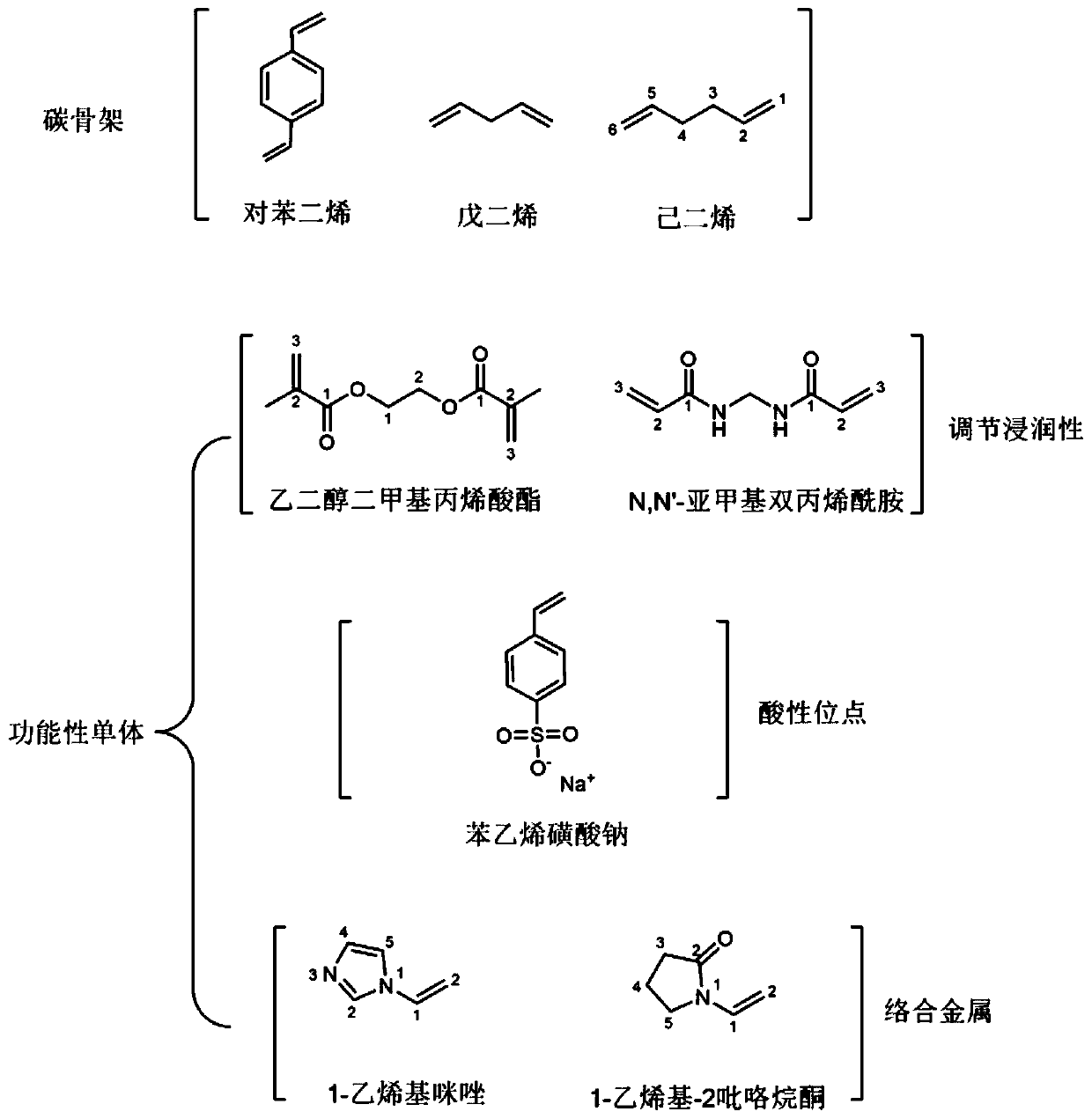
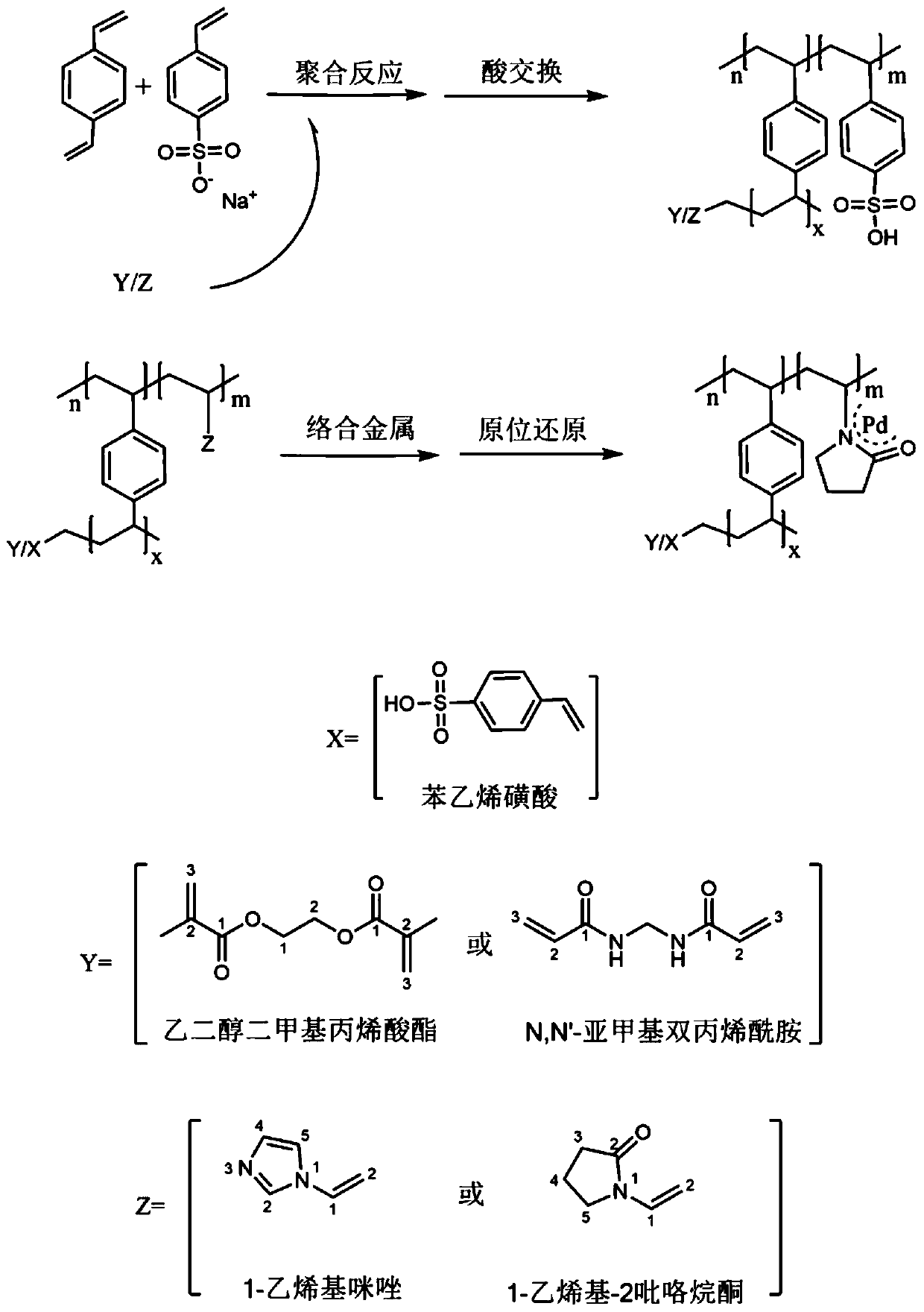
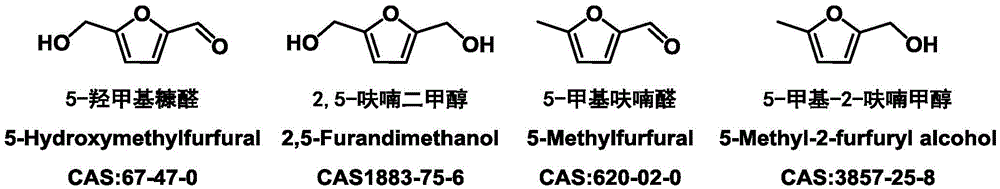
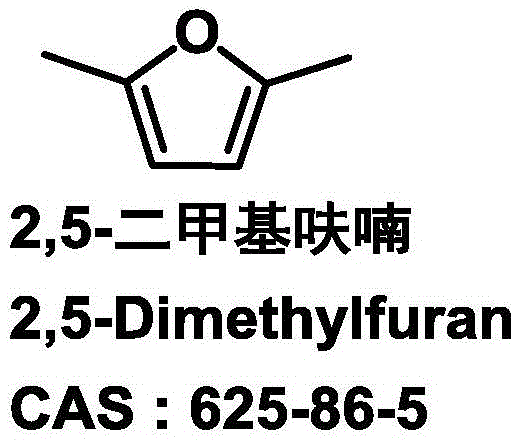
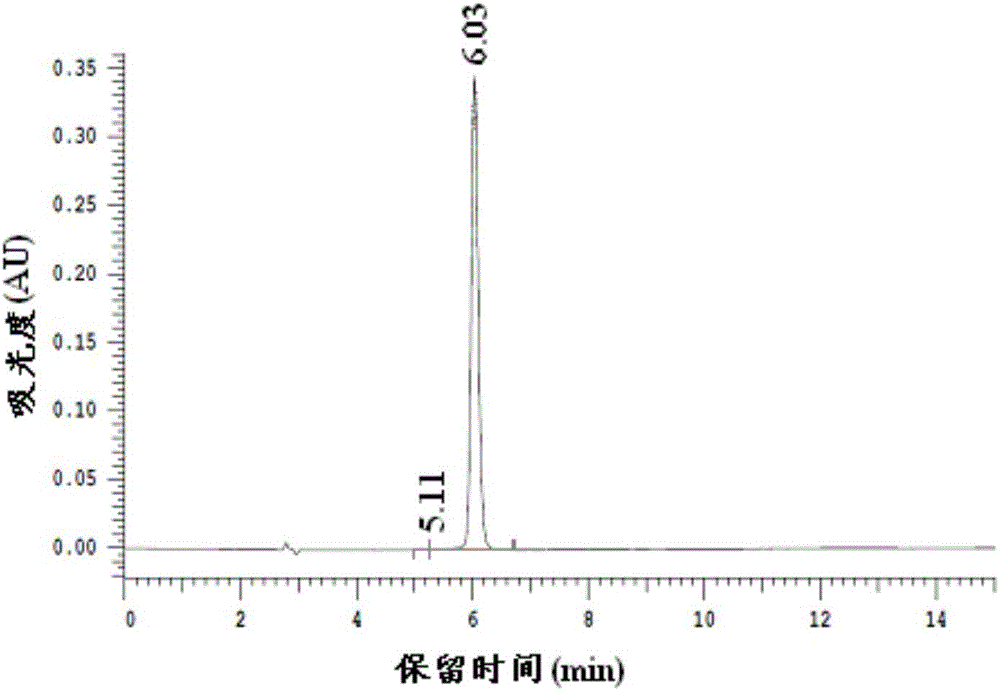
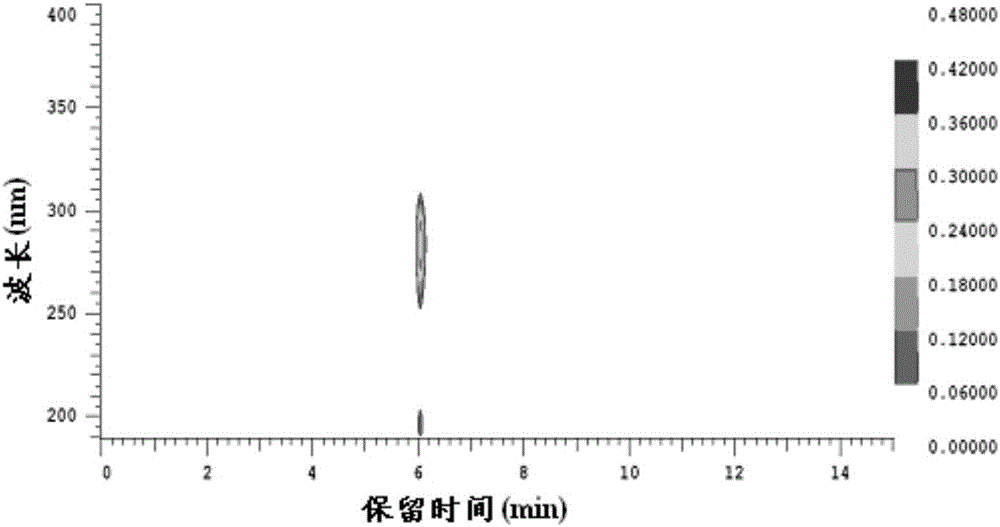
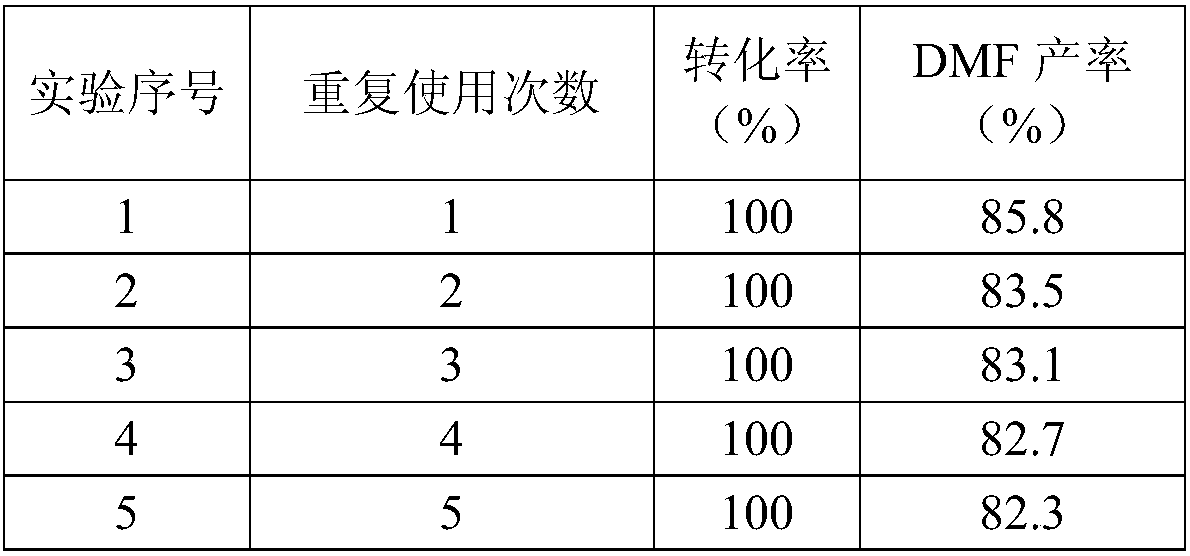
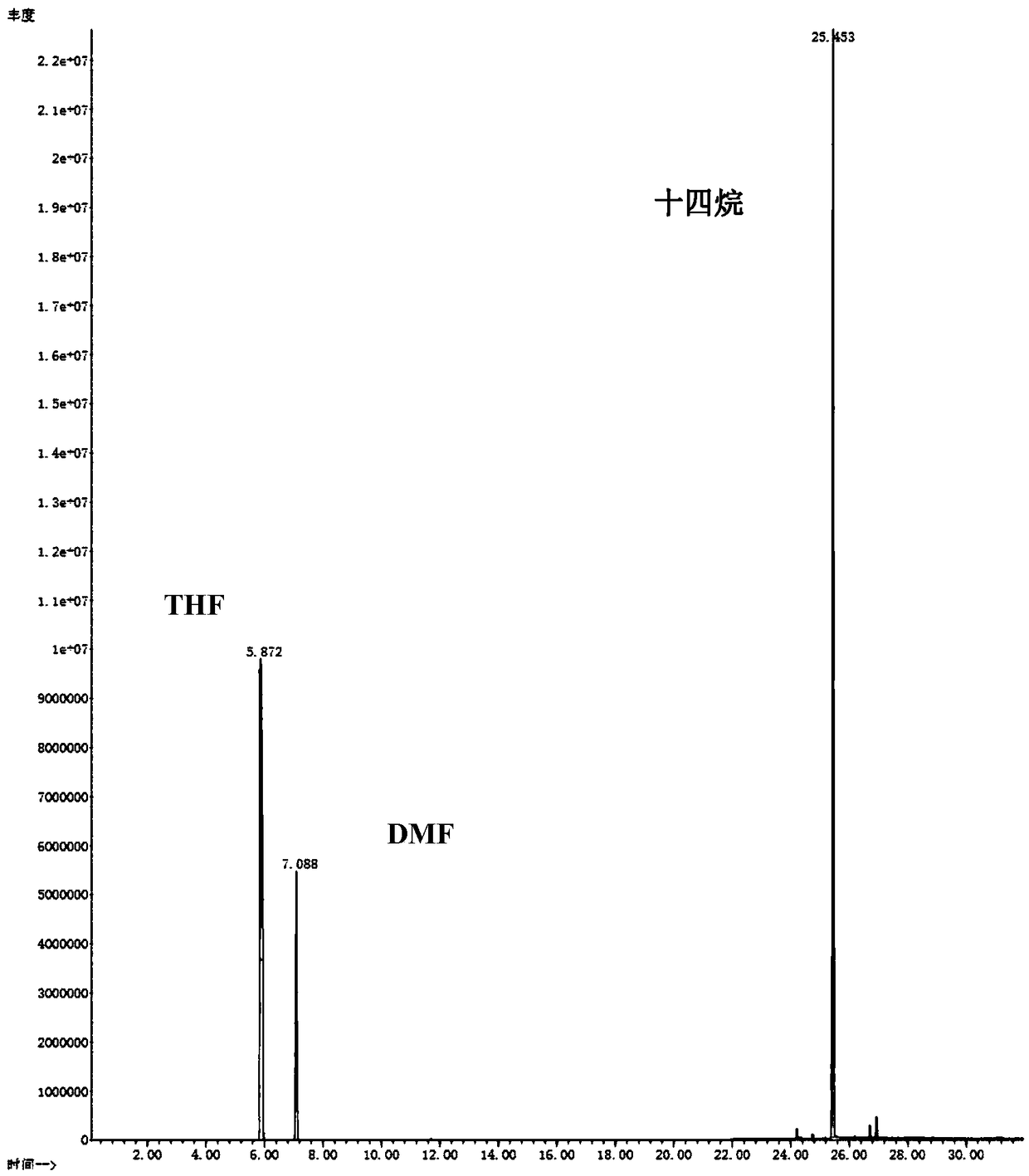
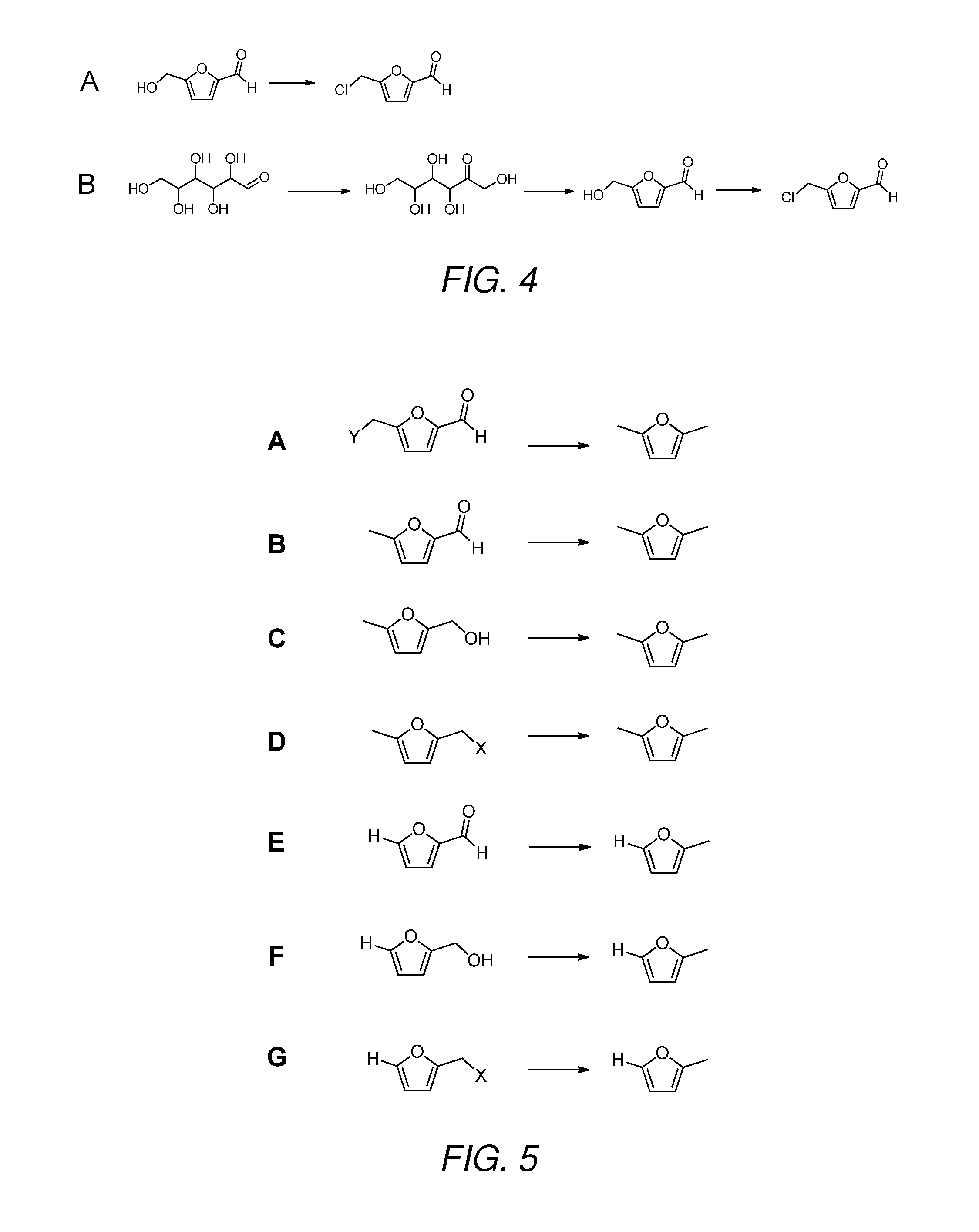Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "2,5-Dimethylfuran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
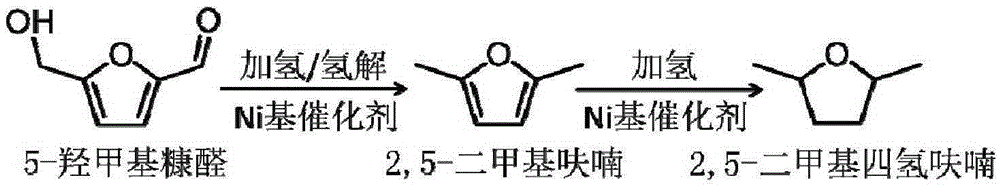
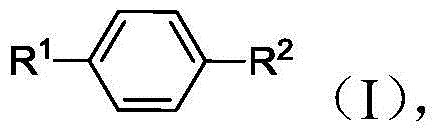
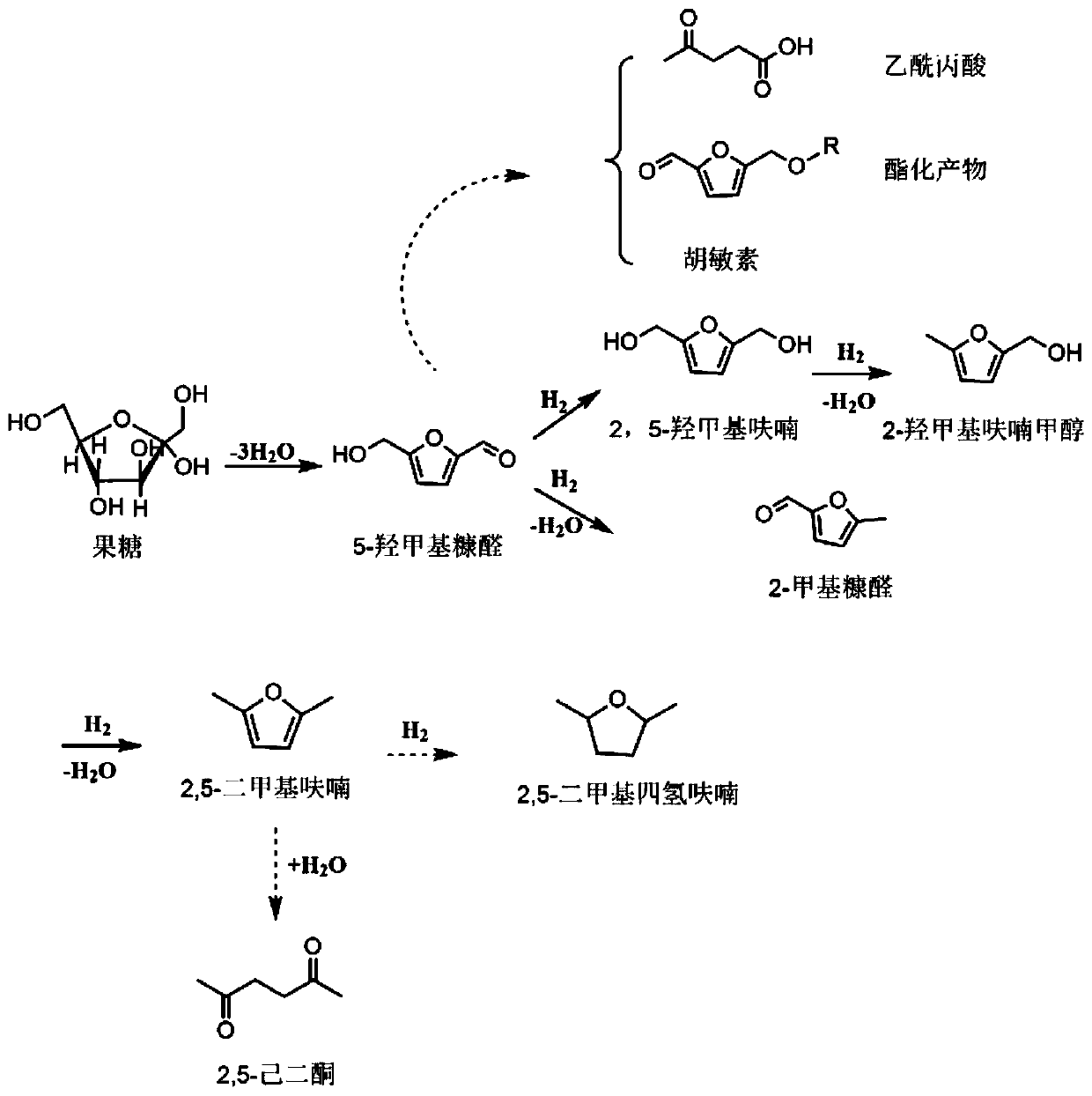
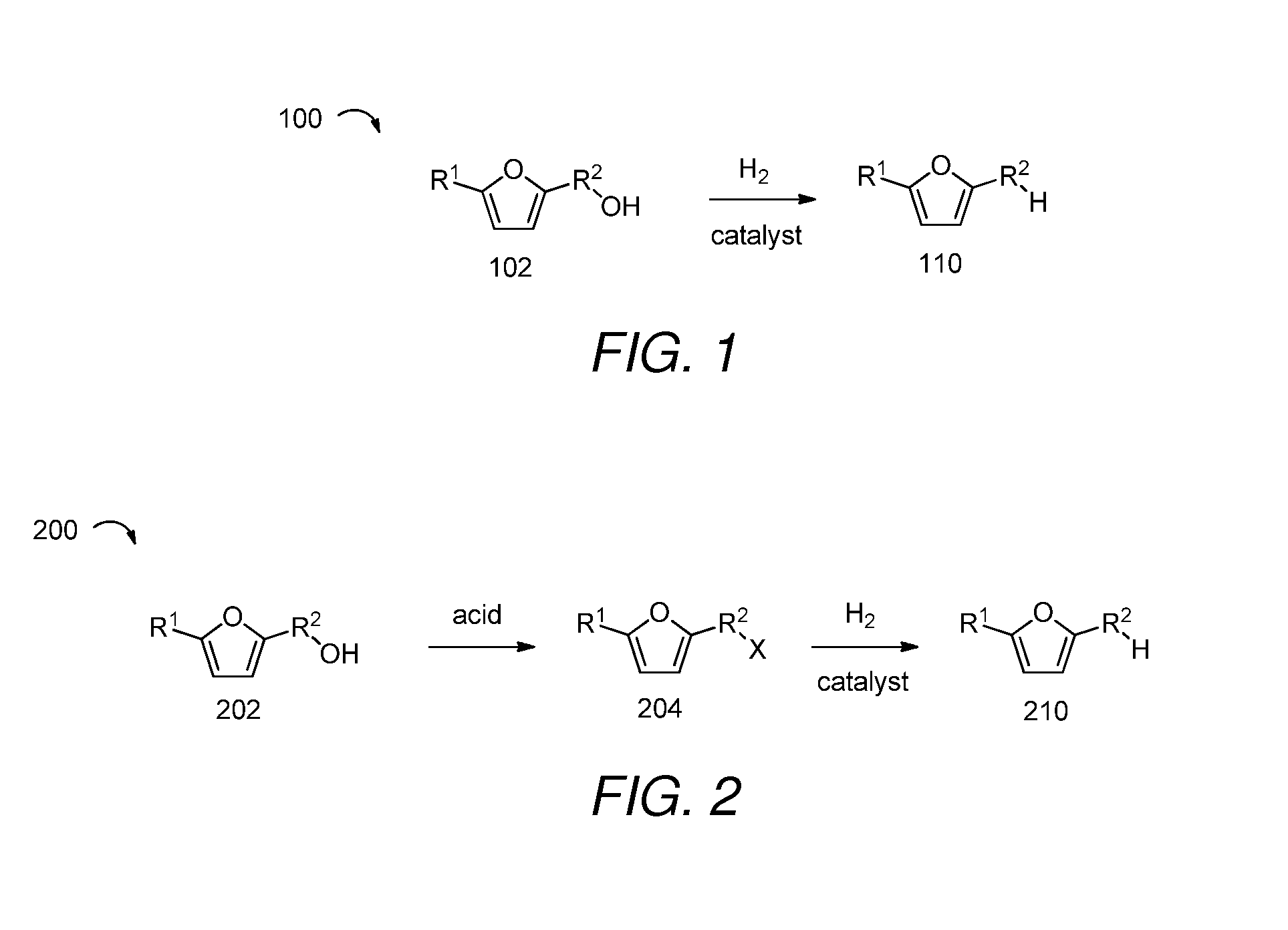
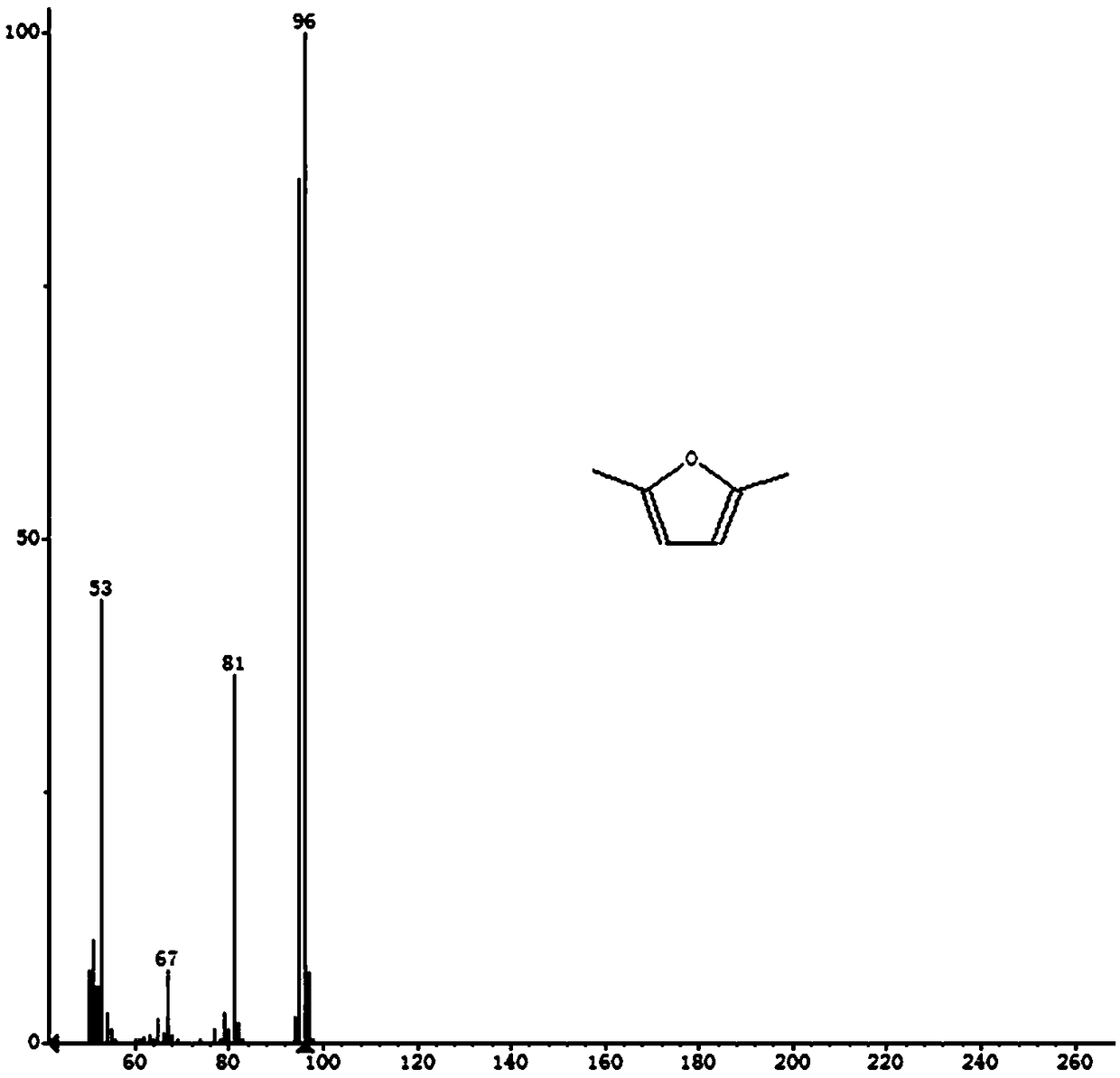
2,5-Dimethylfuran is a heterocyclic compound with the formula (CH₃)₂C₄H₂O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.
