Method for preparing catalyst and 2,5-dimethylfuran for hydrogenolysis
A dimethylfuran and catalyst technology, which is applied in the field of hydrogenolysis catalysts and the preparation of 2,5-dimethylfuran, can solve the problems of reduced reaction efficiency, high cost of precious metals, and restrictions on practical applications, and achieve high catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention has no special requirements for the preparation method of the catalyst, and those skilled in the art can select suitable raw materials to prepare according to the common knowledge in the field. The present invention is preferably prepared according to the following method:
[0024] Dissolve nickel nitrate, iron nitrate and lanthanum nitrate in deionized water, add citric acid and polyethylene glycol, stir vigorously for 12 hours, then place in an oil bath at 80°C until a foamy solid forms, then dry the foamy solid, Calcination and reduction to obtain a catalyst; wherein, the drying temperature is 100-130°C, and the drying time is preferably 20-30 minutes; the calcination temperature is preferably 700-800°C, and the calcination time is preferably 3 ~5 hours; the reduction conditions are preferably: hydrogen flow rate, 100ml / min, nitrogen flow rate, 20ml / min, temperature rise from room temperature 10°C / min to 500°C, keep for 4h, take it out after cool...
Embodiment 1
[0032] Catalyst preparation
[0033] Weigh 2.17mmol of nickel nitrate, 10mmol of iron nitrate, and 10mmol of lanthanum nitrate into a 100mL round-bottomed flask, and add deionized water until the solids are completely dissolved. Add 26.6 mmol of citric acid and 5.32 mmol of polyethylene glycol to the above nitrate solution. The resulting mixture was stirred vigorously for 12 h and placed in an oil bath at 80° C. until a foamy solid was formed. The foamy solid was dried in an oven at 120 °C for 24 h. Then transfer to muffle furnace for calcination at 750°C for 5h. After it is taken out, it is reduced in a reduction tube. The reduction conditions are as follows: hydrogen flow rate, 100ml / min, nitrogen flow rate, 20ml / min, temperature rises from room temperature 10°C / min to 500°C, retains for 4h, takes it out after cooling down to room temperature, and obtains Ni A perovskite-type oxide catalyst with a loading capacity of 5 wt%, marked as LF-N5;
[0034]Perovskite-type oxide ...
Embodiment 2
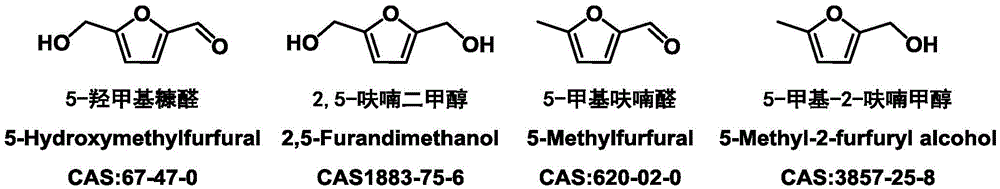
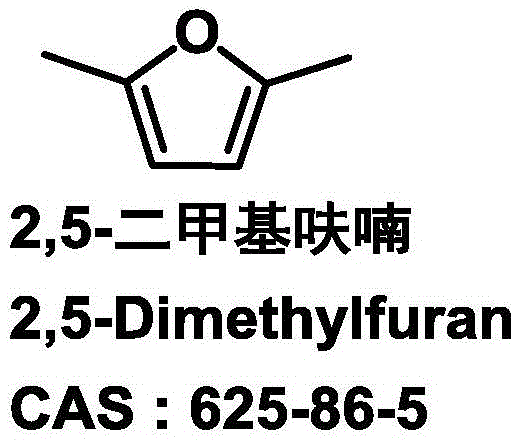
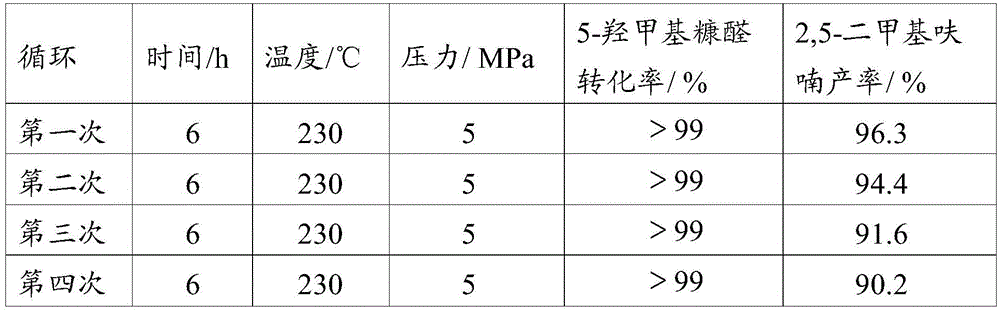
[0036] In a 25ml autoclave, add 1mmol 5-hydroxymethylfurfural, the catalyst prepared in 100mg Example 1 and 12ml reaction solvent ethanol, then seal the autoclave, fill it with hydrogen to 5MPa, and the heating program is from room temperature through 30min to 230 DEG C, keep 6h, use Shimadzu gas chromatograph (model GC2014, Wax chromatographic column) to carry out the quantitative analysis of raw material and product after the reaction finishes, analysis result sees Table 1, and Table 1 is the different Ni content that the embodiment 1 of the present invention provides Application data of the catalyst for the preparation of 2,5-dimethylfuran.
[0037] Table 1 The application data of the preparation of 2,5-dimethylfuran by catalysts with different Ni contents provided in Example 1 of the present invention
[0038] catalyst
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com