to the contaminated water to increase the size of solid particulates in the water and to thereby allow the solid particulates to be filtered or to otherwise be removed from the water are disclosed herein. While the inorganic coagulant can be any suitable coagulant, in some cases the coagulant is selected from aluminum chlorohydrate, polyaluminum chloride, aluminum sulfate, and ferric sulfate. Similarly, the polymer can comprise any suitable polymer, such as epi / dma, a condensation product of epichlorohydrin and dimethyl amine. The described systems and methods can clean the contaminated water so that the water can then be treated in a variety of other manners, which may include reverse osmosis, deionization, treatment with mixed bed deionizers, electro-separation, fraction distillation, distillation, and other suitable water cleansing processes.
Owner:WATER SOLUTIONS TECH LLC
Method for removal of phosphate from bodies of water by topical application of phosphate scavenging compositions with a hand held, hose end sprayer
ActiveUS7481939B2Quick and easy applicationReduce concentrationWater contaminantsSeparation devicesPhosphate ClearanceSprayer
Owner:HALEY PATRICK
Method for paint detackification using compositions containing chitosan
InactiveUS6858093B2Shorten the timeHollow article cleaningChemical paints/ink removersWater basedSodium Bentonite
A composition and method for treating oversprayed paints in paint spray booths is provided. The composition includes an aqueous solution of a compound such as chitosan, and a complex metal salt, such as aluminum chlorohydrate, capable of flocculating the oversprayed paint, and optionally, bentonite clay. The composition is useful for detackifying and flocculating oversprayed paint, and is particularly useful as a liquid concentrate for the addition to wash systems in paint spray booths for water-based and solvent-based paints. The composition is also useful in decreasing the time for phase separation of the organic phase and the aqueous phase in solvent-based removal processes.
Owner:PPG IND OHIO INC
Polymetal Hydroxychloride Processes and Compositions: Enhanced Efficacy Antiperspirant Salt Compositions
InactiveUS20070196302A1Materials and reduced eliminatedSteps reduced eliminatedCosmetic preparationsSemi-permeable membranesAluminium chlorideZirconium compounds
The invention describes processes for the production of basic aluminum compounds, including aluminum chlorohydrate, basic zirconium compounds, and basic aluminum zirconium compounds. The process produces products of a wide range of basicities. The products formed by the present invention are comprised of low molecular weight species characteristic of enhanced efficacy antiperspirant salt compositions. The products of this process are suitable for use as water purification agents, as binders in catalyst applications, and in antiperspirant applications. In addition, the invention is directed to the products made by the disclosed process.
Owner:NEXT CHEM LLC
High quality porous ink-jet media
A print medium for ink-jet printing comprises a photobase substrate, a porous ink-receiving layer and a subbing layer. The photobase substrate can include paper and a moisture barrier layer coated on at least one side of the paper. The porous ink-receiving layer can include metal oxide or semi-metal oxide particulates treated with an organosilane reagent and aluminum chlorohydrate, and a polyvinyl alcohol binder. The subbing layer can be disposed between the moisture barrier layer of the photobase and the ink-receiving layer and include a polyvinyl alcohol or a copolymer of a polyvinyl alcohol.
Owner:HEWLETT PACKARD DEV CO LP
Polyaluminum Chloride and Aluminum Chlorohydrate, Processes and Compositions: High-Basicity and Ultra High-Basicity Products
ActiveUS20070187256A1Increase concentrationIncrease hydroxide contentCosmetic preparationsElectrolysis componentsAntiperspirantsAlkalinity
The invention relates generally to processes for the production of high-basicity and ultr-high basicity polyaluminum chlorides including aluminum chlorohydrate. The processes can produce products of a wide range of basicities and are particularly useful in producing high basicity products. The process can produce a wide range of solution concentrations and are particularly useful in producing high solution concentrations. The processes described generate high purity products, which are free of by-product salt(s). The processes described herein can also be utilized to produce enhanced efficacy polyaluminum chlorides including aluminum chlorohydrate. When compared to conventional processes for manufacturing these compounds the processes disclosed herein are unique in so far as the disclosed processes do not require aluminum metal as a starting material. The products of the processes are suitable in applications including water purification, catalysts, and antiperspirants. In addition, the invention is directed to the products prepared by the processes described herein.
Owner:NEXT CHEM LLC
Polyaluminum chloride and aluminum chlorohydrate, processes and compositions: high-basicity and ultra high-basicity products
The invention relates generally to processes for the production of high- basicity and ultr- high basicity polyaluminum chlorides including aluminum chlorohydrate. The processes can produce products of a wide range of basicities and are particularly useful in producing high basicity products. The process can produce a wide range of solution concentrations and are particularly useful in producing high solution concentrations. The processes described generate high purity products, which are free of by-product salt(s). The processes described herein can also be utilized to produce enhanced efficacy polyaluminum chlorides including aluminum chlorohydrate. When compared to conventional processes for manufacturing these compounds the processes disclosed herein are unique in so far as the disclosed processes do not require aluminum metal as a starting material. The products of the processes are suitable in applications including water purification, catalysts, and antiperspirants. In addition,the invention is directed to the products prepared by the processes described herein.
Owner:NEXT CHEM LLC
Water filtration process and apparatus
InactiveUS20070007213A1Quality improvementSimple processSpecific water treatment objectivesTreatment involving filtrationAluminium chlorideFiltration
A water filtration process and apparatus which includes the steps of treating well water with a coagulating agent or flocculant such as aluminum sulfate and / or potassium permanganate and aluminum chlorohydrate and directing the treated effluent water stream containing positively charged coagulated contaminant particles into a series of filter chambers packed with finely crushed rocks, sand and / or negatively-charged glass filter medium of random particle size. The water is filtered through the glass filter medium from top to bottom and the filtered potable effluent removed from the bottom of the filter chambers and typically directed to a storage tank. A closed-system, clarified water recycle backwash system is also provided, wherein water clarified in a series of interconnected backwash tanks is introduced into the well water effluent line and filtered as the potable filtered effluent is back-flushed through backwashed filter chamber or chambers in a desired sequence, to remove the impurities, residue and chemicals from the glass filter media. The accumulated impurities, residue and chemicals introduced with the backwash water into the backwash tanks are periodically removed from the tanks, typically by vacuum trucks.