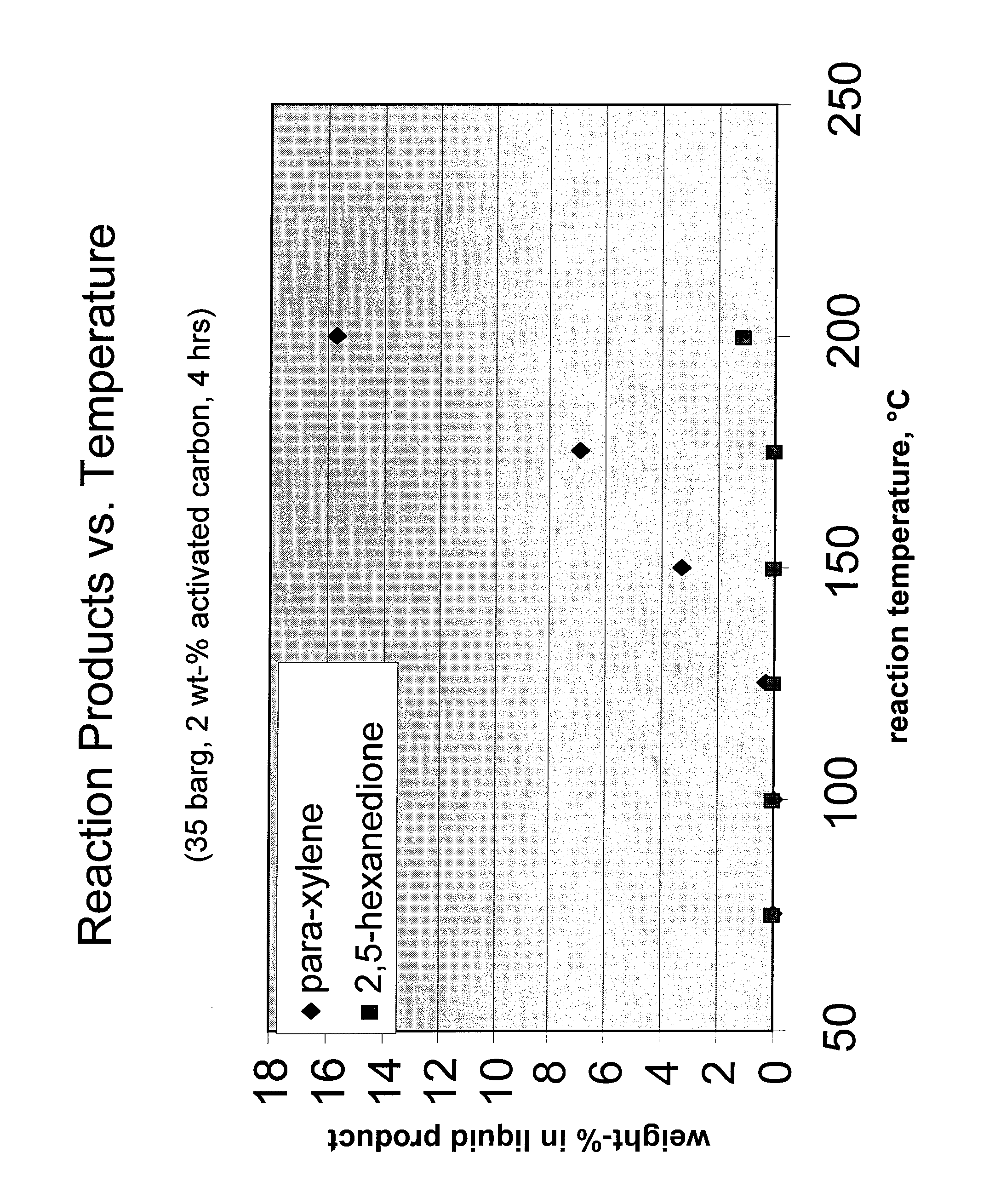
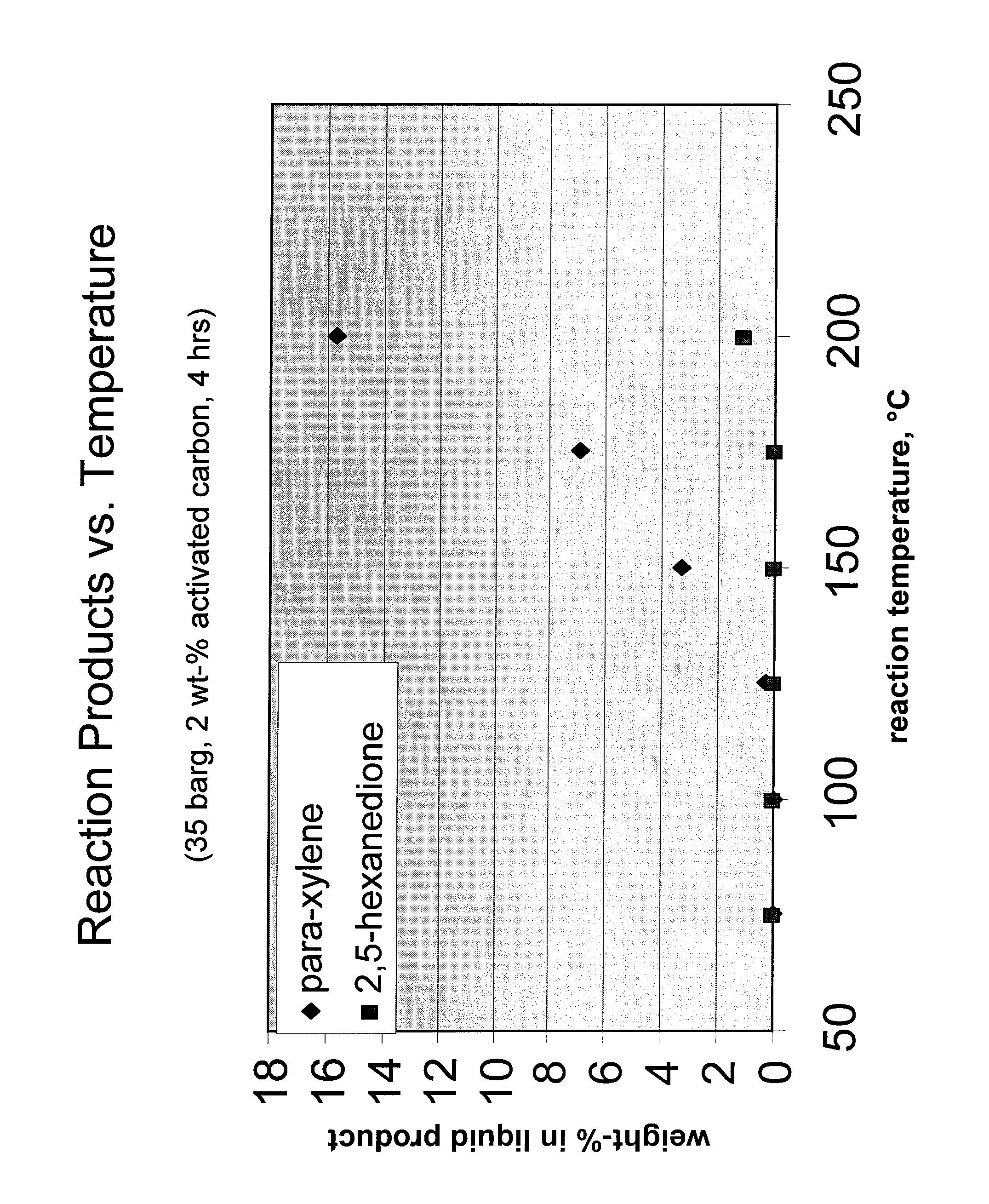
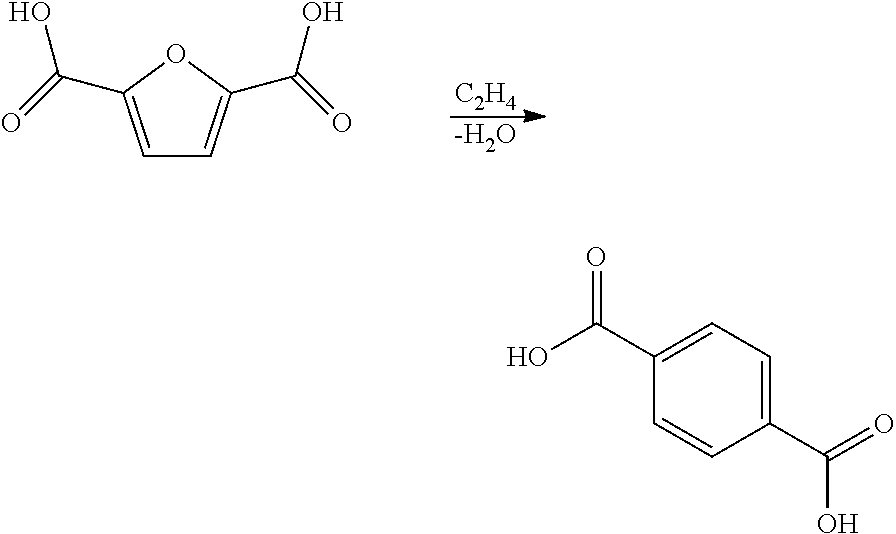
Carbohydrate route to para-xylene and terephthalic acid
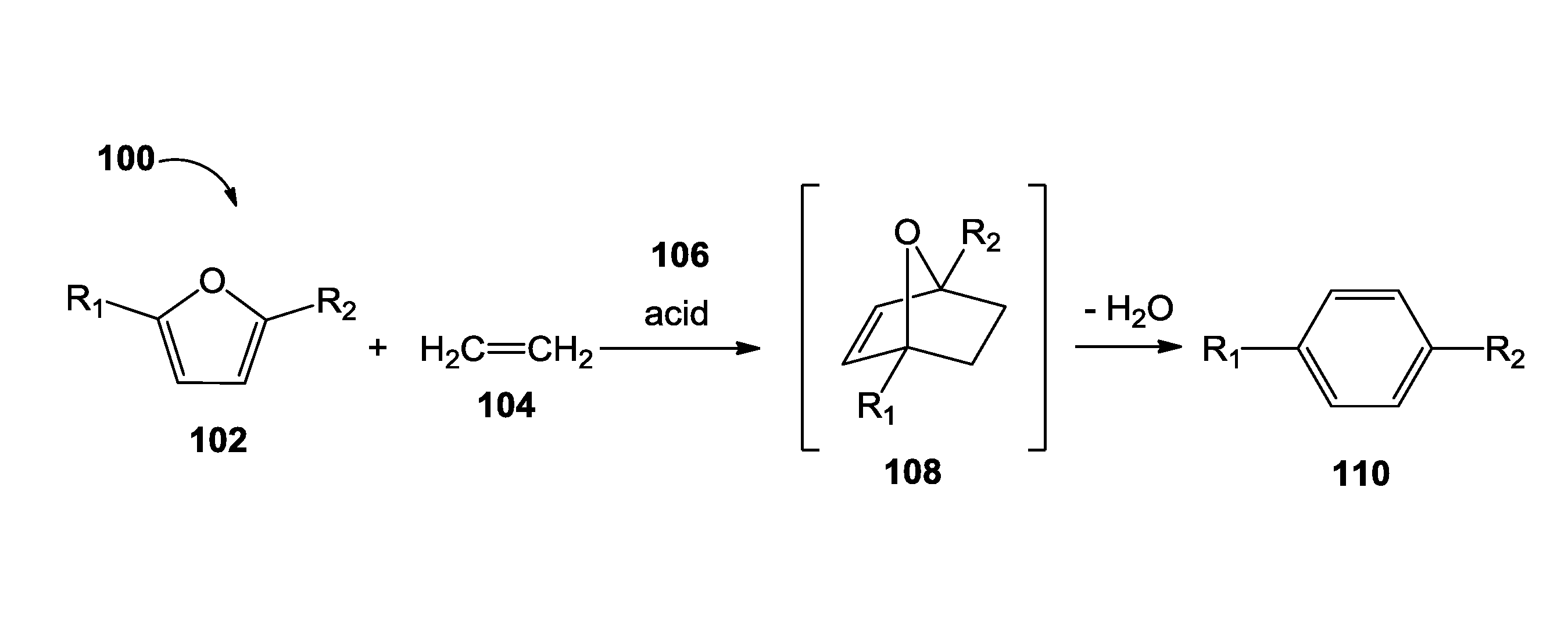
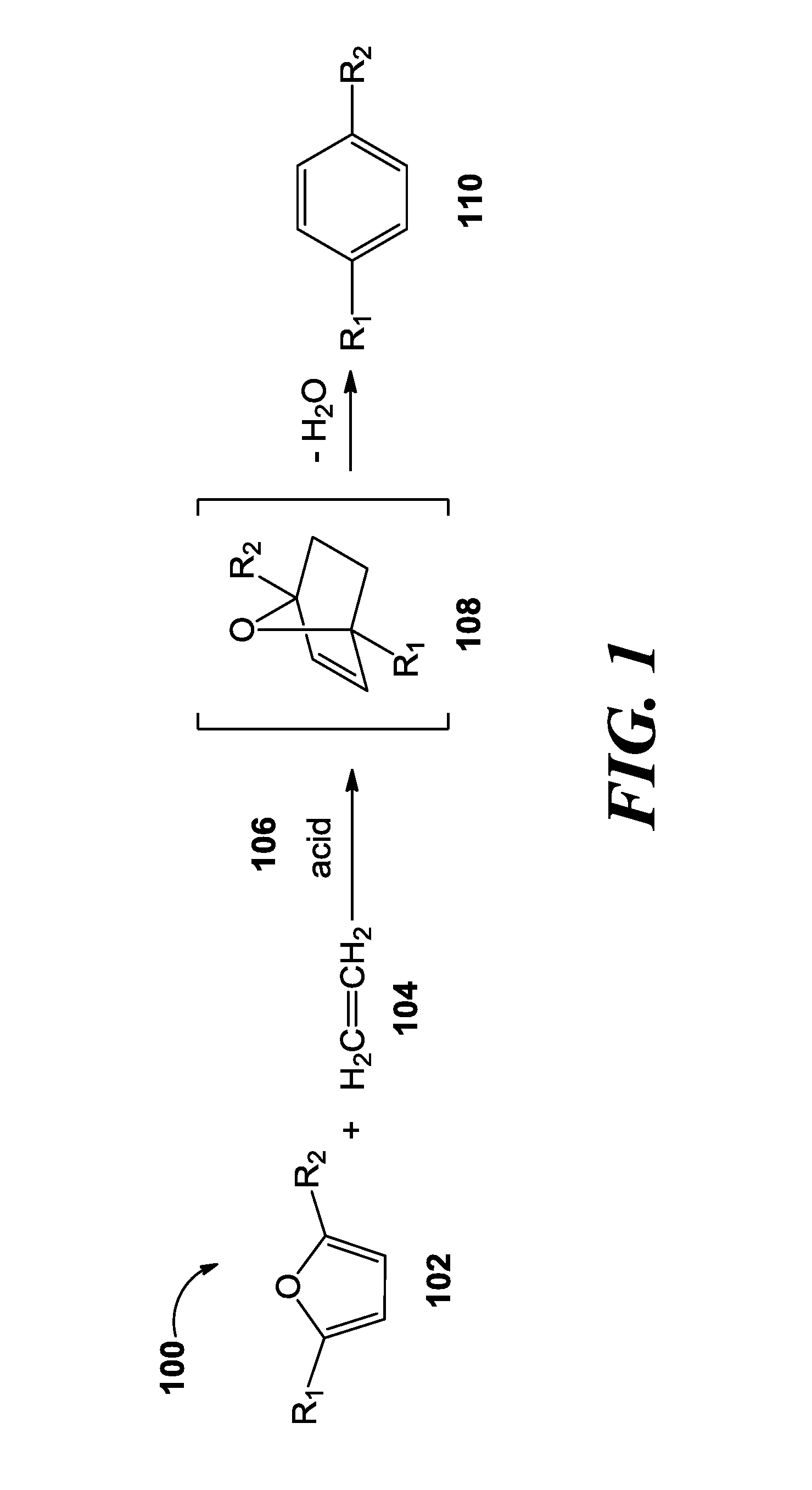
Catalytic processes for the conversion of 2,5-dimethyl furan (DMF) to para-xylene are described. Para-xylene is a key product that is currently obtained commercially from petroleum sources. However, it has now been determined that the cycloaddition of ethylene to DMF provides an alternative route to para-xylene. Advantageously, the DMF starting material for the processes may be synthesized from carbohydrates (e.g., glucose or fructose), thereby providing a pathway that relies at least partly, if not completely, on renewable feedstocks.
Owner:UOP LLC
Carbohydrate route to para-xylene and terephthalic acid
ActiveUS8314267B2Organic compound preparationHydrocarbon by hydrocarbon and non-hydrocarbon condensationFuranCycloaddition
Catalytic processes for the conversion of 2,5-dimethyl furan (DMF) to para-xylene are described. Para-xylene is a key product that is currently obtained commercially from petroleum sources. However, it has now been determined that the cycloaddition of ethylene to DMF provides an alternative route to para-xylene. Advantageously, the DMF starting material for the processes may be synthesized from carbohydrates (e.g., glucose or fructose), thereby providing a pathway that relies at least partly, if not completely, on renewable feedstocks.
