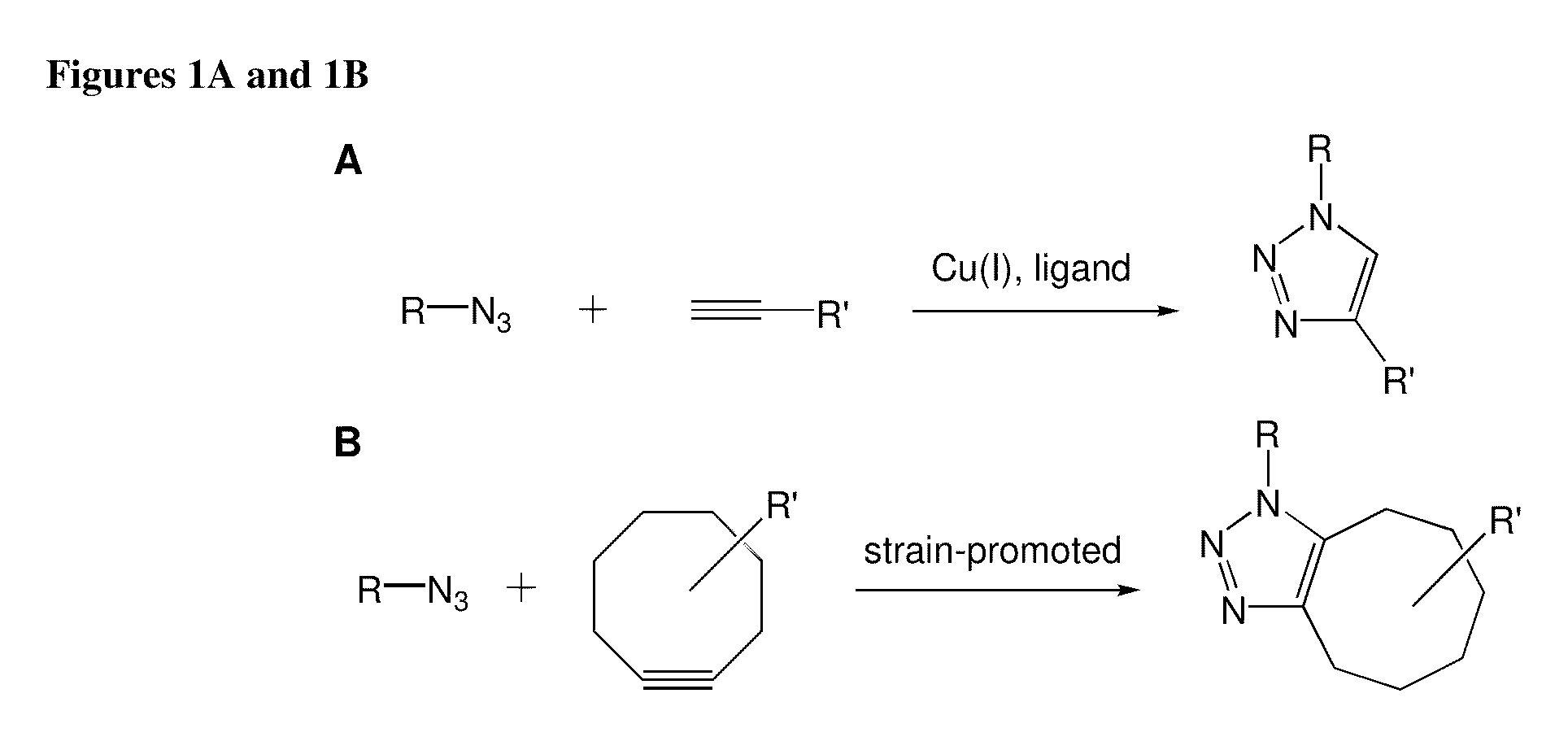
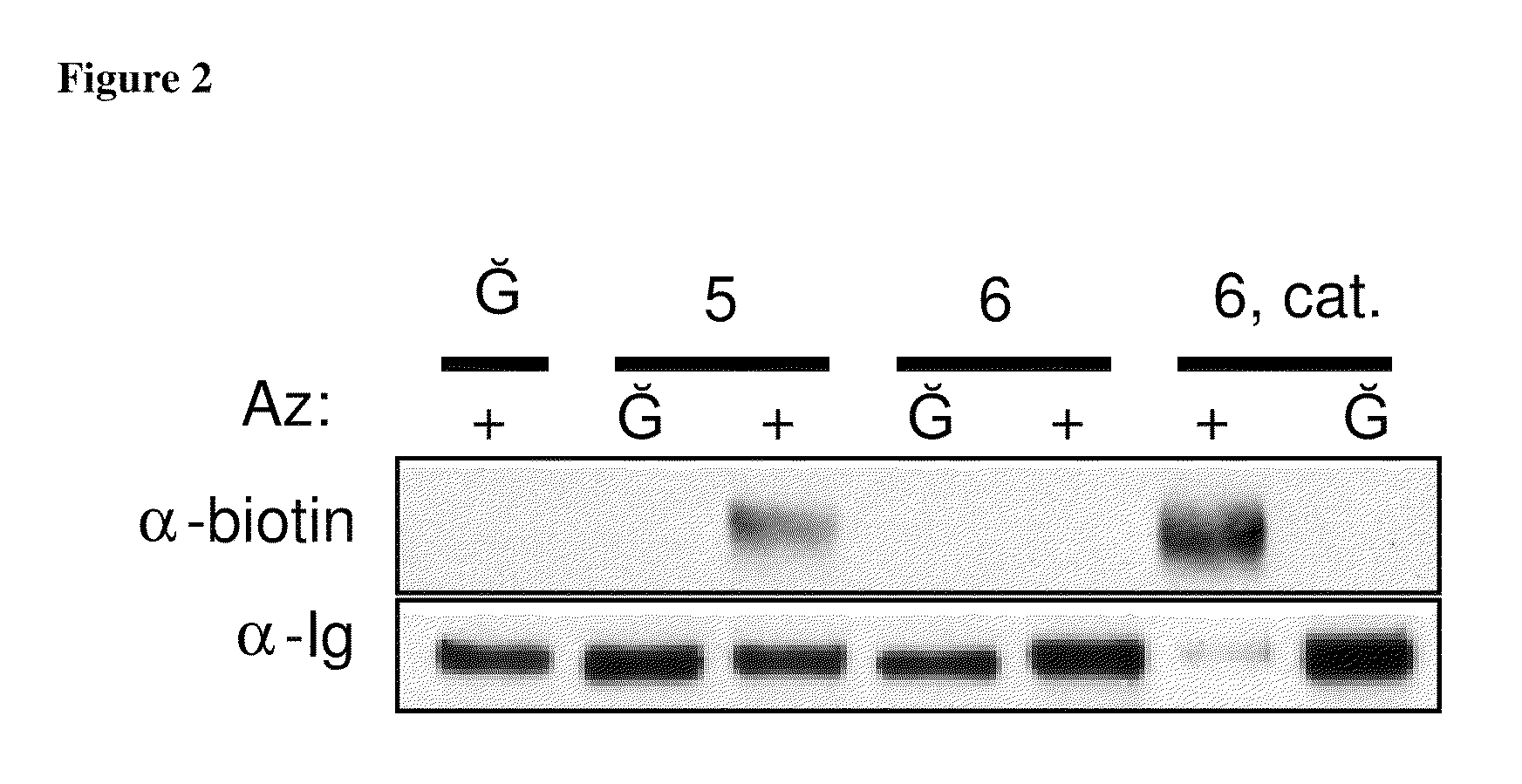
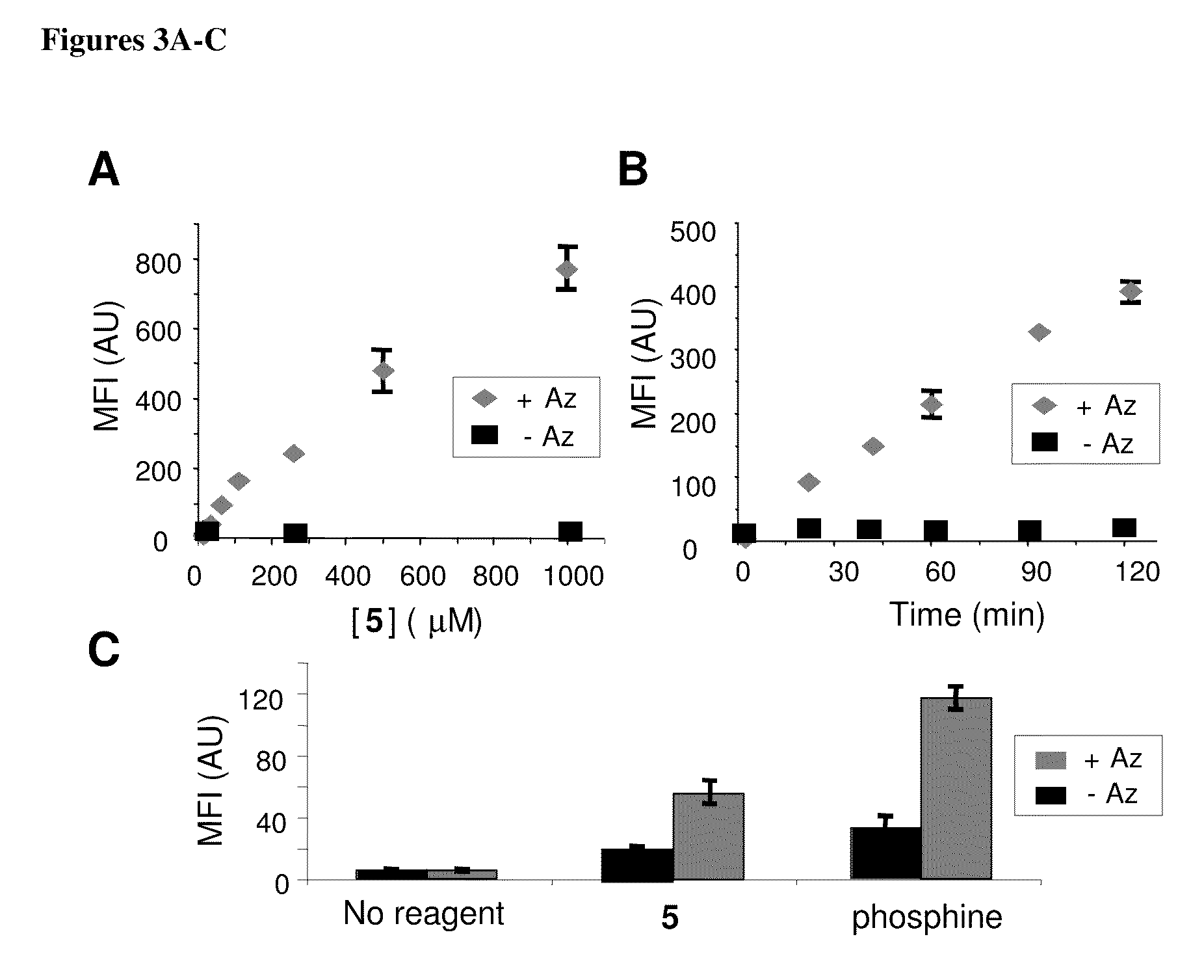
Compositions and methods for modification of biomolecules
a biomolecule and composition technology, applied in the field of compositions and methods for modification of biomolecules, can solve the problems of limited applicability of reaction in biological systems, increased reaction rate has proved synthetically challenging, and cells cannot be viable,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Modification of Biomolecules Using a Modified Cyclooctyne
Materials and Methods
[0238]All chemical reagents were purchased from Aldrich and used without further purification. All solvents were distilled under a N2 atmosphere. CH2Cl2, toluene, and pyridine were dried over CaH2. Thin layer chromatography was carried out on Analtech Uniplate® silica gel plates. Flash chromatography was performed using Merck 60 Å 230-400 mesh silica gel. All 1H and 13C NMR spectra were acquired on Bruker AVB-400® or DRX-500® as noted. 1H chemical shifts are reported as δ referenced to solvent and coupling constants (J) are reported in Hz. Compounds 1 and 4 have been previously reported. Skattebol and Solomon (1973) Organic Syntheses 5 / Coll. Volumes:306-310; and Wilbur et al. (1996) Bioconj. Chem. 7:689-702.
