Bifunctional catalyst for catalyzing fructose to directly prepare 2,5-dimethylfuran in one step
A bifunctional catalyst, dimethylfuran technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of catalysts. High cost, many reaction steps, complex operation and other problems, to achieve the effect of low catalyst cost, simple process and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
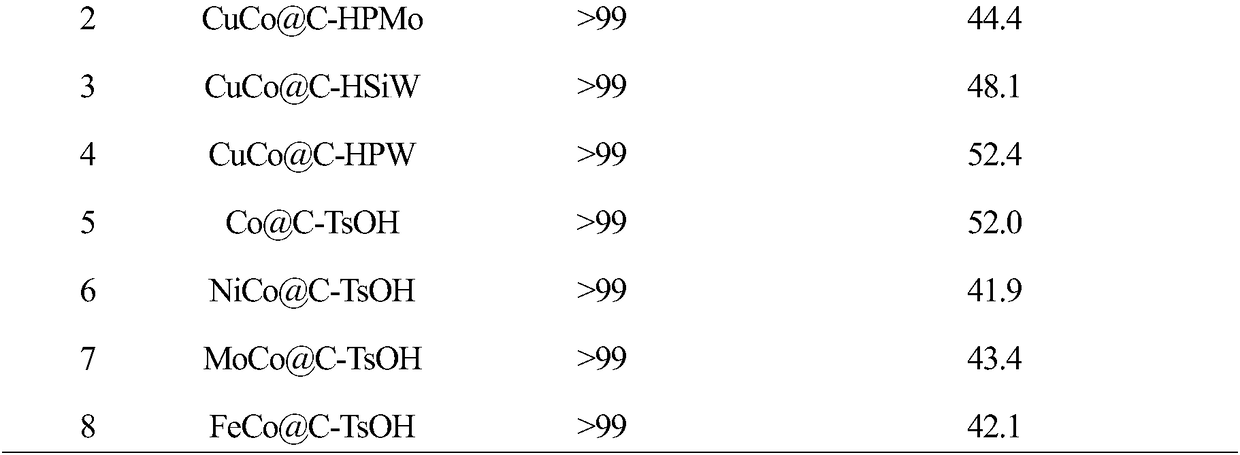
[0020] In this example, the preparation method of CuCo@C is the same as that in Example 1. The same mass of CuCo@C and phosphomolybdic acid (HPMo) are uniformly dispersed in absolute ethanol and immersed for 12 hours. After the impregnation is completed, dry at 80°C for 6 hours to obtain Carbon-based phosphomolybdic acid coated copper-cobalt bimetallic catalyst (CuCo@C-HPMo).
Embodiment 3
[0022] In this example, the preparation method of CuCo@C is the same as that in Example 1. The same mass of CuCo@C and silicotungstic acid (HSiW) are uniformly dispersed in absolute ethanol and immersed for 12 hours. Carbon-based silicotungstic acid coated copper-cobalt bimetallic catalyst (CuCo@C-HSiW).
Embodiment 4
[0024] In this example, the preparation method of CuCo@C is the same as that of Example 1. CuCo@C and phosphotungstic acid (HPW) of equal mass are uniformly dispersed in absolute ethanol and immersed for 12 hours. After the impregnation is completed, dry at 80°C for 6 hours to obtain Carbon-based phosphotungstic acid coated copper-cobalt bimetallic catalyst (CuCo@C-HPW).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com