Owner:MACPHERSON JOHN +2
High performance low environmental impact detackifier
ActiveCN102459090AGood anti-sticking effectEasy to separatePaint waste treatmentWater/sewage treatment by neutralisationWater basedAluminium chlorohydrate
The current invention relates to a composition and a method for treating impurities in a circulating water system. The circulating water system impurities may be oversprayed paint in paint spray booth applications. The composition includes an aqueous solution of a cationized starch, a polybasic aluminum salt such as aluminum chlorohydrate or polyaluminum chloride and a solution of a medium to high MW aqueous flocculant. The composition is particularly useful when added to recirculating scrubber water in paint spray booths for effectively treating both water based and solvent based paints.
Owner:NALCO CO
Polyaluminum chloride and aluminum chlorohydrate, processes and compositions: high-basicity and ultra high-basicity products
ActiveUS7846318B2Increase hydroxide contentCosmetic preparationsChloride preparationAntiperspirantsAlkalinity
The invention relates generally to processes for the production of high-basicity and ultra-high basicity polyaluminum chlorides including aluminum chlorohydrate. The processes can produce products of a wide range of basicities and are particularly useful in producing high basicity products. The process can produce a wide range of solution concentrations and are particularly useful in producing high solution concentrations. The processes described generate high purity products, which are free of by-product salt(s). The processes described herein can also be utilized to produce enhanced efficacy polyaluminum chlorides including aluminum chlorohydrate. When compared to conventional processes for manufacturing these compounds the processes disclosed herein are unique in so far as the disclosed processes do not require aluminum metal as a starting material. The products of the processes are suitable in applications including water purification, catalysts, and antiperspirants. In addition, the invention is directed to the products prepared by the processes described herein.
Owner:NEXT CHEM LLC
Compositions and processes for reducing true color in waste liquids
InactiveUS20020130089A1Reducing true colorTrue colorScale removal and water softeningSeparation devicesSolubilityLiquid waste
Compositions of aluminum chlorohydrate and high molecular weight branched epichlorohydrin amine condensate polymers are applied to waste liquids, such as pulp and paper mill effluents having a lignin content, to reduce true color, The branched epichlorohydrin amine condensate polymer is branched using branching agents which result in a molecular weight greater than about 300,000 and permit solubility. When applied to waste liquids, the high molecular weight branched epichlorohydrin amine condensate polymer and aluminum chlorohydrate react with the lignin components in the waste liquid to form an insoluble agglomerate thus reducing the true color of the waste liquid. The resulting agglomerate, including the colored lignin, may be filtered from the waste liquid.
Owner:STEEN RES
Method of making aluminum-zirconium antiperspirant of enhanced efficacy
InactiveUS7060258B2Improve efficacyPrevent skinPillowsCosmetic preparationsDepolymerizationRoom temperature
A novel efficacious and less irritant aluminum-zirconium antiperspirant composition is provided by the addition of a small amount of AlCl3 and / or HCl to the activated aluminum component. After the heating of diluted basic aluminum chlorohydrate solution, cooling to room temperature, mixing with small amount of AlCl3 or HCl and then reacting with zirconium glycine complex, an aluminum-zirconium salt is produced with a maximum amount of depolymerization aluminum and zirconium species. The addition of a small amount of AlCl3 or HCl to the diluted and activated aluminum chlorohydrate solution accelerates the depolymerization of the activated ACH solution, and upon the addition of zirconium glycinate the solution is further depolymerized and results in the formation of less polymerized zirconium species.
Owner:SUMMIT RES LAB
Antiperspirant sterilization agent, antiperspirant sterilization agent-containing body-odor deodorant and preparation method of antiperspirant sterilization agent-containing body-odor deodorant
InactiveCN110812298AEliminate inflammationImprove secretionCosmetic preparationsToilet preparationsOdor sourceHamamelis virginiana
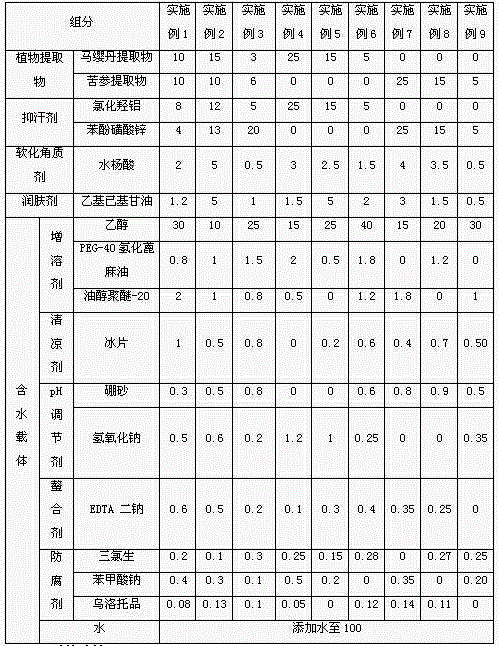
The present invention discloses an antiperspirant sterilization agent, an antiperspirant sterilization agent-containing body-odor deodorant and a preparation method of the antiperspirant sterilizationagent-containing body-odor deodorant, and relates to the field of deodorants. The antiperspirant sterilization agent is composed of four components of hamamelis virginiana water, aluminum chlorohydrate, a herba portulacae extract and zinc phenolsulfonate. The present invention also provides the antiperspirant sterilization agent-containing body-odor deodorant and the preparation method of the antiperspirant sterilization agent-containing body-odor deodorant. The body-odor deodorant is obtained by interaction of the antiperspirant sterilization agent with other substances in a formula, synergistic effects of removing odor, conducting astringency to stop sweat, and preventing microorganisms of the body-odor deodorant are further improved, at the same time, micro-irritation of some raw materials in the formula can also be avoided, and active components in the formula penetrate into tube walls of apocrine sweat glands, directly hit an odor source, eliminate harmful microorganisms and regulate secretion of the sweat glands from a root of the odor source; and generation of unsaturated fatty acids and unsaturated fatty alcohols is avoided, and thus body odor is cured.