Owner:UOP LLC
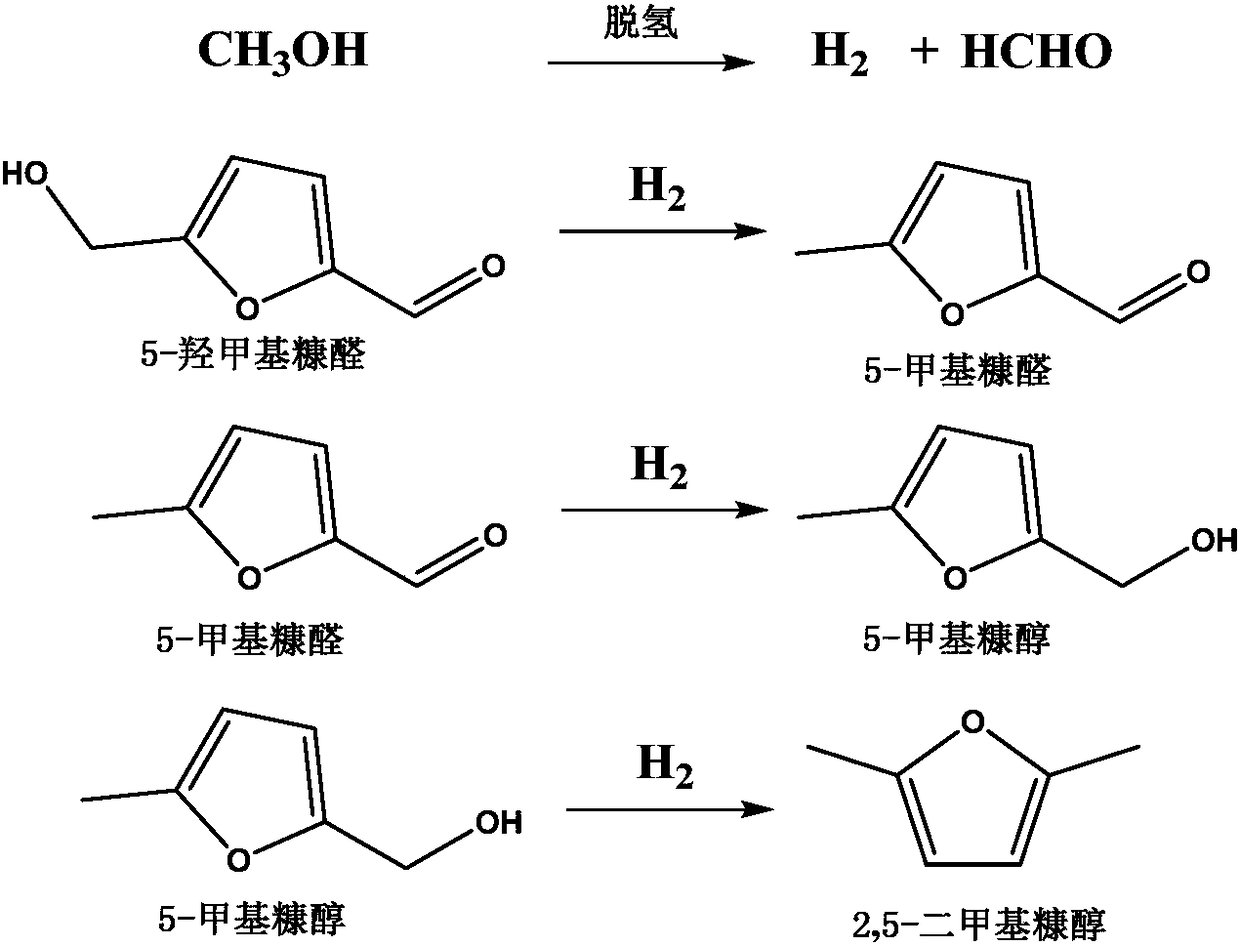
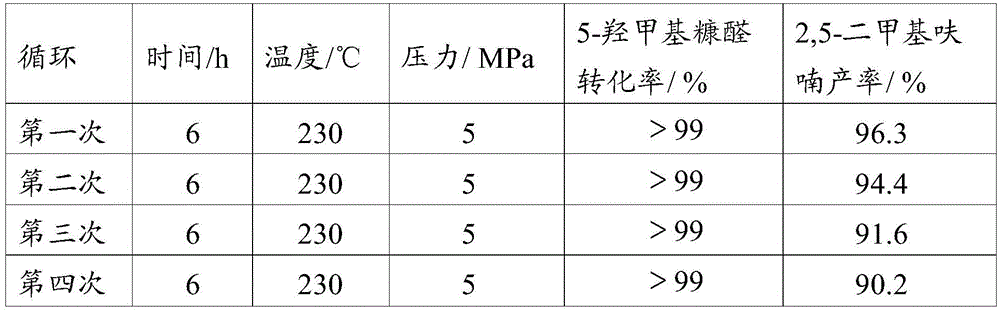
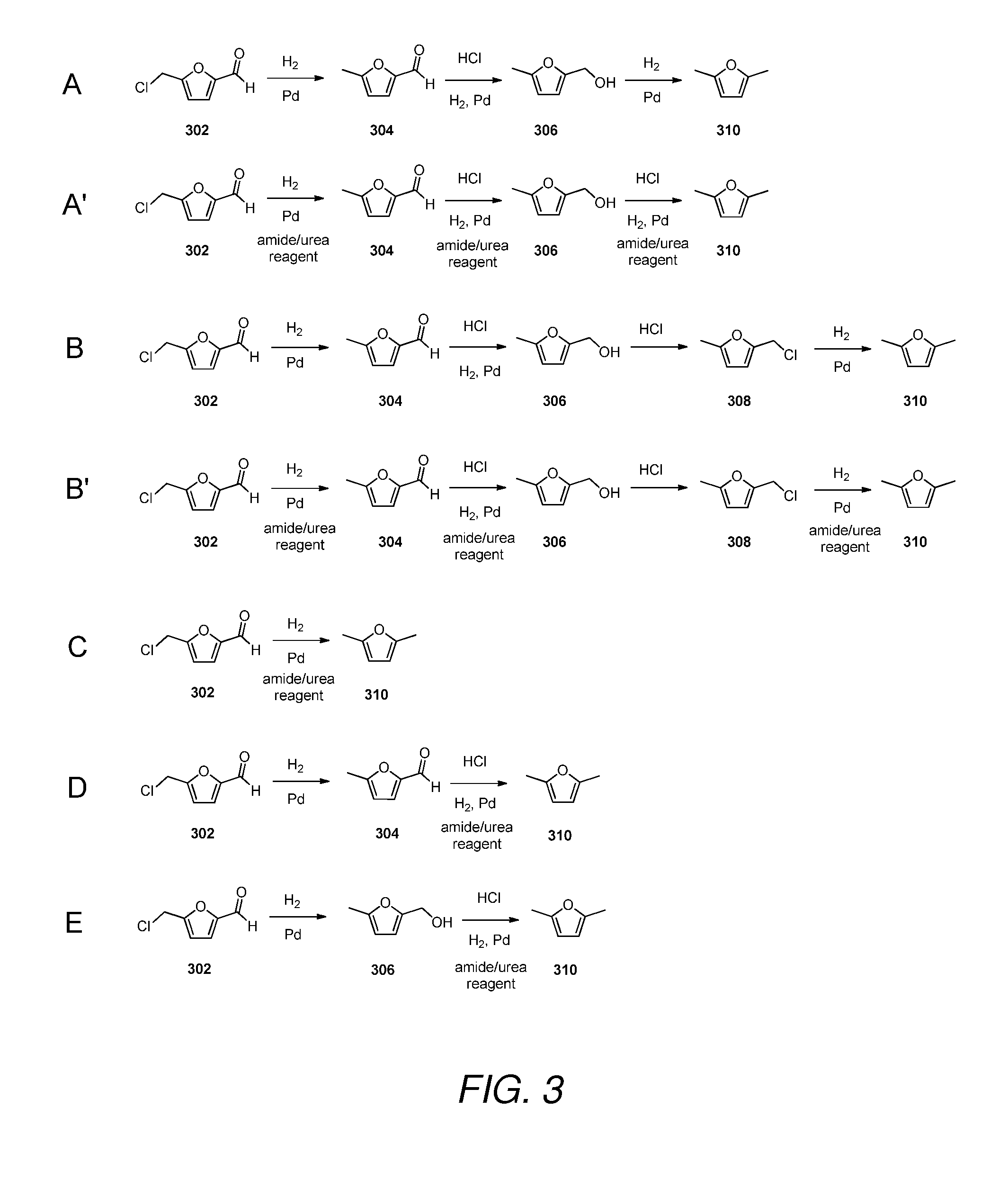
Catalyst for preparing 2,5-dimethylfuran through selective hydrogenolysis of 5-hydroxymethylfurfural and preparation method of catalyst
ActiveCN105251491ALow costPlay a protective roleOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCarbon layerHydroxymethylfurfural
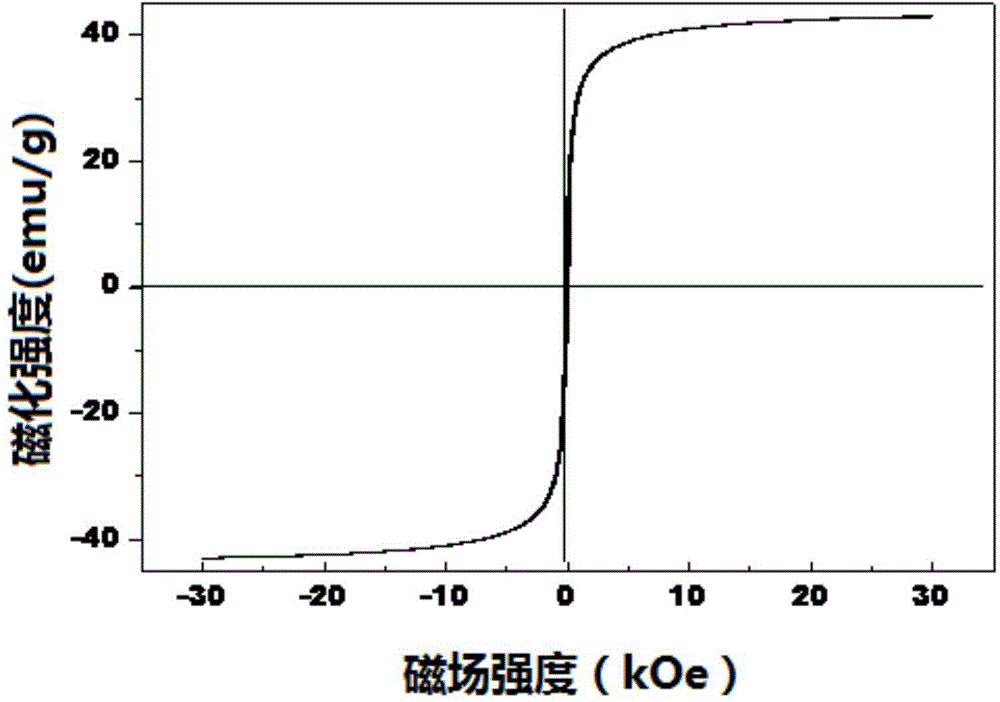
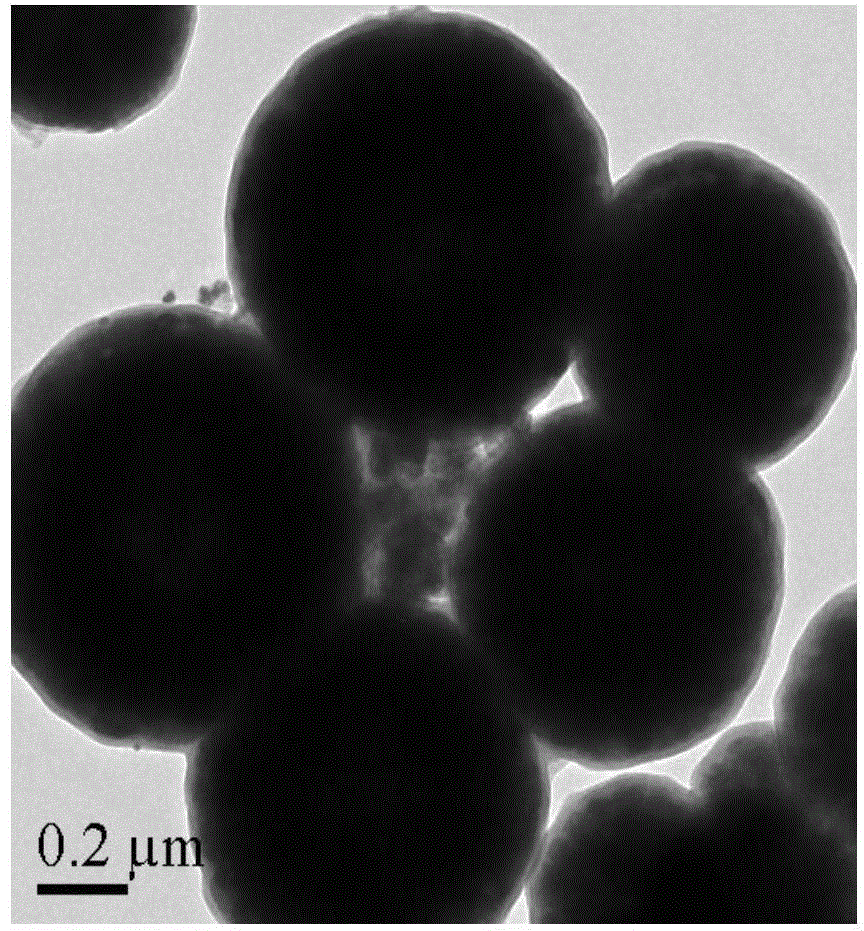
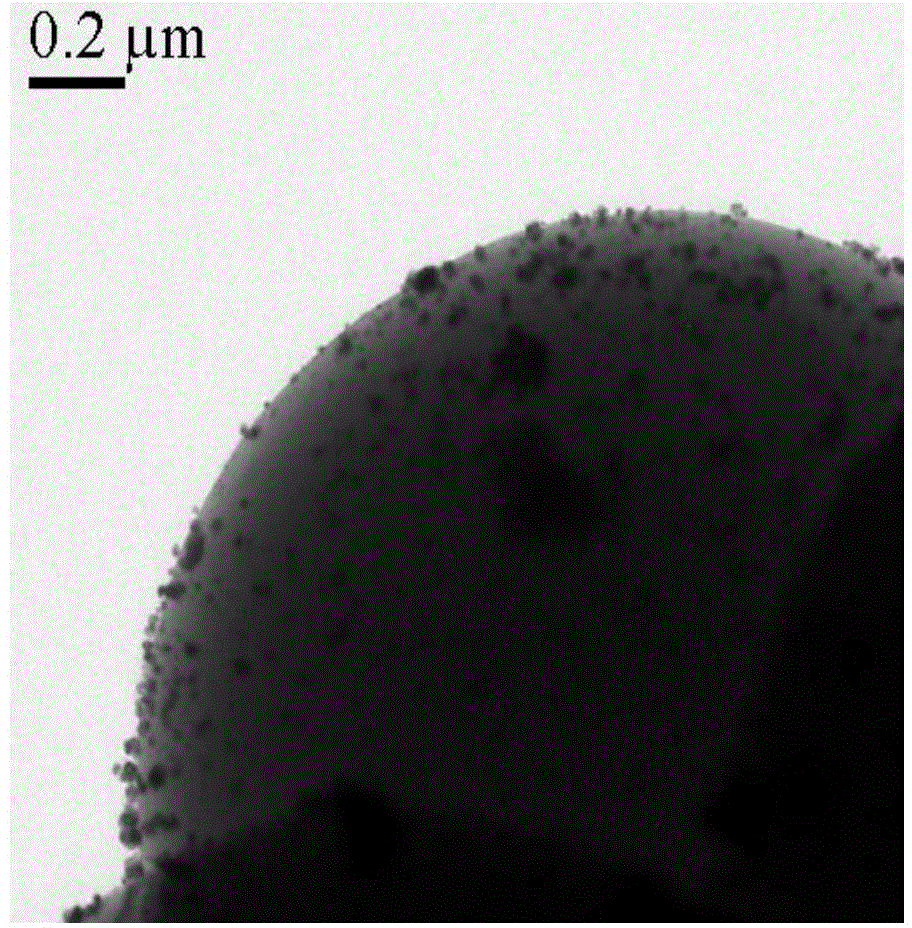
The invention discloses a catalyst for preparing 2,5-dimethylfuran through selective hydrogenolysis of 5-hydroxymethylfurfural and a preparation method of the catalyst. The catalyst consists of a catalyst carrier and carbon layer coated reactive metals, wherein the catalyst carrier comprises a carbon-based carrier, SiO2, TiO2 or Al2O3 and the like; and the reactive metals are selected from Co, Fe, Ni, Cu or Zn and other base metals. Compared with the conventional catalyst system, the catalyst disclosed by the invention has the advantages that (1) the reactive metal of the catalyst refers to a first transition metal, and the raw materials are cheap and readily available; (2) compared with a noble metal catalyst, the catalyst disclosed by the invention has extremely high activity and selectivity; and (3) the catalyst has magnetism and is easy to recycle.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for the preparation of 2,5-furandicarboxylic acid and for the preparation of the dialkyl ester of 2,5-furandicarboxylic acid
ActiveUS20120271060A1High yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsFuranDicarboxylic acid
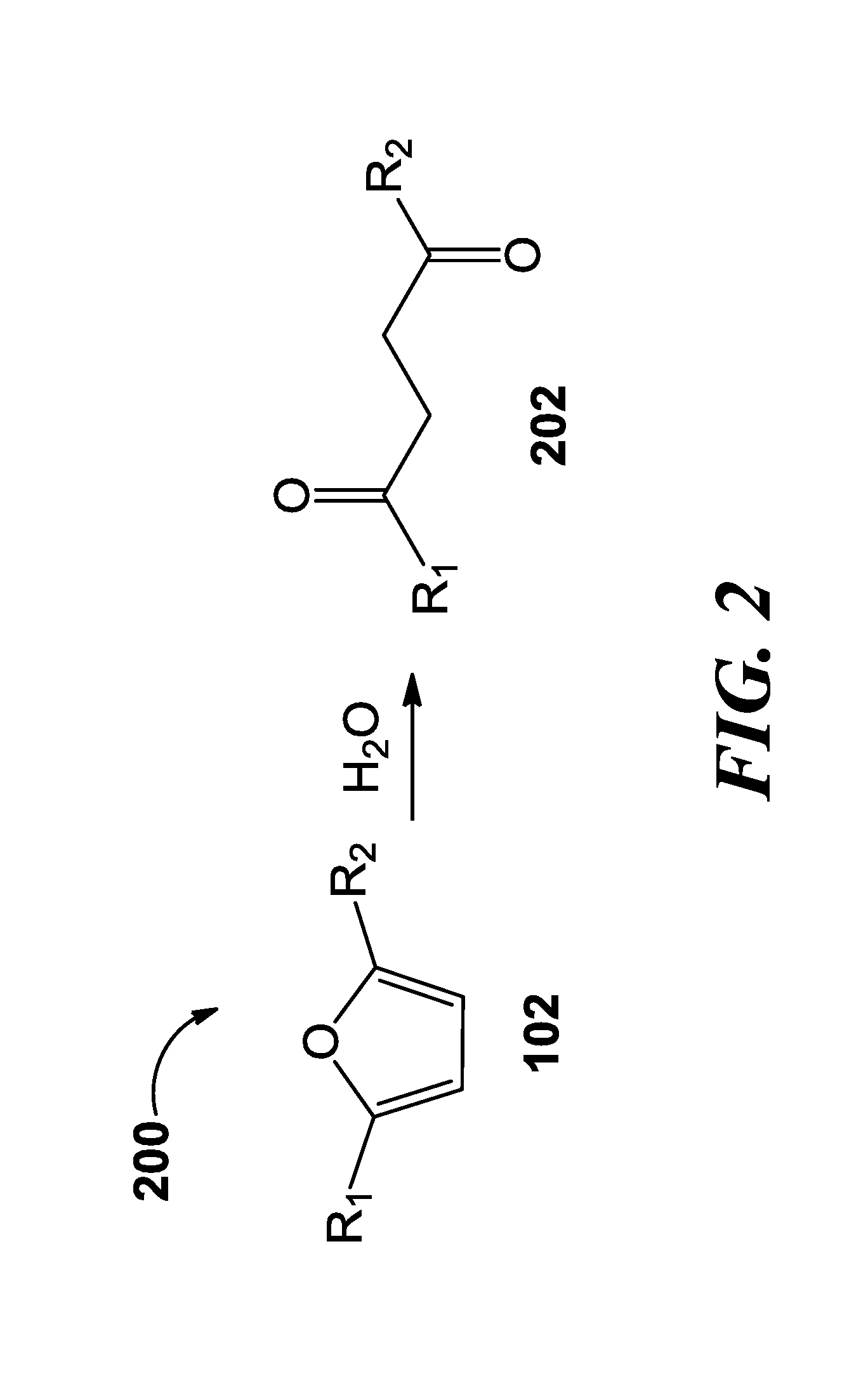
A method for the preparation of 2,5-furan dicarboxylic acid includes the step of contacting a feed comprising a compound selected from the group consisting of 5-hydroxymethylfurfural (“HMF”), an ester of 5-hydroxymethyl-furfural, 5-methylfurfural, 5-(chloromethyl)furfural, 5-methylfuroic acid, 5-(chloromethyl)furoic acid, 2,5-dimethylfuran and a mixture of two or more of these compounds with an oxidant in the presence of an oxidation catalyst at a temperature higher than 140° C.