[0239]RPMI 1640 media and phosphate-buffered saline (PBS) were purchased from Invitrogen Life Technologies. Fetal calf serum (FCS) was from Hyclone. FITC-conjugated avidin and bovine serum albumin (BSA)...
example 2
Synthesis of Additional Cyclooctyne Compounds and their Use in Labeling of Living Cells
[0262]This example presents the synthesis of compounds 3a and 3b (shown below), the evaluation of their kinetic parameters in reactions with small organic azides, and their use in bioorthogonal labeling of living cells.
[0263]
Materials and Methods
[0264]All chemical reagents were of analytical grade, obtained from commercial suppliers, and used without further purification unless otherwise noted. With the exception of reactions performed in aqueous media, all reaction vessels were flame-dried prior to use. Reactions were performed in a N2 atmosphere, except in the case of reactions performed in aqueous media, and liquid reagents were added with a syringe unless otherwise noted. Tetrahydrofuran (THF) was distilled under N2 from Na / benzophenone immediately prior to use, and CH2Cl2 was distilled from CaH2 immediately prior to use. Chromatography was carried out with Merck 60 230-400 mesh silica gel acc...
example 3
Synthesis of an Aryl-Less Cyclooctyne Compound and its Use in Labeling of Living Cells
[0306]This example describes synthesis of an “aryl-less” octyne (ALO; shown below) and the use of a conjugated ALO to label living cells in vivo. The aryl-less octyne was synthesized; then a maleimide group was introduced. The maleimide functionality allows for specific conjugation to the thiol of a cysteine containing peptides. Described below are the synthesis of the octyne, the octyne-linked maleimides, their conjugation to a FLAG peptide, and the subsequent use of these conjugates on cell surfaces and in living mice.
[0307]
Materials and Methods
Synthesis of ALO
[0308]
[0309]Compound 14. AgClO4 (2.40 g, 11.6 mmol) was added to a solution of dibromobicycle 1 (1.00 g, 3.72 mmol) and methyl glycolate (6.0 ml, 78.2 mmol) dissolved in toluene (4 mL) in a flame-dried, aluminum-foil-wrapped flask. The reaction was stirred for 2 h, diluted with pentane (20 mL), and filtered to remove insoluble silver salts....
PUM
| Property | Measurement | Unit |
|---|---|---|
| bond angle | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com