Owner:广州市妆妍生物技术有限公司
Composition for treating beriberi and foot stench, and preparation method thereof
ActiveCN103908491AGood compatibilityImprove comfortSalicyclic acid active ingredientsAntimycoticsCutinPhenol
The invention discloses a composition for treating beriberi and foot stench. The composition has the functions of suppressing bacteria and relieving itching, stopping perspiration and deodorizing. The composition contains a plant extract, a perspiration-suppressing agent, a cutin-softening agent and a skin-moistening agent. On basis of preferably selecting conventional deodorizing and itching-relieving traditional Chinese medicines, aluminum chlorohydrate and zinc phenolsulfonate which have the perspiration-stopping function are added. The composition has good functions for suppressing bacteria and relieving itching, stopping perspiration and deodorizing, can effectively improve foot skin environment, and can prevent and control beriberi and foot stench relatively well.
Owner:广西厚德药业有限公司
Feed capable of improving egg laying quality of chicken and preparation method of feed
InactiveCN106804982AIncrease egg productionQuality improvementFood processingAnimal feeding stuffAnimal scienceLiver and kidney
The invention provides feed capable of improving the egg laying quality of chicken and a preparation method of the feed. The feed comprises, by weight, 6000-6500 parts of corns, 1500-2000 parts of bean pulp, 250-500 parts of mountain flour, 300-600 parts of paludina powder, 180-420 parts of corn protein powder, 80-350 parts of pork scraps, 80-130 parts of calcium hydrophosphate, 50-100 parts of feather protein meal, 10-25 parts of vitamin, 0.1-1 part of aluminum chlorohydrate, 0.1-1 part of natural amino acid, 20-35 parts of trace elements, 20-60 parts of food attractant, 30-50 parts of medical stone, 30-50 parts of sodium chloride and 20-50 parts of Chinese herbal medicine additives. According to the feed, the laying rate of the chicken and the egg quality can effectively improved, immunities and stress resistance of the chicken can be improved, Chinese herbal medicine has the functions of reinforcing qi and blood, nourishing liver and kidney and strengthening spleen and stomach, so that the chicken can be integrally adjusted, and the death rate of the chicken is reduced.
Owner:嵊州市派特普科技开发有限公司
Wet tissue for making body have aroma
InactiveCN103845241ASoft towelCosmetic preparationsToilet preparationsAdditive ingredientHamamelis virginiana leaf extract
The present invention relates to the field of wet tissues, specifically to a wet tissue for making body have aroma. The preparation method comprises: taking 3-5 parts by weight of aluminum chlorohydrate, 3-5 parts by weight of dipropylene glycol, 3-5 parts by weight of methyl gluceth, 3-5 parts by weight of PEG-40 chlorinated castor oil, 3-5 parts by weight of glycerin, 3-5 parts by weight of phenoxyethanol, 3-5 parts by weight of EDTA-2Na, 5-10 parts by weight of an oriental cherry blossom extract, 5-10 parts by weight of an orchid extract, and 5-10 parts by weight of a hamamelis mollis oliver leaf extract, adding to purified water 3-5 times the volume of the above mixture to prepare a solution, adopting a spunlaced non-woven fabric as a base cloth, cutting into the predetermined size, sterilizing, disinfecting, soaking the base cloth in the prepared solution for 4-6 h, and carrying out sealing aseptic packaging to obtain the wet tissue. According to the present invention, the wet tissue is made by adopting the spunlaced non-woven fabric, and the texture is soft; the adopted Chinese herbal ingredients are the pure nature plant extract essence and do not have side effects; and the Chinese herb technology and the existing wet tissue technology are combined to obtain the technical scheme of the present invention.
Owner:高峰
Antihidrotic deodorization antiperspirant for human bodies and method for preparing antihidrotic deodorization antiperspirant
InactiveCN105963205AAdd lessImprove immunityCosmetic preparationsToilet preparationsAntiperspirantsAlcohol
The invention aims to provide antihidrotic deodorization antiperspirant for human bodies and a method for preparing the antihidrotic deodorization antiperspirant. The antihidrotic deodorization antiperspirant comprises, by weight, 0.3-0.7 part of Virginia witch hazel extract, 0.1-0.3 part of golden chamomile extract, 0.1-0.3 part of aloe vera powder, 0.2-0.4 part of essence, 12-17 parts of aluminum chlorohydrate, 0.05-0.15 part of bisabolol, 17-23 parts of ethyl alcohol, 2.5-4 parts of propylene glycol, 1-2 parts of solubilizers and 50-70 parts of water. The antihidrotic deodorization antiperspirant and the method have the advantages that the antihidrotic deodorization antiperspirant contains diversified safe and effective plant components, the additive amounts of chemical substances can be reduced, accordingly, hazards to the health of the human bodies due to the chemical substances can be prevented to a certain extent, and the method for preparing the antihidrotic deodorization antiperspirant is simple and is easy to implement.
