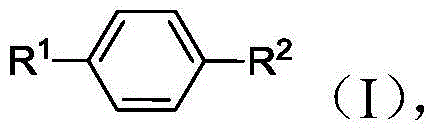
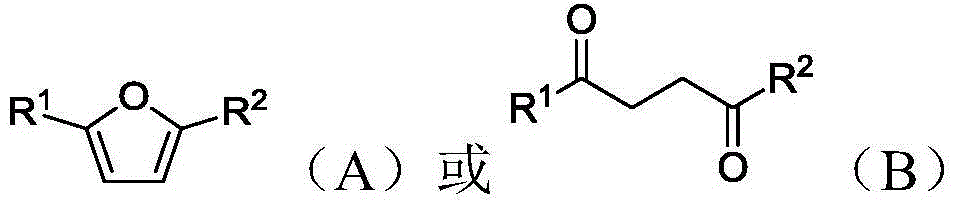
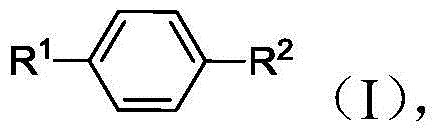
Methods of producing para-xylene and terephthalic acid
A technology of p-xylene and sulfonic acid, which is applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve the problem of reduced selectivity of xylene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0333] The embodiments listed below represent some aspects of the invention.
[0334] 1. A method for producing p-xylene, comprising:
[0335] a) providing 2,5-hexanedione;
[0336] b) providing ethylene;
[0337] c) providing a catalyst;
[0338] d) combining 2,5-hexanedione, ethylene and catalyst to form a reaction mixture; and
[0339] e) generating p-xylene from at least a portion of the 2,5-hexanedione in the reaction mixture.
[0340] 2. The method of embodiment 1, further comprising separating paraxylene from the reaction mixture.
[0341] 3. The method of embodiment 1, further comprising providing a solvent system, and combining the 2,5-hexanedione, ethylene, the catalyst, and the solvent system to form a reaction mixture.
[0342] 4. The method of embodiment 3, wherein the solvent system comprises an aprotic solvent.
[0343] 5. The method of embodiment 3, wherein the solvent system comprises an ether solvent.
[0344] 6. The method according to embodiment 3, w...
Embodiment 1
[0469] Preparation of PX from DMF
[0470] A mixture of 5.0 g of DMF, 0.025 g of copper triflate, and 100 mL of dioxane was charged into an autoclave equipped with a gas-entraining impeller. The autoclave was purged three times with nitrogen, once with ethylene, and then pressurized to 500 psig (3,447 kpas) with ethylene. The autoclave was heated to 250°C at which point the pressure was increased to 2250 psig (15,513 kpas). The reactor was kept pressurized at 250°C for 7 hours with the heater turned off and the reactor was allowed to cool at RT. The pressure was released and the reaction solution was poured into a storage bottle. The reaction mixture was a pale yellow solution with a small amount of black precipitate. pass 1 H and 13 C NMR spectroscopy of the solution phase identified residual DMF, PX, ethylene and HD as major components and dioxane as solvent. These components pass 1 The H NMR spectra were quantified and the values are given in Table 1 below. About ...
Embodiment 2
[0476] Preparation of PX from DMF and HD
[0477] A mixture of 8.0 g DMF, 2.0 g HD, 0.5 g yttrium triflate and 200 g dioxane was charged to an autoclave equipped with a gas entrainment propeller. The autoclave was purged three times with nitrogen, once with ethylene, and then pressurized to 500 psig (3,447 kpas) with ethylene. The autoclave was heated to 250°C at which point the pressure was increased to 2,000 psig (13,790 kpas). The reactor was kept pressurized at 250°C for 7 hours. Samples were taken at hourly intervals and analyzed for conversion and selectivity by NMR spectroscopy. After 7 hours, the heater was turned off and the reactor was allowed to cool to RT. The pressure was released and the reaction solution was poured into a storage bottle. After 7 hours 100% conversion was obtained, the molar selectivity of PX based on HD and DMF was 90%, the yield was equal to 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com