Owner:FURANIX TECH BV
Nickel-based catalyst and its preparation method and use in 5-hydroxymethylfurfural hydrogenation
ActiveCN105289619AHigh activityHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrotalciteEconomic benefits
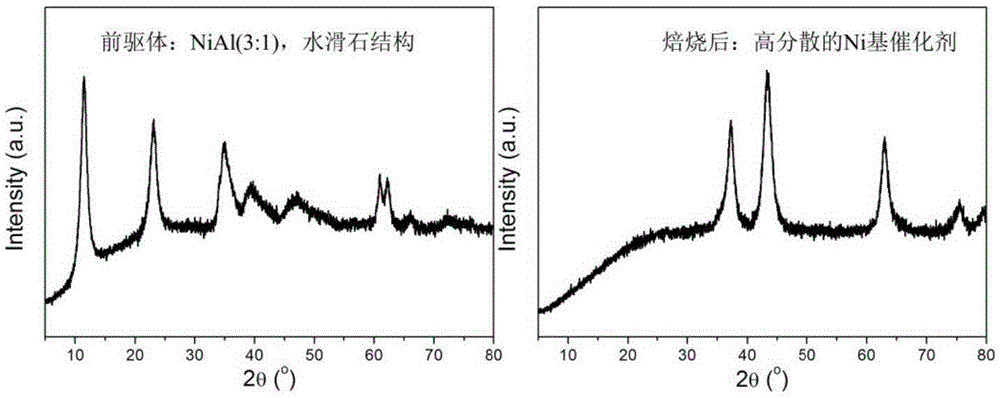
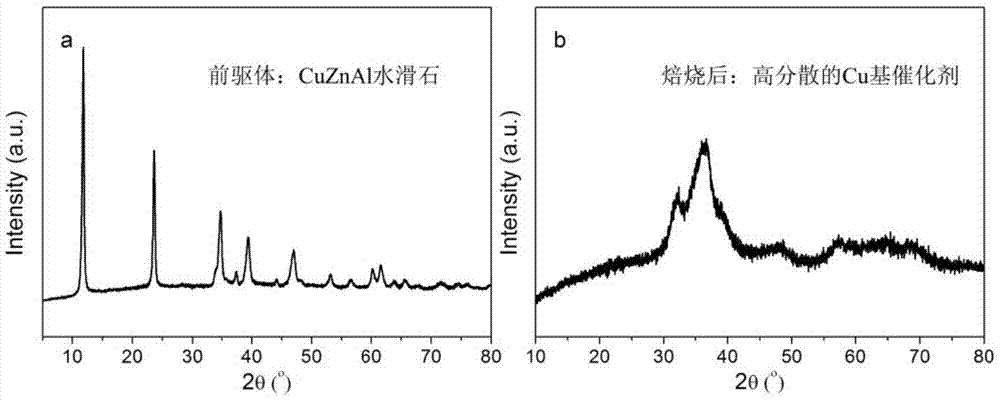
The invention discloses a nickel-based catalyst and its preparation method and use in 5-hydroxymethylfurfural hydrogenation. The nickel-based catalyst has a general formula of NiAl or NiMAl, wherein M represents a metallic element selected from Zn, Mg and Mn, in NiAl, a mole ratio of Ni to Al is 1-3: 1, and in NiMAl, a mole ratio of Ni, M to Al is 1: 1: 1. Ni-based hydrotalcite as a precursor is roasted to form a high-dispersibility Ni-based catalyst with a high 5-hydroxymethylfurfural conversion rate (greater than 99%) and high 2, 5-dimethylfuran or 2, 5-dimethyltetrahydrofuran selectivity (greater than 95%). The nickel-based catalyst has the advantages of easily available raw materials, simple preparation method, operation convenience, low cost, repeatable utilization and latent economic benefits.
Owner:SYNFUELS CHINA TECH CO LTD
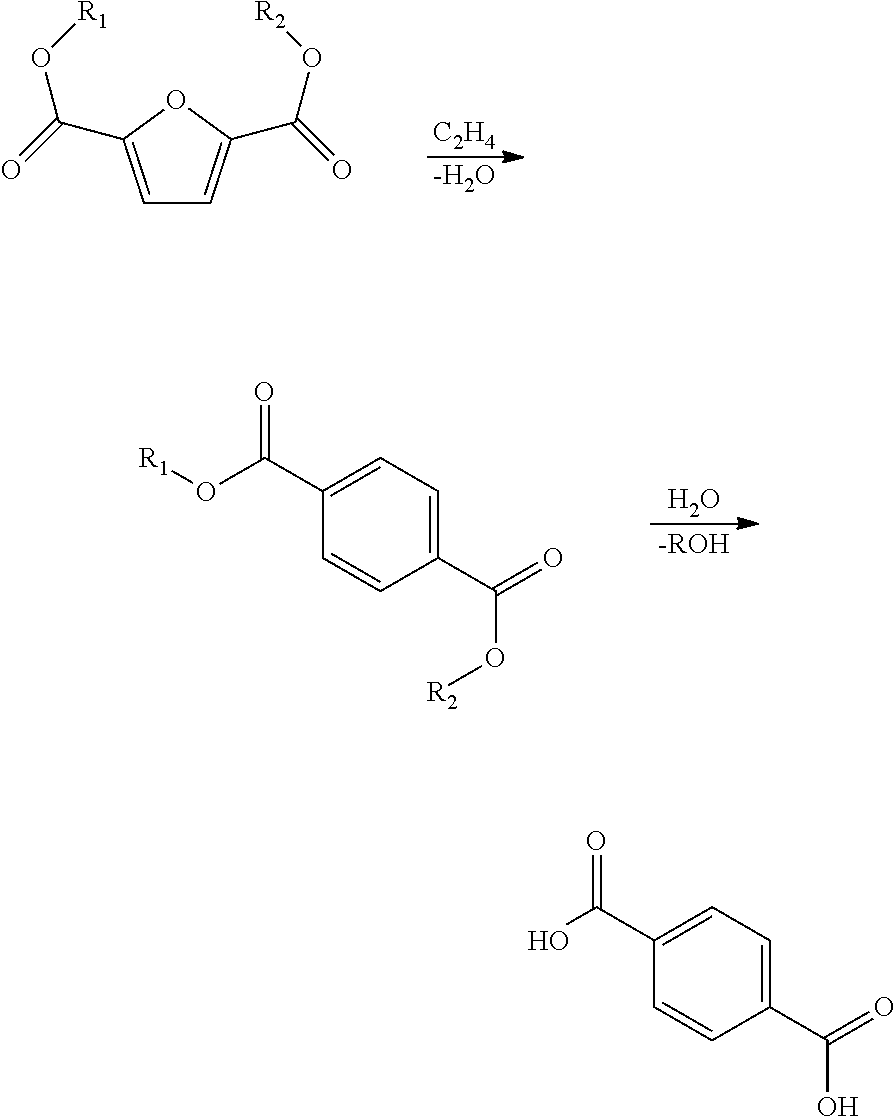
Processes and catalysts for conversion of 2,5-dimethylfuran derivatives to terephthalate
InactiveUS9321714B1Organic compound preparationCarboxylic acid esters preparation2,5-DimethylfuranOrganic chemistry
A process of making terephthalic acid or a derivative of terephthalic acid is described. The process includes reacting a derivative of 2,5-dimethylfuran, with a dienophile containing an unsaturated 2-carbon unit, in the presence of a catalyst having Brönsted acidity to form a para-xylene derivative; and optionally reacting the para-xylene derivative to terephthalic acid.
Owner:UOP LLC
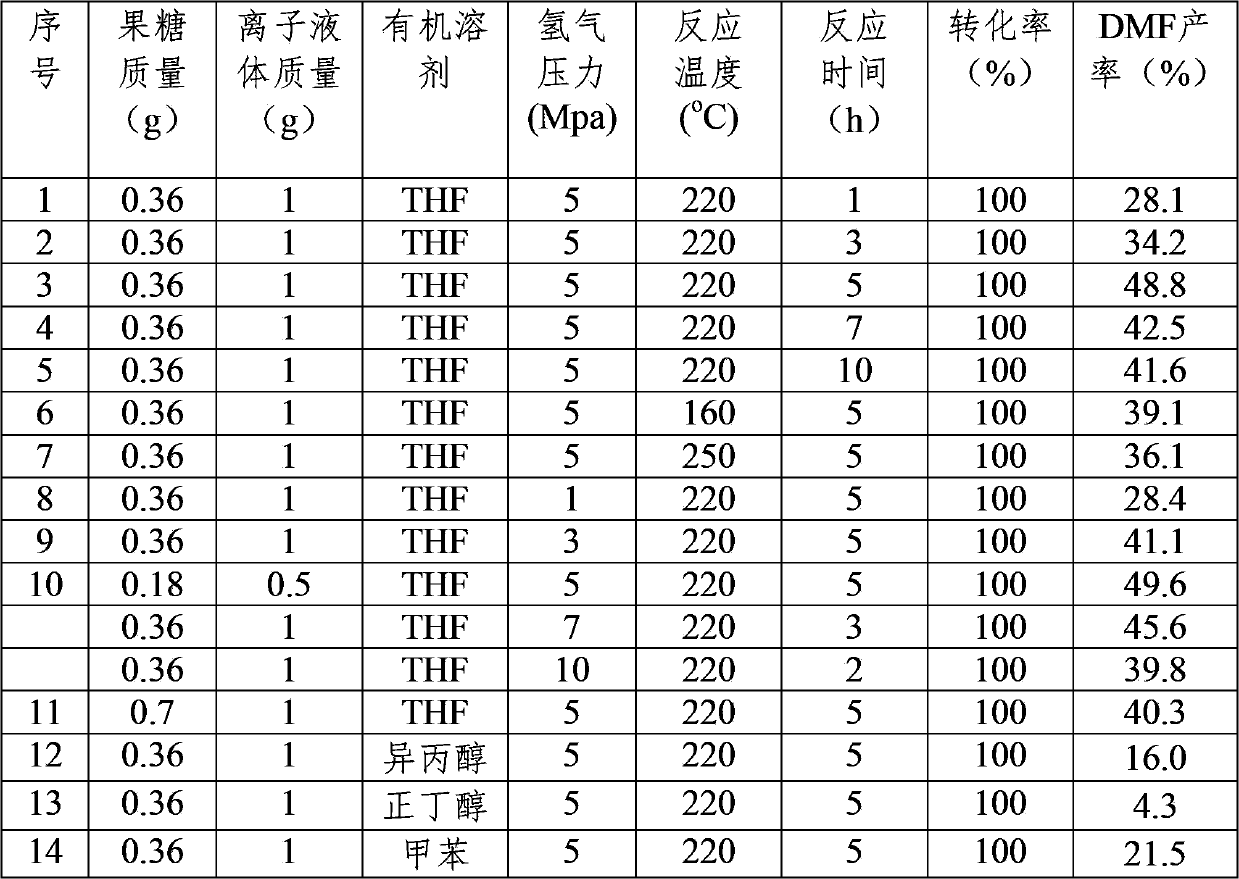
Method for preparing 2,5-dimethyl furan by use of fructosyl biomass
The invention relates to a method for preparing a 2,5-dimethyl furan by use of a fructose and a fructosyl biomass, and specifically relates to a method which comprises the following step: on the basis of adopting a mixed reaction medium of an ionic liquid and an organic solvent, and under the conditions of appropriate temperature and pressure, performing continuous dehydration and hydrogenation reactions on the fructose and the fructosyl biomass in the presence of a catalyst so as to convert the fructose and the fructosyl biomass into the 2,5-dimethyl furan. The method is used for preparing the target product by use of the biomass which is rich in fructose and is taken as the raw material by virtue of a one-pot method; a plurality of steps of reactions are coupled, the reaction steps are simple, the raw materials is cheap and regenerative, the operation is convenient and the product yield is high; a new method for preparing chemicals directly by use of the biomass is provided.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method to prepare 2,5-dimethylfuran by in-situ hydrogenation of 5-hydroxymethylfurfural
InactiveCN108586392AImprove hydrogen production activityHigh activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsAlcoholHydroxymethylfurfural
The invention relates to a method to prepare 2,5-dimethylfuran by in-situ hydrogenation of 5-hydroxymethylfurfural. The method includes: mixing 5-hydroxymethylfurfural, primary alcohol and a Cu-basedcatalyst, heating to 150-250 DEG C, and enabling reaction to occur for 0.5-7 h to obtain 2,5-dimethylfuran; the Cu-based catalyst is three-way catalyst Cu-ZnO-CoOx. The method gains further increasedconversion rate of 5-hydroxymethylfurfural and further improved selectivity of 2,5-dimethylfuran as preparation conditions are optimized.
Owner:ZHEJIANG UNIV
Catalyst used for preparing 2,5-methyl furan and preparation method thereof
InactiveCN105435800AEasy to prepareEasy to operateOrganic chemistryHeterogenous catalyst chemical elementsFuranEconomic benefits
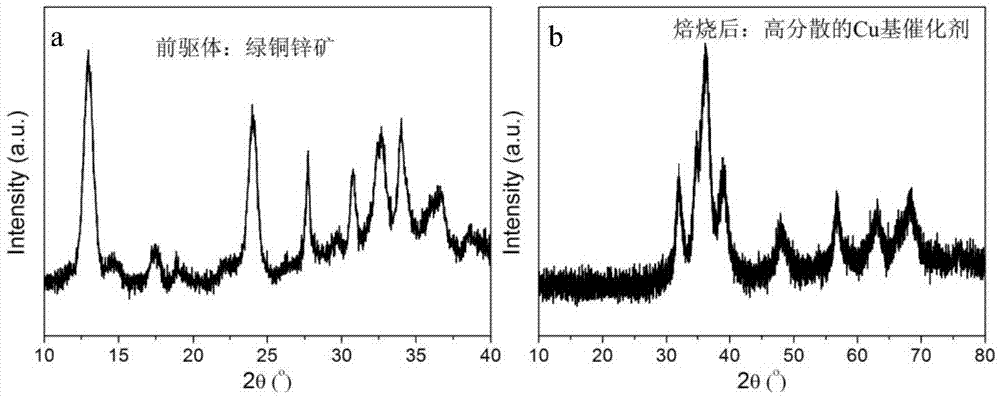
The invention discloses a catalyst used for preparing 2,5-methyl furan and a preparation method thereof. A copper-based catalyst is composed of a copper element and a metal element M, wherein the metal element M is selected from at least one of zinc element and aluminum element and the mole ratio of copper element to metal element M is (1-4):1. According to the invention, a coprecipitation method is used for preparing a copper-based mineral salt precursor; after the copper-based mineral salt precursor is roasted, the high-dispersion copper-based catalyst is acquired, with higher 5-hydroxymethyl furfural conversion rate (99%) and 2,5-methyl furan selectivity (85%). Besides, the raw materials required by the preparation for the copper-based catalyst are easily acquired; the preparation method is simple; the operation is convenient; the cost is low; the catalyst has potential economic benefit.