Owner:郑州雷曼药业有限公司
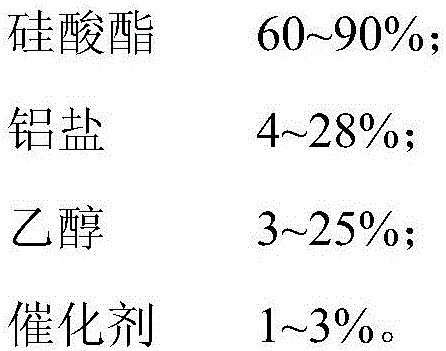
Liquid hardening agent with high wear resistance and preparation method thereof
The invention relates to a liquid hardening agent with high wear resistance and a preparation method thereof. In terms of 100% of the mass of the hardening agent, the liquid hardening agent is prepared from the following raw materials: 50-90% of silicate ester, 1-30% of aluminum salts, 1-30% of alcohol and 1-5% of a catalyst, wherein the silicate ester is selected from one or a combination of more of methyl silicate, ethyl silicate, tri(methyl silicate), and preformed polymer of methyl silicate and ethyl silicate; the aluminum salt is selected from one or a combination of more of aluminum chloride, aluminum nitrate, aluminum chlorohydrate, polymer aluminum nitrate, aluminum formate, aluminum acetate, aluminum methoxide, aluminum ethylate and aluminum isopropoxide. According to the liquid hardening agent, silicate ester and the aluminum salt are adopted as the main ingredients, so that the content of the active ingredients is higher than that of a common water-based product, and the permeability is extremely high, and therefore, the hardness and wear resistance of cement can be remarkably improved. Meanwhile, the preparation method is simple and is beneficial to reduction in the industrial production cost.
Owner:HANGZHOU GELING NEW MATERIAL TECH
Special powder paint for outdoor metal guardrail
InactiveCN106752192AStrong adhesionImprove the safety of useAnti-corrosive paintsPowdery paintsPhosphateALUMINUM CHLOROHYDRATE
The invention discloses special powder paint for an outdoor metal guardrail and relates to the technical field of processing of metal paint. The special powder paint is prepared from the following raw materials in parts by weight: 15 to 20 parts of thermosetting acrylic resin, 10 to 15 parts of aromatic hydrocarbon modified terpene resin, 3 to 6 parts of bismaleimide resin, 2 to 4 parts of C5 hydrogenation petroleum resin, 2 to 4 parts of pentaerythritol ester of hydrogenated rosin, 1 to 2 parts of waste tire rubber powder, 1 to 2 parts of asbestos powder, 1 to 2 parts of sepiolite fiber, 0.5 to 1 part of poly-dimethyl-diallyl-ammonium chloride, 0.5 to 1 part of N-methylol acrylamide, 0.5 to 1 part of aluminum chlorohydrate, 0.3 to 0.5 part of tri(2-chloroethyl) phosphate, 0.3 to 0.5 part of dilauryl thiodipropionate, 0.1 to 0.3 part of tribasic lead sulfate and 0.1 to 0.3 part of triphenyl phosphite. A coating formed by the powder paint has excellent adhesion property, wearing resistance and weather resistance and can be firmly attached on the surface of the metal guardrail; a rusting speed is slow and powdering and falling phenomena of the coating are avoided.
Owner:ANHUI ZHENXIN PAINT
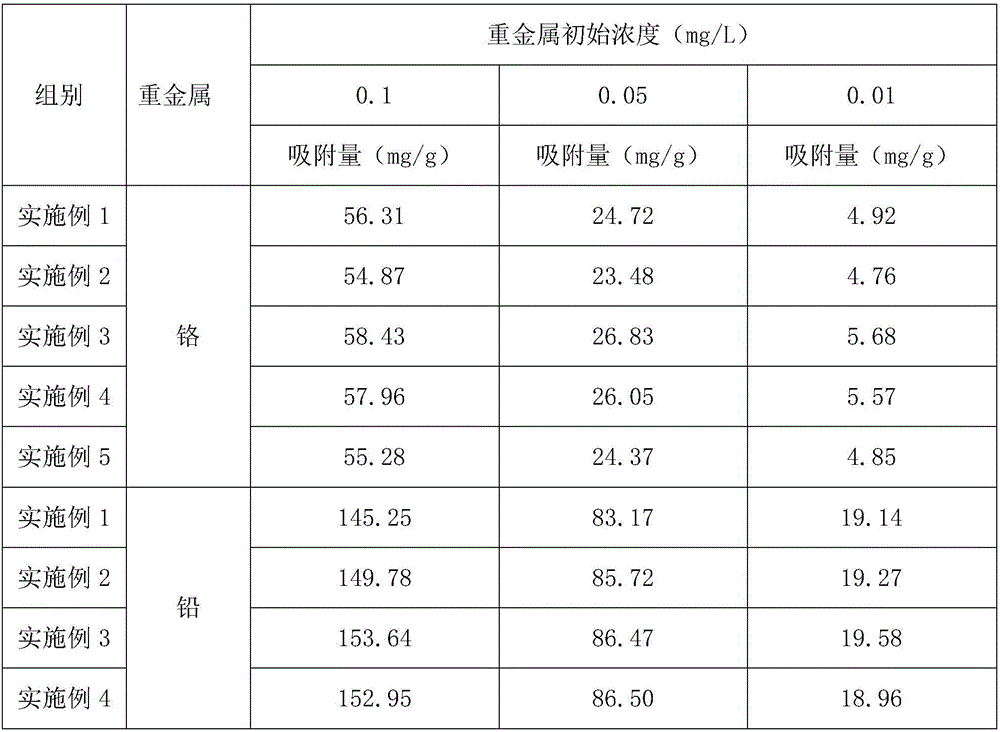
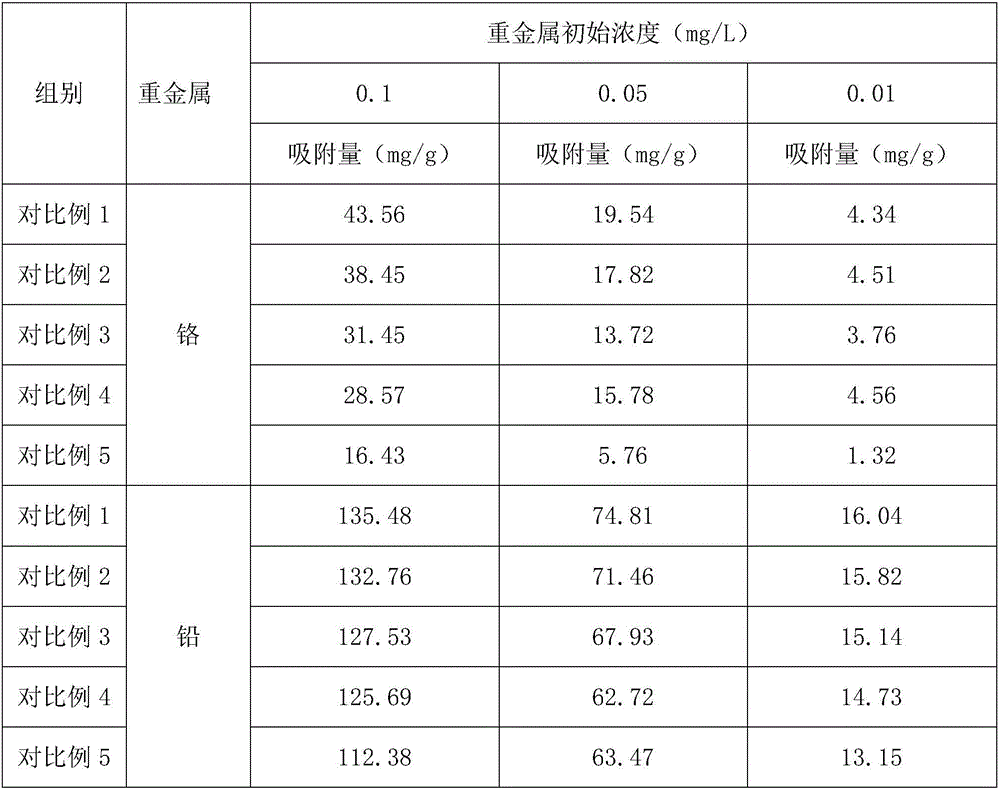
Composite heavy metal adsorption material based on volcanic ash and preparation method of same
InactiveCN106552594AImprove adsorption capacitySimple ingredientsOther chemical processesWater contaminantsAmmonium bromideAdsorption effect
The invention discloses a composite heavy metal adsorption material based on volcanic ash, which is prepared from, by weight, 32-37 parts of volcanic ash, 8-12 parts of chitosan, 5-8 parts of cetyl ammonium bromide, 3-6 parts of aluminum chlorohydrate, 2-5 parts of urea, and 1-3 parts of 11-carbonyl-[beta]-masticinic acid. The invention also discloses a preparation method of such composite heavy metal adsorption material