Owner:SYNFUELS CHINA TECH CO LTD
Methods of producing para-xylene and terephthalic acid
InactiveCN104918901AOrganic compound preparationMolecular sieve catalystCycloadditionRenewable resource
The present disclosure provides methods to produce para-xylene, toluene, and other compounds from renewable sources (e.g., cellulose, hemicellulose, starch, sugar) and ethylene in the presence of a catalyst. For example, cellulose and / or hemicellulose may be converted into 2,5-dimethylfuran (DMF), which may be converted into para-xylene by cycloaddition of ethylene to DMF. Para-xylene can then be oxidized to form terephthalic acid.
Owner:MICROMIDAS INC
Magnetic nanomaterial supported ruthenium catalyst and application of magnetic nanomaterial supported ruthenium catalyst in preparation of 2, 5-dimethylfuran by catalyzing 5-hydroxymethylfurfural
InactiveCN104607202ALow priceImprove stabilityOrganic chemistryChemical recyclingHydroxymethylfurfural2,5-Dimethylfuran
The invention relates to the technical field of preparation and application of a novel catalyst material, in particular to a magnetic nanomaterial supported ruthenium catalyst and an application of the magnetic nanomaterial supported ruthenium catalyst in preparation of 2, 5-dimethylfuran by catalyzing 5-hydroxymethylfurfural. The magnetic nanomaterial supported ruthenium salt Fe3O4@C-Ru is taken as a catalyst, and a magnetic nanomaterial can be easier to recycle under the external magnetic field, so that the problem of production cost increase caused by the fact that the catalyst is difficult to recycle in the process of 2, 5-dimethylfuran preparation through hydrogenation reduction of 5-hydroxymethylfurfural; the catalyst has the numerous advantages that the catalytic reaction yield is high, products are easy to separate, the catalyst is simple and convenient to recycle and the like, and has a wide application prospect.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Methods of producing para-xylene and terephthalic acid
ActiveUS9260359B2High yieldMolecular sieve catalystOrganic compound preparation2-MethylfuranRenewable resource
The present disclosure provides methods to produce para-xylene, toluene, and other compounds from renewable sources (e.g., cellulose, hemicellulose) and ethylene in the presence of an acid, such as a Lewis acid. For example, cellulose and / or hemicellulose may be converted into 2,5-dimethylfuran (DMF) and 2-methylfuran, which may be converted into para-xylene and toluene, respectively. In particular, para-xylene can then be oxidized to form terephthalic acid.
Owner:ORIGIN MATERIALS OPERATING INC
Hydrophobic palladium/metal organic framework material, preparation method thereof and application of hydrophobic palladium/metal organic framework material to synthesis of 2,5-dimethylfuran
ActiveCN108654693AImprove hydrophobicityEasy to prepareOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholHydrogen
The invention discloses a hydrophobic palladium / metal organic framework material which is a solid catalytic material prepared by taking a porous metal organic framework as a carrier, introducing elemental palladium by using an impregnation-reduction method, and then, carrying out polydimethylsiloxane coating treatment. The invention further discloses a method for preparing 2,5-dimethylfuran by selectively catalyzing hexose by using the hydrophobic palladium / metal organic framework material. The method comprises the steps: dissolving hexose into alcohol, and carrying out a reaction at 70-130 DEG C for 0.25-12 h under the action of an acidic additive by taking the hydrophobic palladium / metal organic framework material as a catalyst and polymethylhydrosiloxane as a hydrogen donor, wherein theconcentration of hexose in alcohol is 0.2-10wt%, and the total quantity of Pd contained in the used hydrophobic palladium / metal organic framework material is 0.1-5mol% relative to hexose. The hydrophobic palladium / metal organic framework material disclosed by the invention is stable in structure, and the catalytic efficiency is obviously higher than that of a commercially available palladium carbon and common palladium / metal organic framework material under the same condition.
Owner:NANJING AGRICULTURAL UNIVERSITY
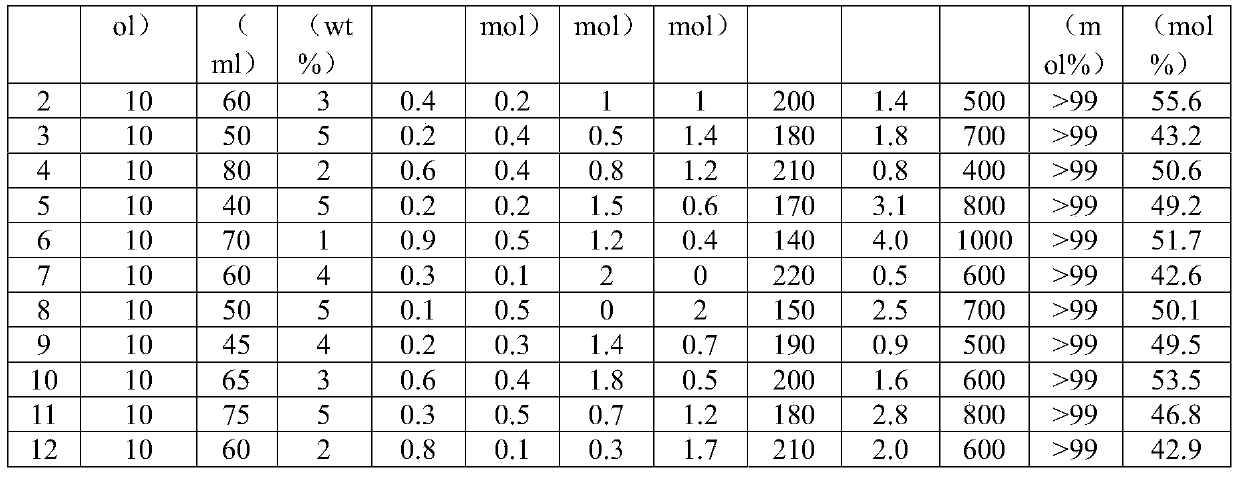
Method for producing 2,5-dimethyl furan (2,5-DMF) by fructose one-step process
ActiveCN105175366AWide variety of sourcesResolve separation difficultiesOrganic chemistryFuranN dimethylformamide
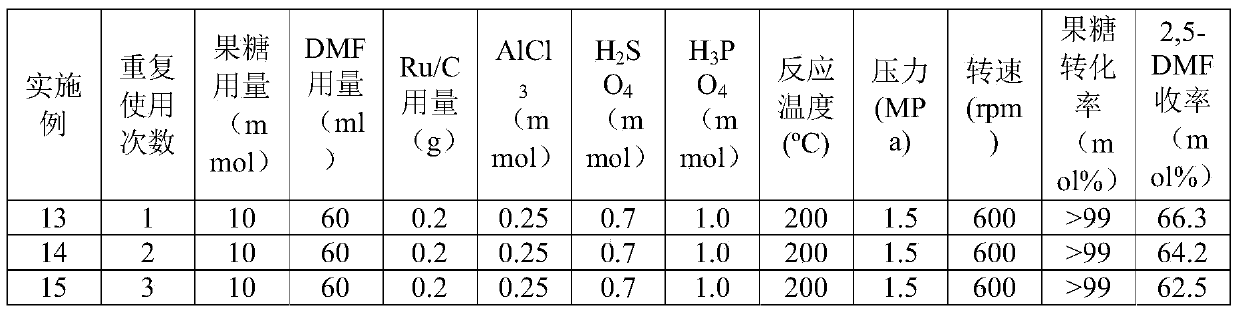
The invention discloses a method for producing 2,5-dimethyl furan (2,5-DMF) by a fructose one-step process. The method comprises the following steps: reacting under the conditions of 140 to 220 DEG C, 400 to1000rpm (revolutions per minute) and 0.5 to 4MPa by taking fructose as a raw materials, Ru / C and mixed liquid of AlCl3 and inorganic acid as catalysts and N,N-dimethylformamide as solvent; after reaction is ended, performing suction filtration on reaction liquid, and recovering Ru / C from a filter cake; performing aftertreatment on filtrate to obtain the product 2,5-dimethyl furan. According to the method disclosed by the invention, 2,5-DMF is produced from the fructose, which is wider in source and lower in price, instead of 5-HMF (5-hydroxymethylfurfural); the method has greater industrial application value; the course of the original two-step process is integrated into one step, therefore the problem that the intermediate product 5-HMF is difficult to separate can be effectively solved; the method has obvious innovation and simple and easy experiment operations; good 2,5-DMF yield can be obtained, and the molar yield reaches 66.3 percent.
Owner:ZHEJIANG UNIV
Method for preparing DMF (2,5-dimethylfuran) from HMF (5-hydroxymethylfurfural) by catalytic hydrogenation
ActiveCN109384750AExpensive to fixHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsReduction treatmentHydrogen pressure
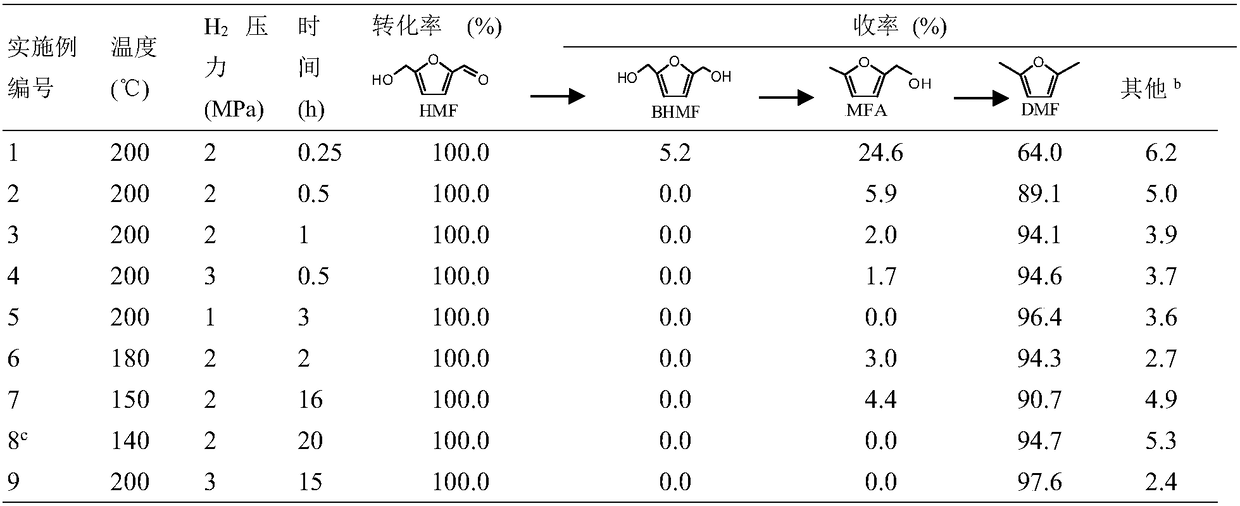
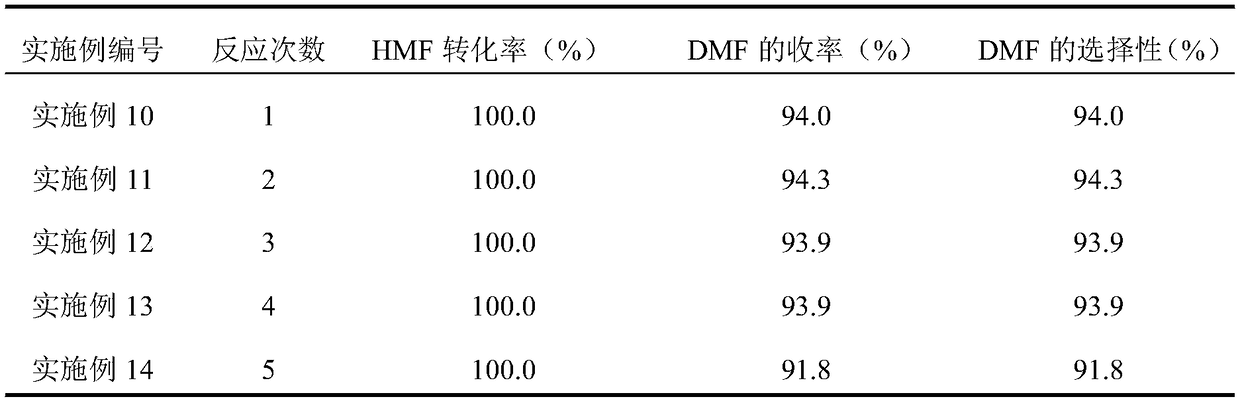
The invention relates to a method for preparing DMF (2,5-dimethylfuran) from HMF (5-hydroxymethylfurfural) by catalytic hydrogenation. According to the method, a Co / rGO (cobalt and reduced graphene oxide) composite is used as a catalyst, the Co / rGO catalyst does not need reduction pretreatment, the conversion rate of HMF can reach 100% at 140-200 DEG C and under 1-2 MPa hydrogen pressure, and theyield of DMF exceeds 90%. The Co / rGO catalyst is cheaper than noble metal catalysts such as Pt and Pd, only C=O / C-O bond is dissociated, and the furan ring and C-C bond are not damaged, so that the catalyst has high selectivity to DMF, does not need pre-reduction treatment and has industrial application value.
Owner:DALIAN UNIV
Methods of producing para-xylene
The present disclosure provides methods to produce para-xylene, toluene, and other compounds from renewable sources (e.g., cellulose, hemicellulose, starch, sugar) and ethylene in the presence of a catalyst. For example, cellulose and / or hemicellulose may be converted into 2,5-dimethylfuran (DMF), which may be converted into para-xylene by cycloaddition of ethylene to DMF. Para-xylene can then be oxidized to form terephthalic acid.
Owner:ORIGIN MATERIALS OPERATING INC
Acidic solid catalyst used for catalyzing conversion of fructose into 2,5-dimethylfuran in one step
ActiveCN109985664AHigh activityImprove stabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalysts2,5-DimethylfuranCobalt
The invention relates to an acidic solid catalyst used for catalyzing conversion of fructose into 2,5-dimethylfuran in one step as well as a preparation method and application of the catalyst. The catalyst is composed of an acidic porous polymer loaded with metal nanoparticles, wherein the porous polymer is a multifunctional polymer, has different wettability and acidity, and can anchor metal ruthenium, platinum, rhodium, palladium, nickel and cobalt under milder conditions. The catalyst provided by the invention is used for catalyzing conversion of the fructose into the 2,5-dimethylfuran in one step, has excellent catalytic activity, high selectivity and strong stability, and provides a novel idea and method for conversion of bio-based materials into high-value-added chemicals.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing catalyst and 2,5-dimethylfuran for hydrogenolysis
ActiveCN105597771AHigh activityHigh catalytic activityOrganic chemistryChemical recycling2,5-DimethylfuranPerovskite
The invention provides a method for preparing a catalyst and 2,5-dimethylfuran for hydrogenolysis. The catalyst for hydrogenolysis is composed of a perovskite oxide carrier LaFeO3 and Ni loaded on the carrier. The catalyst is applied to preparing 2,5-dimethylfuran, the conversion rate of a biomass derivative containing a furan ring reaches 99% or above, and the yield of 2,5-dimethylfuran is high. Besides, the activity of the catalyst recycled after reaction is still high.
Owner:UNIV OF SCI & TECH OF CHINA
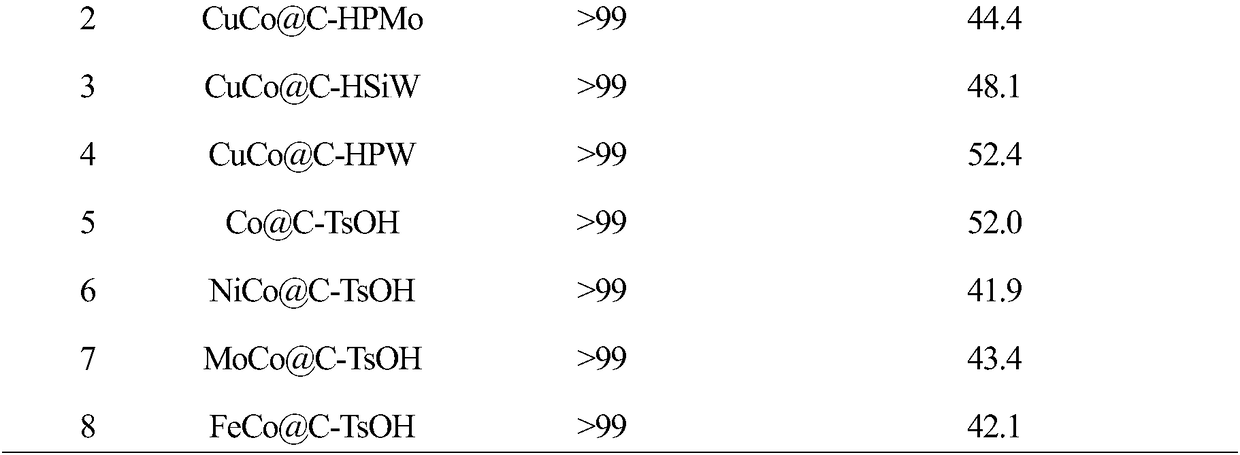
Bifunctional catalyst for catalyzing fructose to directly prepare 2,5-dimethylfuran in one step
ActiveCN108722495ALow costSimple processOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCelsius DegreeHeteropoly acid
The invention discloses a bifunctional catalyst for catalyzing fructose to directly prepare 2,5-dimethylfuran. The bifunctional catalyst is carbon-based solid acid wrapped non-noble metal core-shell type catalyst, wherein carbon-based solid acid is carbon-based p-toluenesulfonic acid, carbon-based heteropoly acid, carbon-based niobic acid or carbon-based sulfonic acid, and non-noble metal is at least one of Cu, Co, Ni, Mn, Mo, Fe and Zn. The preparation method firstly adopts a Pechini sol-gel method to prepare a carbon-coated non-noble metal core precursor; the precursor is pyrolyzed at 400-800 Celsius system in an inert atmosphere to obtain a carbon-coated non-noble metal core, then impregnated with solid acid, and dried to obtain the carbon-based solid acid wrapped non-noble metal core-shell catalyst. The catalyst disclosed by the invention has low cost, the preparation method is simple , and one-step high yield preparation of 2,5-dimethylfuran can be realized through catalyzing fluctose without purification of an intermediate 5-hydroxymethyl furfural; the highest yield of 2,5-dimethylfuran is 71.1%; the operation is simple and safe, and the prospect of industrial application isgood.
Owner:SHAANXI NORMAL UNIV
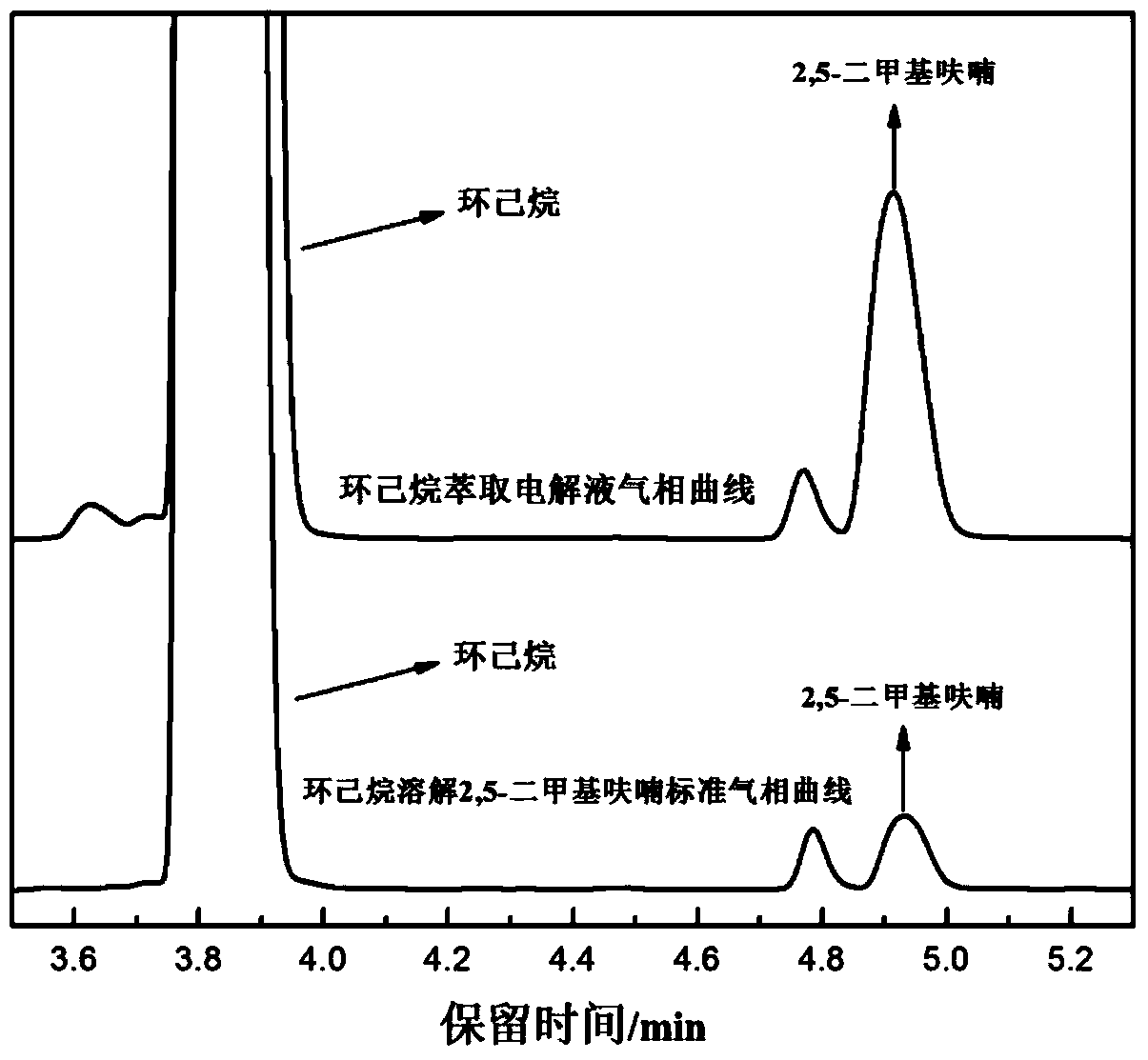
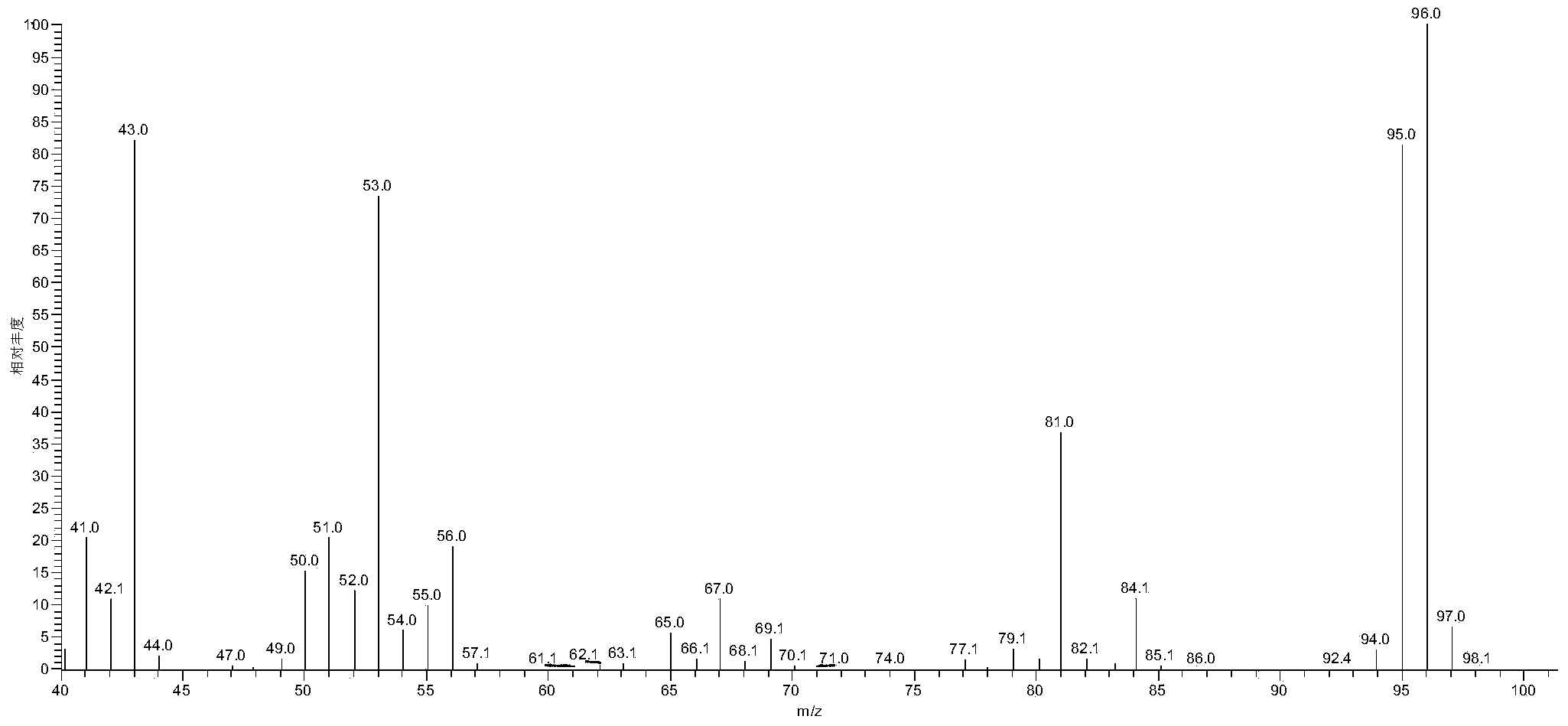
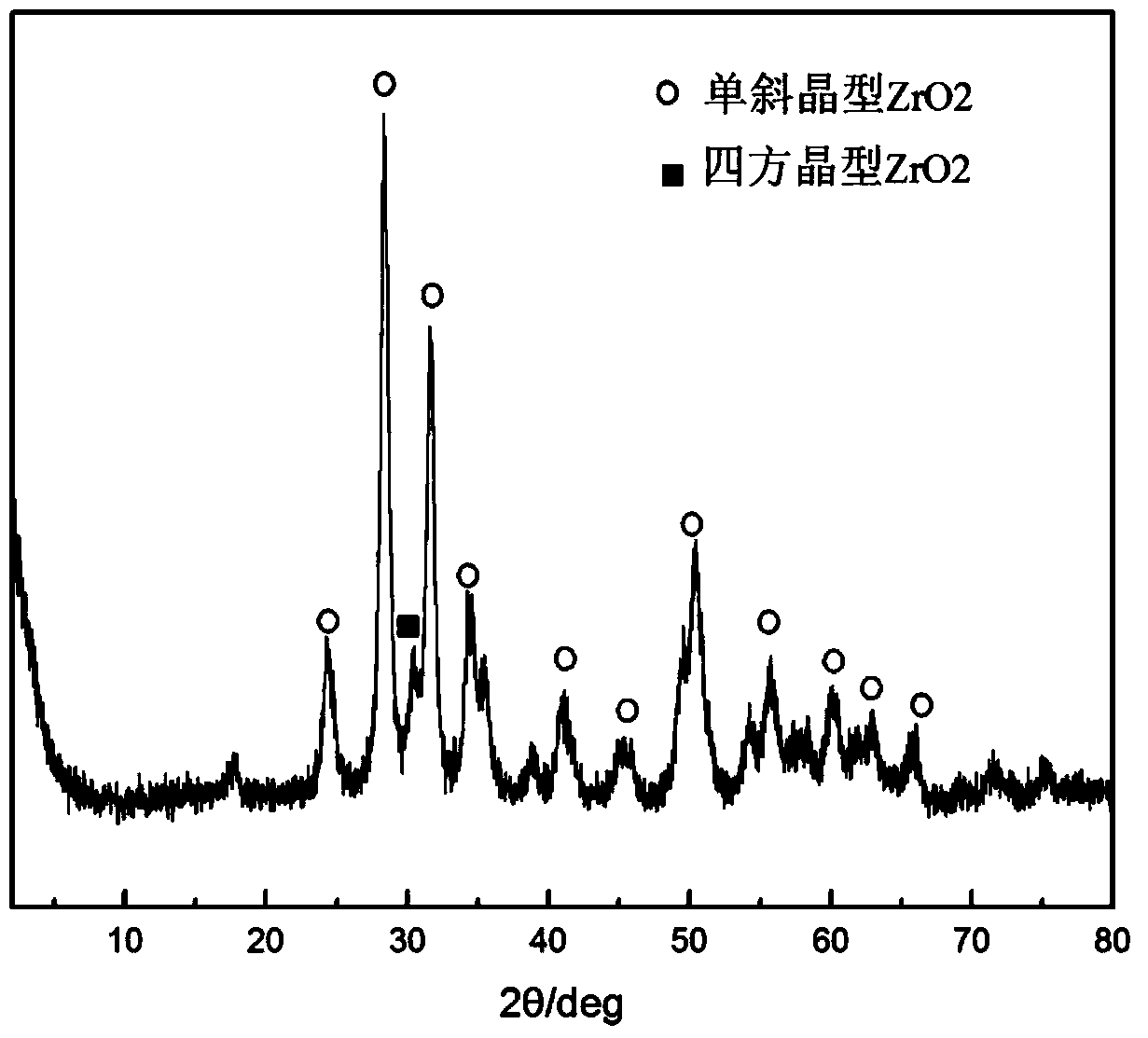
Method for preparing 2,5-dimethyl furan through electrocatalytic reduction of 5-hydroxymethyl furfural by ZrO2-doped graphite electrode
InactiveCN103774173AReduce usageNo pollution in the processElectrolytic organic productionElectrodesFuranElectrolysis
The invention discloses a method for preparing 2,5-dimethyl furan through electrocatalytic reduction of 5-hydroxymethyl furfural by a ZrO2-doped graphite electrode. The method comprises the steps of preparing a 5-hydroxymethyl furfural / N,N-dimethyl acetamide stock solution through catalyzing dehydration of fructose; and implementing electrocatalytic reduction on the 5-hydroxymethyl furfural in an electrolytic bath by taking a ZrO2-doped graphite electrode as a negative electrode and a platinum electrode as a positive electrode, thus preparing the 2,5-dimethyl furan. The method disclosed by the invention successfully solves a problem of a 2,5-dimethyl furan preparation process through hydrogenation reduction of 5-hydroxymethyl furfural which is relatively high in equipment and catalyst expenses. The method, by taking ZrO2 as a catalyst, avoids use of precious metal (ruthenium, palladium and the like) in a conventional 5-hydroxymethyl furfural hydrogenation process, and simultaneously avoids direct use of H2 so as to effectively reduce cost of producing the 2,5-dimethyl furan; and the method disclosed by the invention is free from environmental pollution, relatively moderate in reaction condition and good in selectivity.
Owner:HEBEI UNIV OF TECH
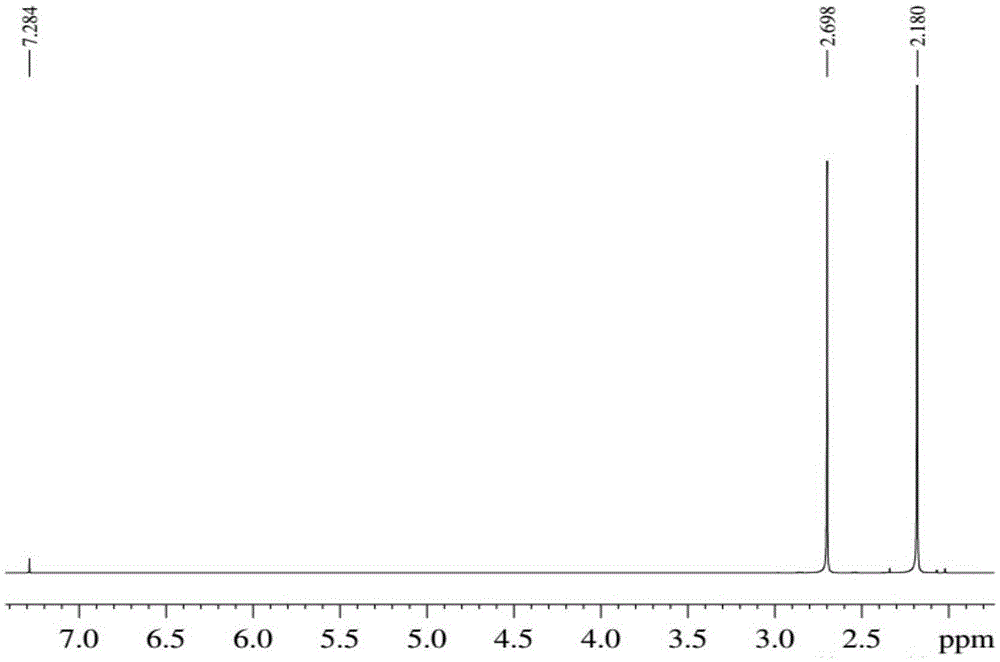
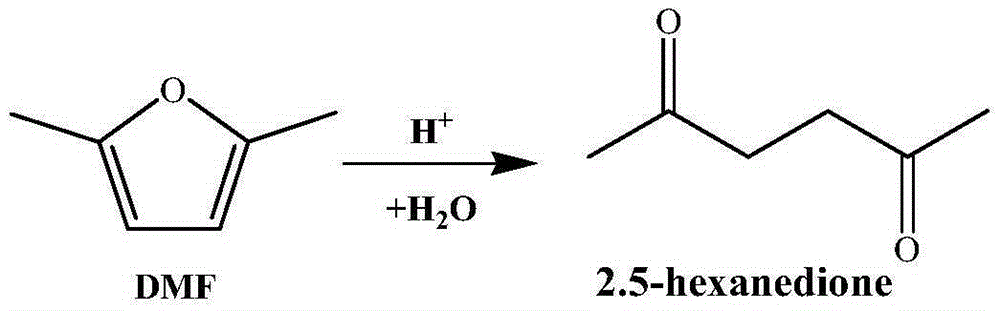
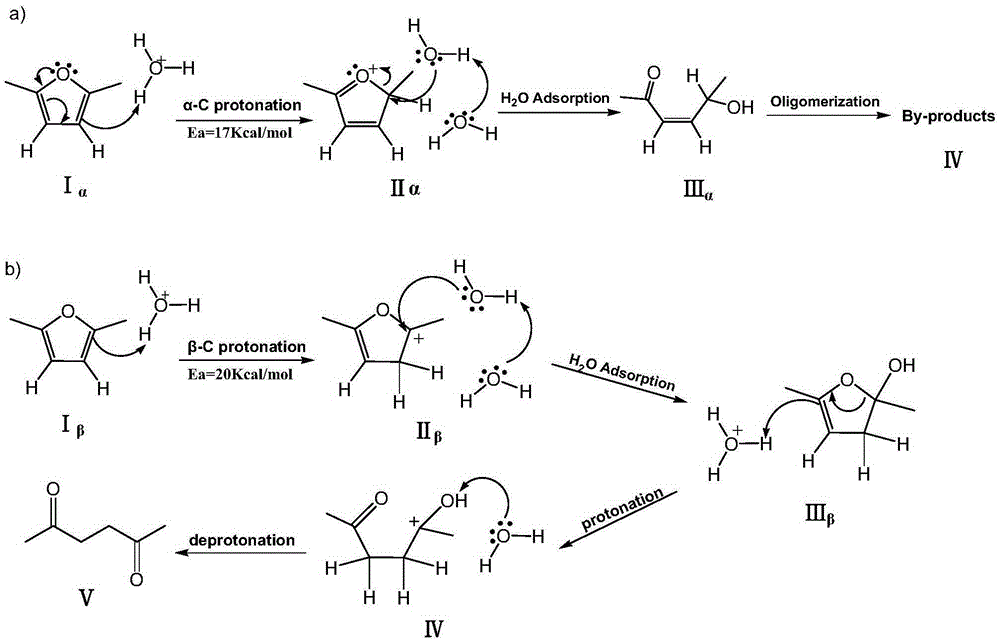
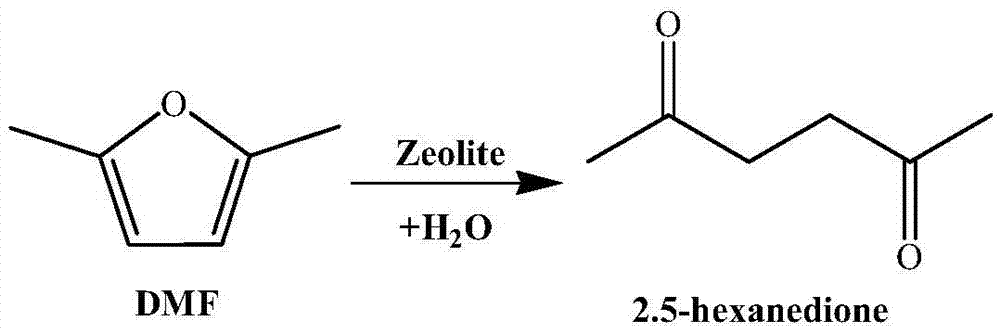
Method for synthesizing 2,5-hexanedione by two-phase process
ActiveCN105348056AAvoid formingHigh catalytic efficiencyCarbonyl compound separation/purificationPreparation from heterocyclic compoundsOrganic solventDistillation
The invention provides a method for synthesizing 2,5-hexanedione by a two-phase process, which comprises the steps of mixing 2,5-dimethylfuran with a weak-polarity organic solvent, adding an aqueous solution of an acid catalyst, quenching a reaction system, quickly cooling the system temperature to the room temperature, and taking the reaction liquid out, wherein the reaction temperature is 60 to 180 DEG C, and the reaction time is 30 minutes to 24 hours. An organic phase is split; an aqueous phase is filtered or separated through centrifugation; organic phases are collected. The obtained organic phases are subjected to reduced pressure distillation, and the organic solvent is removed so that the product 2,5-hexanedione is obtained. The method has the advantages of efficiency, economy, environment friendliness, and high yield.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Preparation method for 2,5-dimethylfuran
InactiveCN108863996AShort preparation timeGood dispersionOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsGas phaseHigh pressure
The invention discloses a preparation method for 2,5-dimethylfuran. The preparation method comprises the following steps: adding 0.5 mmol of 5-hydroxymethylfurfural to 20 mL of tetrahydrofuran to prepare 5-hydroxymethylfurfural solution; mixing the 5-hydroxymethylfurfural solution and 0.1 g of a load-type metal catalyst and placing in a closed high-pressure reaction kettle, replacing air for 3-4 times by using H2 or a mixed gas of H2 / Ar, re-charging the H2 or H2 / Ar, heating to 200-350 DEG C in a high pressure of 0.8-2 MPa, stirring and performing a hydrodeoxygenation reaction for 12 h, whereina gas flow rate is 20-100 ml / min, and detecting by using a gas chromatography and gas chromatography-mass spectrometer. The preparation method has the beneficial effect that a non-noble metal catalyst is developed, the 2,5-dimethylfuran is efficiently and continuously prepared in a moderate reaction condition, and selectivity of the 5-hydroxymethylfurfural is greater than 95%.