based on volcanic ash. With the volcanic ash as a main raw material, the composite heavy metal adsorption material has excellent adsorption effect on heavy metals, and the adsorption performance is better than that of modified volcanic ash in the prior art. The material has important market value and social value.
Owner:ZHENGZHOU YUANRAN BIOLOGY TECH CO LTD
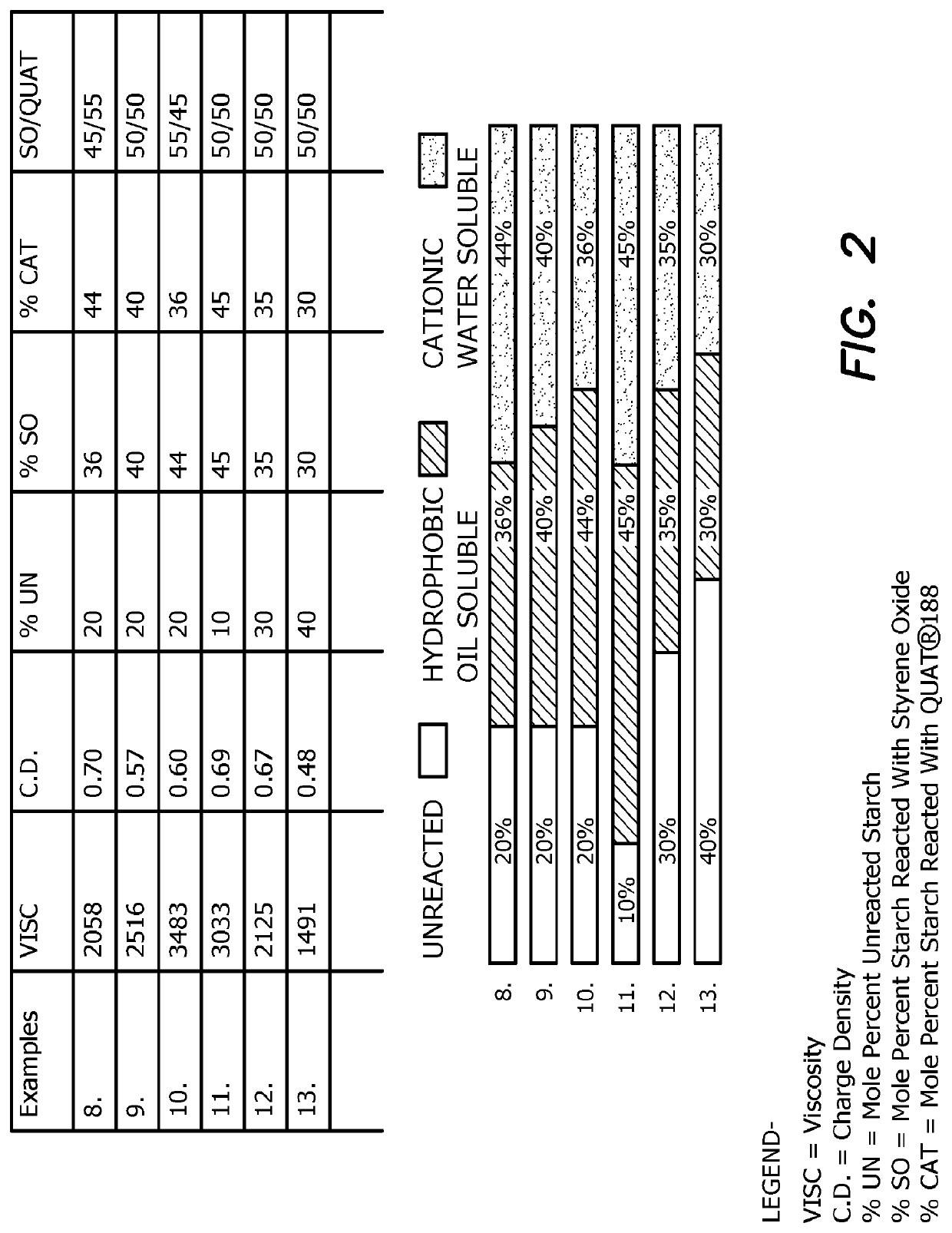
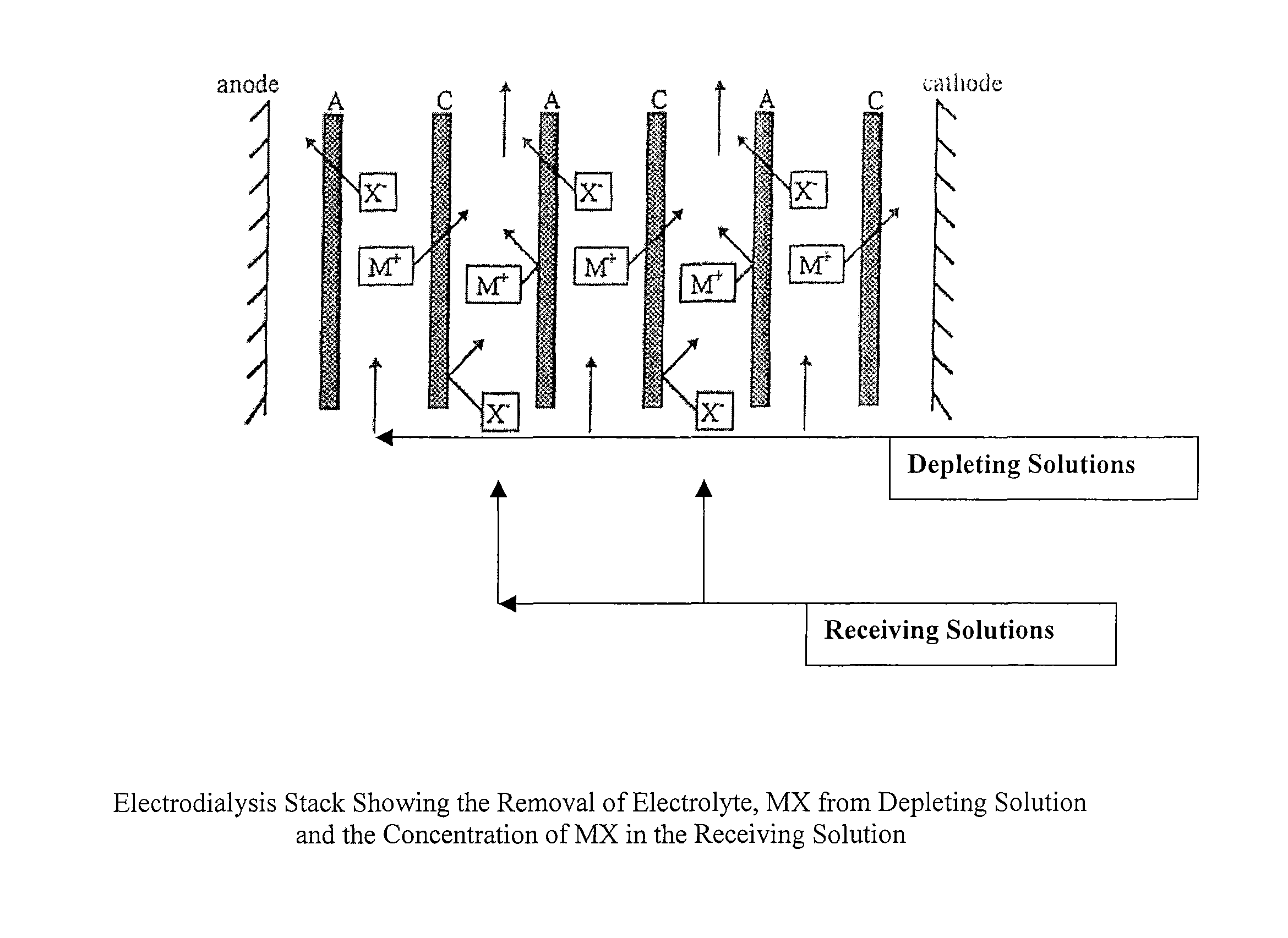
Water-Enriching and Water Depleting Compositions and Methods
ActiveUS20190374877A1Pollution minimizationIncrease the amount of waterDewatering/demulsification with chemical meansNon-miscible liquid separationSolubilityPoly(diallyldimethylammonium chloride)
Compositions are disclosed for dewatering mixtures of petroleum and water. The compositions comprise one or more of the following: an unreacted polysaccharide component; and one or both of a polysaccharide component reacted with a hydrophilic component and a polysaccharide component reacted with a hydrophobic component. The compositions may also include viscosifying agents or stabilizers to stabilize the compositions against separation, for example, prior to use. In particularly preferred embodiments the invention is drawn to compositions for breaking an emulsion; such compositions comprising a carbohydrate component containing a cationic starch joined to a hydrophobic moiety, providing the carbohydrate component oil solubility. The composition may optionally comprise one or more additional demulsifier selected from, without limitation, salts (such as a polyaluminum chloride, an aluminum chlorohydrate, an alum, etc.), metal salts (such as iron and zinc salts), dithiocarbamate, tannin, and organic demulsifiers such as poly-DADMAC and similar compounds.