Owner:枣庄九星生物科技有限公司
Method for preparing 2,5-hexanedione under catalysis of solid acid
ActiveCN105439836AInhibition of polymerizationEasy to separatePreparation from heterocyclic compoundsOrganic solventDistillation
The invention provides a method for preparing 2,5-hexanedione under the catalysis of solid acid. The method comprises the following steps: adding an organic solvent with weak polarity, a solid acid catalyst and distilled water into a raw material 2,5-dimethylfuran and carrying out a reaction at a temperature of 80 to 200 DEG C for 1 to 12 h; quenching a reaction system so as to allow the temperature of the system to rapidly drop to normal temperature, taking out a reaction solution, then subjecting the reaction solution to filtering or centrifugation, drying the solid catalyst and recovering the dried solid catalyst; and collecting an organic phase, subjecting the obtained organic phase to reduced-pressure distillation and removing the organic solvent so as to obtain 2,5-hexanedione. The method provided by the invention has the advantages of easiness in separation and no pollution.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Composite catalytic material and preparation method as well as application of composite catalytic material to mediated preparation of 2,5-dimethylfuran by in situ dehydrogenation-hydrogenation reaction
ActiveCN109675638AStrong magnetic separation characteristicsEasy to separate and recycleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTemperature controlHydrogenation reaction
The invention discloses a composite catalytic material and a preparation method as well as application of the composite catalytic material to mediated preparation of 2,5-dimethylfuran by an in situ dehydrogenation-hydrogenation coupling reaction. A catalytic material comprises active thermometal and a magnetic inorganic-organic hybrid polymer carrier with Lewis acid alkaline site and Br-nsted acidsite; in addition, the catalytic material has higher 1,4-butanediol dehydrogenation capability and 5-hydroxymethylfurfural hydrogenation capability; by adopting a segmented temperature control method, the catalytic performance and the synergistic effect of the active thermometal and the magnetic inorganic organic hybrid polymer carrier can be selectively regulated, the formation of by-products iseffectively avoided, the yield of a target product is improved, and good industrial application potential is achieved.
Owner:HUAIYIN TEACHERS COLLEGE
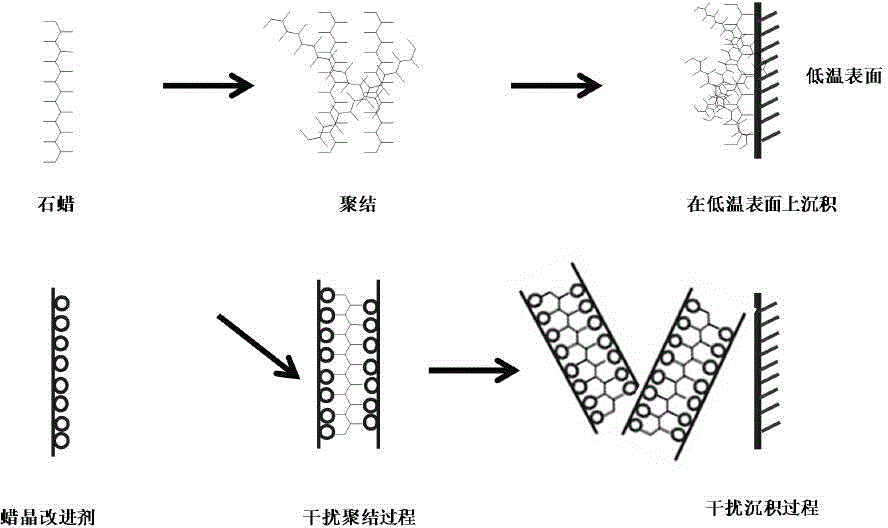
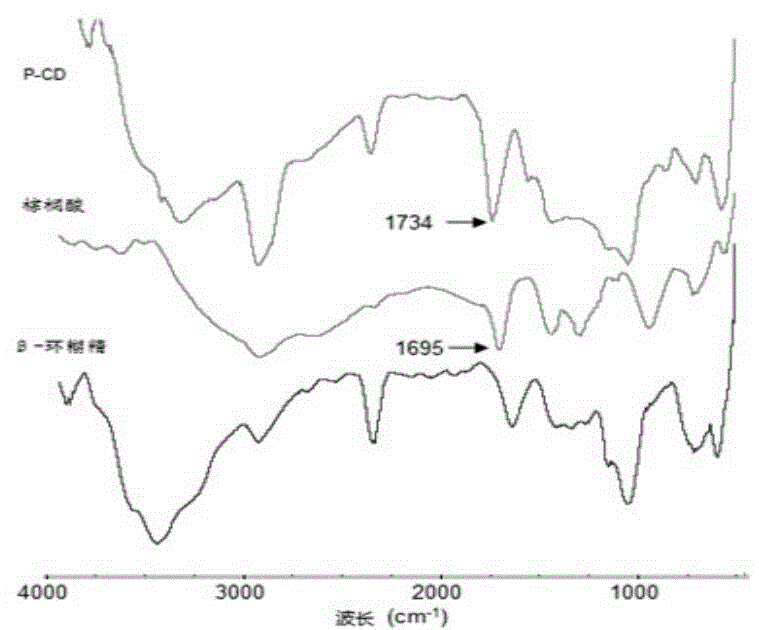
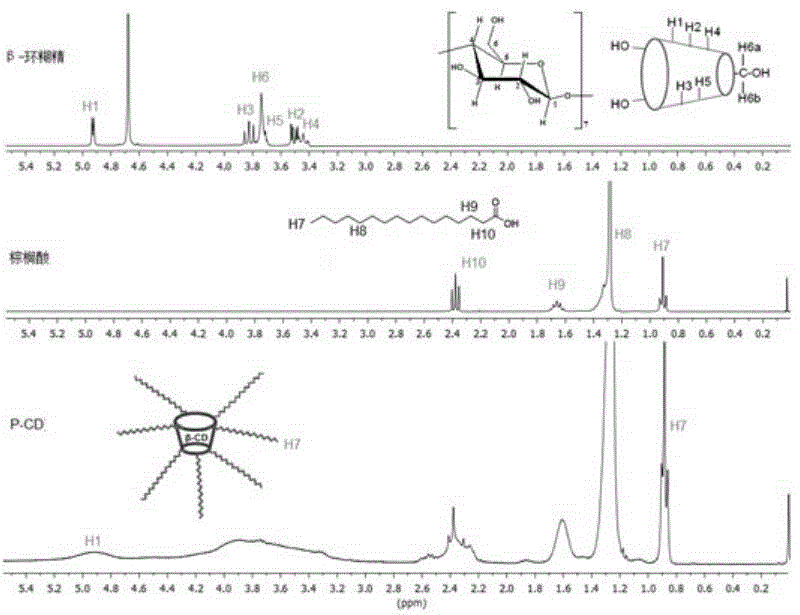
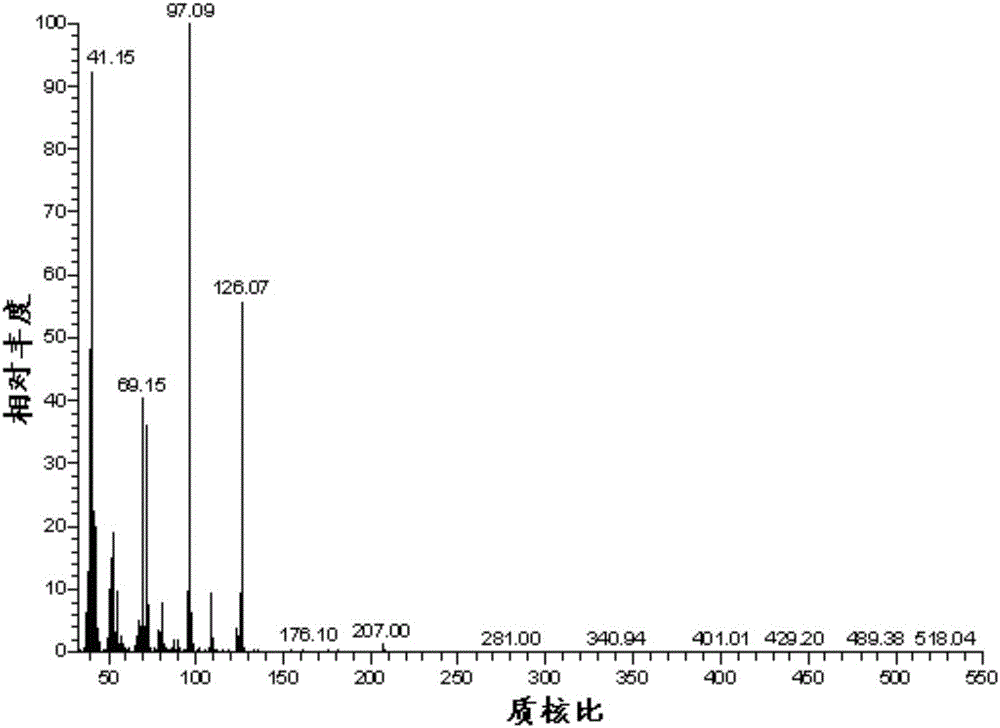
Novel star-shaped wax crystal modifier and synthetic method thereof
The invention discloses a novel star-shaped wax crystal modifier and a synthetic method thereof. The molecular formula of the star-shaped wax crystal modifier is C [42+n (x+1)] H [70+2nx] O [35+n]. The synthetic method includes the steps: (1) respectively dissolving palmitic acid and beta-cyclodextrin in 2, 5-dimethyl-furan; (2) slowly adding ethyl dimethyl amino propyl carbodiimide into palmitic acid solution to form a mixed system, and hermetically placing and stirring the mixed system in an ice-water bath; (3) adding 4-(dimethylamino) pyridine into the mixed system; (4) slowly dropping beta-cyclodextrin solution into the solution, sealing the solution, and uniformly increasing reaction temperature to reach room temperature; (5) performing reaction for 25-30 hours and placing a product in a drying oven for drying after purification, separation and vacuum filtration. The wax crystal modifier has alkyl multi-branched star-shaped space structure characteristics, crystallization deposition of wax is effectively suppressed, and the synthetic method is reliable in principle, convenient to operate and wide in application prospect.
Owner:SOUTHWEST PETROLEUM UNIV
A kind of preparation method of 2,5-dimethylfuran
The invention provides a method for preparing 2,5-dimethyl furan. The method comprises the following steps: under the action of a nickel metal catalyst, performing a hydrogenolysis reaction on 5-hydroxymethyl furfural in a solvent so as to obtain the 2,5-dimethyl furan, wherein the nickel metal catalyst is a loaded type double-metal catalyst; the nickel metal catalyst comprises effective active components including nickel and tungsten. According to the method provided by the invention, the nickel metal catalyst which takes nickel and tungsten as the effective components is adopted to catalyze HMF (Hydroxymethyl Furfural) to perform hydrogenolysis so as to obtain DMF (Dimethyl Formamide); the component nickel has a good hydrogenation capability, and can perform hydrogenation on an aldehyde group to be a hydroxymethyl group; the component tungsten has good Lewis acidity, can prompt breakage of a carbon-oxygen bond on the hydrogenolysis process of HMF, and can convert the hydroxymethyl group into a methyl group; under the dual functions of nickel and tungsten, the HMF can be efficiently and selectively converted into the DMF, and therefore, the yield of the DMF is relatively high.
Owner:UNIV OF SCI & TECH OF CHINA
A kind of method that catalyzes 5-hydroxymethylfurfural to prepare 2,5-dimethylfuran
ActiveCN106279075BEasy to makeEasy to operatePhysical/chemical process catalystsOrganic chemistryHydroxymethylfurfural2,5-Dimethylfuran
The invention provides a method for preparing 2,5-dimethylfuran by catalyzing 5-hydroxymethylfurfural. The method includes the following steps that firstly, 5-hydroxymethylfurfural is added into organic solvent to prepare a 5-hydroxymethylfurfural solution; secondly, the 5-hydroxymethylfurfural solution is catalyzed by means of a heterogeneous iron-based catalyst for hydrodeoxygenation, and 2,5-dimethylfuran is obtained. According to the method, catalytic active metal of the heterogeneous iron-based catalyst in use is iron, and compared with noble metal ruthenium, palladium, platinum and the like, cost is low; compared with other non-noble metal catalysts, the heterogeneous iron-based catalyst is easy to prepare and convenient to operate, and catalytic activity and selectivity are high; the catalyst is used for catalyzing 5-hydroxymethylfurfural (HMF) to prepare 2,5-dimethylfuran (DMF), an efficient environment-friendly method is provided for preparation of DMF, and the heterogeneous iron-based catalyst recycled after catalytic reaction can be repeatedly used.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Method for preparing 2,5-dimethylfuran through hydrogenation of 5-hydroxymethyl furfural
ActiveCN109503525AHigh selectivityHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogenHydroxymethylfurfural
The invention discloses a method for preparing 2,5-dimethylfuran through hydrogenation of 5-hydroxymethyl furfural. The method comprises the following steps: 1) charging an autoclave with hydrogen gasof 1MPa prior to a reaction, exhausting the gas for 5 times, and then, charging the autoclave with hydrogen gas; 2) putting HCP-Co, which serves as a catalyst, into the autoclave together with a tetrahydrofuran solvent, then, adding the 5-hydroxymethyl furfural, then, introducing hydrogen gas of 1.6MPa to 2.2MPa, and carrying out a reaction at the temperature of 160 DEG C to 200 DEG C for 1.5 to5.0 hours while maintaining the stirring rate of 500r / min, thereby obtaining the 2,5-dimethylfuran, wherein the mole ratio of the HCP-Co to the 5-hydroxymethyl furfural is 1: (1 to 5). According to the method, the process flow is simple in operation, the selectivity is high, and the cost is low; compared with a ruthenium catalyst, the catalyst used in the method is higher in selectivity and higherin DMF yield and is more economical.
Owner:YANTAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com