Owner:DOBER CHEMICAL CORPORATION
Body-flavoring liquid capable of removing bromhidrosis and application method of body-flavoring liquid
ActiveCN104856907AReduce odorBody odor eliminationCosmetic preparationsToilet preparationsGlycyrrhiza glabra RootBody fluid
The invention discloses a body-flavoring liquid capable of removing bromhidrosis and an application method of the body-flavoring liquid. The body-flavoring liquid is prepared from the following raw materials in parts by weight: 77-85 parts of water, 5-10 parts of aluminum chlorohydrate, 6-7 parts of propylene glycol, 4-5 parts of butanediol, 0.2-0.3 part of bis(hydroxymethyl) imidazolidinyl urea, 5*10<-3> to 8*10<-3> parts of iodopropynol butylcarbamate, 1*10<-3> to 9*10<-3> parts of skullcap root extract liquid and 3*10<-3> to 1*10<-2> parts of a glycyrrhiza glabra root extract; the application method comprises the following steps: (1) cleaning sweating skin; (2) painting the body-flavoring liquid under arms or on other excessively sweating parts. The body-flavoring liquid is capable of rapidly relieving odor of the bromhidrosis, and eliminating the bromhidrosis after being used for a plurality of times for a certain period of time, and has the characteristics of low sensitization and significant effect.
Owner:广州薇意丰华生物科技有限公司
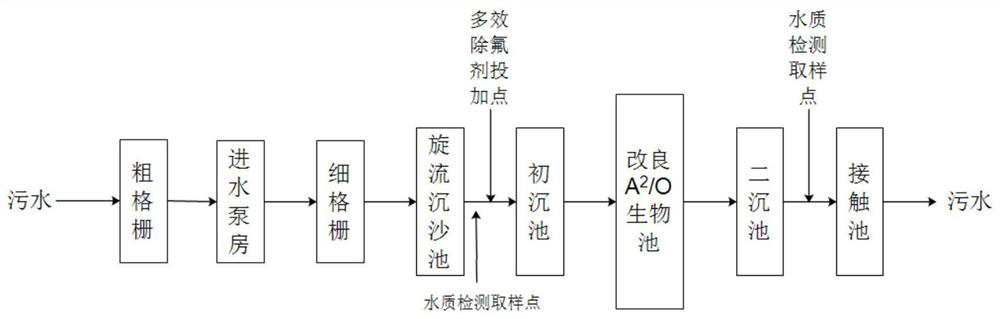
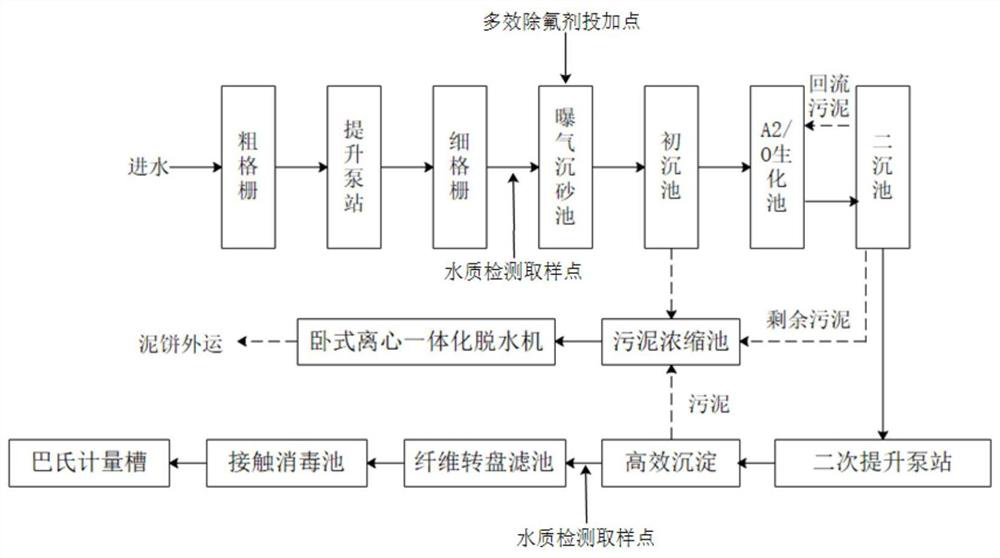
Multi-effect defluorination agent as well as preparation method and application thereof
ActiveCN113213607AGood defluoridation effectGood removal effectWater contaminantsWater/sewage treatment by flocculation/precipitationWater chlorinationTreated water
The invention relates to the technical field of fluorine-containing wastewater treatment, in particular to a multi-effect defluorination agent as well as a preparation method and application thereof. The invention relates to a multi-effect defluorination agent which comprises the following components in parts by weight: 3-10 parts of polymeric aluminum salt (in terms of Al2O3), 8-15 parts of polymeric aluminum ferric salt (in terms of Al2O3), 2-10 parts of an oxidizing agent, 3-6 parts of acetate, 0.5-1.5 parts of poly dimethyl diallyl ammonium chloride, 1-3 parts of iminodiacetic acid and 2-3 parts of aluminum chlorohydrate. According to the defluorination agent, the concentration of fluorine ions in treated water can be smaller than or equal to 1.0 mg, the requirement of surface water class III specified by GB3838-2002 Environmental Quality Standard for Surface Water can be met, thedefluorination agent has the effects of removing fluorine, nitrogen, phosphorus and COD, the removal effect is stable, the high removal rate can be achieved under the condition of the low dosage, the dosage is small, the amount of sludge generated in the treatment process is small, the sludge is easy to treat, and the comprehensive cost is low.
Owner:CSD BEIJING E P DEV CO LTD
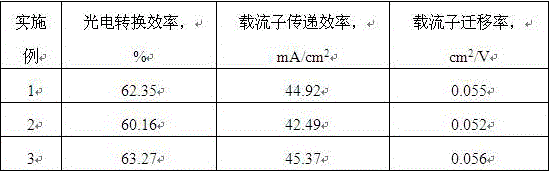
Novel wide spectrum solar cell material
InactiveCN106299140AGood electron stacking propertiesImprove photoelectric conversion efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSodium BentoniteCharge carrier mobility
The invention discloses a novel wide spectrum solar cell material. The material includes the following raw matrials: a [Beta]-chloromethyl thiophene, a 2-amino-5-sulfydryl-1, 3, 4-thiadiazole, a trimethylolpropane, an epoxy-silane coupling agent, a maleic anhydride graftingcompatilizer, an acrylic bridging agent, a platinum catalyst, a graphene, a carbon black, a silicon carbide, an aluminium nitride, a silicon nitride, an aluminium oxide, a silicon dioxide, a bentonite, a carbon fiber, a glass fiber, a methylcellulose, a dibasic lead phosphate, an acrylics conditioning agent, an epoxypropoxypropyl-trimethoxysilane, a 701 powder, an aluminum chlorohydrate, an ethylene propylene rubber, a bis(2,2,6,6-tetramethyl-4-piperidyl) sebacate, a dibasic lead stearate, a 2-Hydroxy-4-methoxybenzophenone, and an ammonium polyphosphate. According to the invention, the material can effectively use the energy of wavelengths that include ultraviolet rays and infrared rays in the sunlight, and increases photovoltaic conversion efficiency, current carrier transmission efficiency and current carrier migration efficiency.
Owner:广西南宁荣威德新能源科技有限公司
Preparation process of toning cream with functions of hidroschesis and deodorization
InactiveCN105662953AAntiperspirant fastLong lastingCosmetic preparationsToilet preparationsDicaprylyl carbonateSweat gland
The invention discloses a preparation process of an antiperspirant and deodorant toning cream. The mixture comprises the following components in proportions: water: 25-40 parts, butane: 2-5 parts, butanediol: 5-10 parts, citric acid: 5-10 parts, aluminum chloride hydroxy: 10-15 parts, dioctyl carbonate: 2-5 parts, licorice hydrosol: 13-28 parts, stearyl alcohol: 5-8 parts , sunflower seed oil: 8-15 parts, menthol: 3-8 parts, dipotassium glycyrrhizinate: 5-8 parts, farnesol: 2-6 parts, distearyl ether: 3-5 parts, only Perspiration agent: 13-28 parts, fragrance agent: 13-18 parts, fungicide: 15-20 parts, black inhibitor: 10-18 parts, vegetable fruit oil: 15-18 parts. The invention can stop perspiration quickly, last for a long time, penetrate deeply, regulate the source of sweat gland secretion, purify odor molecules, solve embarrassing odor, keep fresh and odor-free, safe and mild, colorless paste, light floral fragrance, and will not cause discoloration of skin and clothes .
Owner:陈威宇
Antiperspirant composition friendly to skin and its preparation method
InactiveCN1494896AReduce stimulationNot tenseCosmetic preparationsToilet preparationsAlcoholChloride
An improved skin friendly antiperspirant is provided in which a suitable polyhydric alcohol e.g., glycerin, is complexed with the antiperspirant of the type of activated or nonactivated aluminum or aluminum / zirconium salt that are commonly considered antiperspirant active materials and are covered by FDA OTC Tentative Final Monograph as Category I. Suitable antiperspirant salts include (but are not limited to): aluminum chlorohydrate, aluminum sesquichlorohydrate, aluminum chlorohydrex PG, aluminum dichlorohydrex PG, aluminum sesquichlorohydrex PG, aluminum chlorohydrex PEG, aluminum sesquichlorohydrex PEG, aluminum chloride (15 percent or less aqueous solutions), aluminum zirconium chlorohydrates, aluminum zirconium trichlorohydrate, aluminum zirconium tetrachlorohydrate, aluminum zirconium pentachlorohydrate, aluminum zirconium octachlorohydrate, aluminum zirconium trichlorohydrex Gly, aluminum zirconium penta chlorohydrex Gly, aluminum zirconium tetrachlorohydrex Gly, aluminum zirconium octachlorohydrex Gly, buffered aluminum sulfate, basic aluminum chlorides, zirconium hydroxychloride, zirconyl chloride, basic aluminum nitrates, basic aluminum chlorides combined with zirconyl oxychloride and / or hydroxychloride and organic complexes of each of basic aluminum chlorides with or without zirconyl chloride or zirconium hydroxychloride and mixture of any of the foregoing. Aluminum, zinc or zinc and zirconium complexes or aluminum, zinc and zirconium complexes with having metals / anion ratio of 0.9:1 to 2:1 where the anion is Cl, Br, I and / or NO, with or without additives such as amino acids or polyhydric alcohols.
Owner:RIIHAISU INC
Polymetal hydroxychloride processes and compositions: enhanced efficacy antiperspirant salt compositions
InactiveUS8801909B2Materials and reduced eliminatedSteps reduced eliminatedCosmetic preparationsElectrolysis componentsZirconium compoundsMetal
The invention describes processes for the production of basic aluminum compounds, including aluminum chlorohydrate, basic zirconium compounds, and basic aluminum zirconium compounds. The process produces products of a wide range of basicities. The products formed by the present invention are comprised of low molecular weight species characteristic of enhanced efficacy antiperspirant salt compositions. The products of this process are suitable for use as water purification agents, as binders in catalyst applications, and in antiperspirant applications. In addition, the invention is directed to the products made by the disclosed process.
Owner:NEXT CHEM LLC
Heavy metal adsorbing material prepared from walnut shell and preparation method thereof
InactiveCN106732424AImprove adsorption capacityLow costOther chemical processesWater/sewage treatment by sorptionGuggulsteroneAdsorption effect
The invention discloses a heavy metal adsorbing material prepared from a walnut shell. The heavy metal adsorbing material is prepared from, by weight, 21-25 parts of walnut shell, 12-16 parts of kaolin, 4-7 parts of lignosulfonate, 2-5 parts of aluminum chlorohydrate, 3-6 parts of glutathione and 2-5 parts of guggulsterone. The invention further discloses the preparation method of the heavy metal adsorbing material prepared by the walnut shell. The prepared heavy metal adsorbing material has good adsorption effect on heavy metal lead ions and heavy metal cadmium ions, the adsorption performance is excellent, and the heavy metal adsorbing material and the preparation method can be applied to the field of heavy metal treatment.
Owner:ZHENGZHOU BEIDOU COMM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com