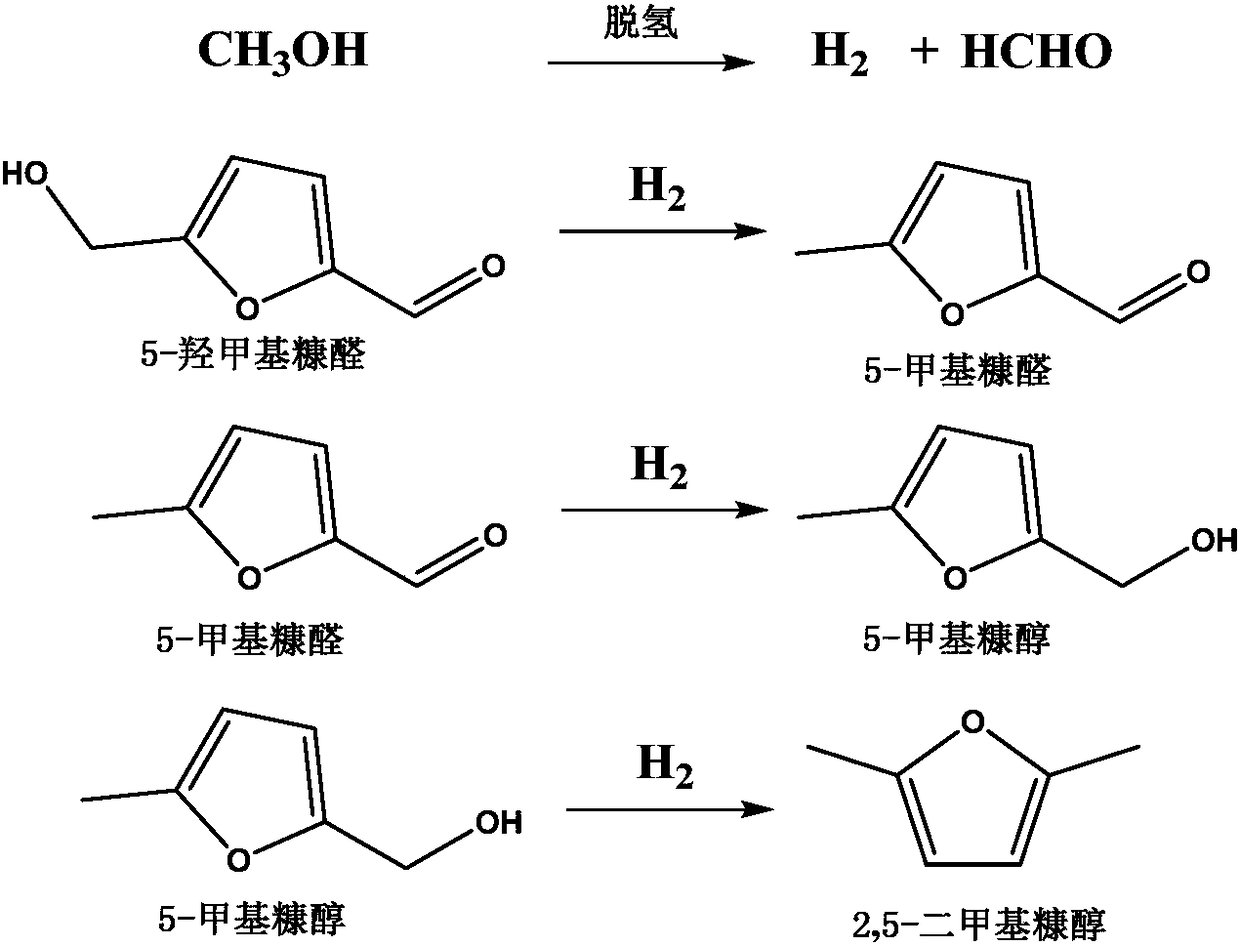
Method to prepare 2,5-dimethylfuran by in-situ hydrogenation of 5-hydroxymethylfurfural
A technology of hydroxymethyl furfural and dimethyl furan, applied in the field of biomass liquid fuel preparation, can solve problems such as hindering the industrialization process, high price of hydrogen supply agent, etc., and achieve the effects of zero hydrogen consumption and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of Cu-ZnO-CoO x catalyst
[0037] Preparation of Cu-ZnO-CoO by coprecipitation method x , where the loading of Cu-ZnO is 20wt%, and the molar ratio of Cu to Zn is 2.5:1.
[0038] The specific preparation process is as follows: 1) Preparation of mixed salt solution: according to the component content calculated in the required catalyst, the corresponding Co(NO 3 ) 2 ·6H 2 O, Zn(NO 3 ) 2 ·6H 2 O and Cu(NO 3 ) 2 ·3H 2 O was dissolved in 500mL deionized water, stirred to make it fully dissolved to make a mixed salt solution.
[0039] 2) Preparation of lye: using NaOH and Na 2 CO 3 The mixed solution is used as a precipitant, and the OH is set according to the molar amount of basic anions required for metal ion precipitation. - and CO 3 2- The molar ratio of the substances is 2:1, the calculated NaOH and Na 2 CO 3 Add 500mL deionized water, stir to fully dissolve to obtain the required lye.
[0040] 3) After the preparation is comp...
Embodiment 2
[0042] Embodiment 2: Preparation of 2,5-dimethylfuran
[0043] Add 5g of 5-hydroxymethylfurfural, 50g of ethanol and 0.5g of CuZnCo-2.5 into a 100mL intermittent high-temperature and high-pressure reactor, start stirring, and raise the temperature to 200°C for 0.5h. After quantitative analysis by GC-FID and HPLC, the conversion rate of 5-hydroxymethylfurfural was calculated to be 9.4%, and the molar yield of 2,5-dimethylfuran was 4.2%.
Embodiment 3
[0044] Embodiment 3: Preparation of 2,5-dimethylfuran
[0045] According to the preparation in Example 2, only changing the reaction time to 3 hours, the calculated conversion rate of 5-hydroxymethylfurfural was 60.1%, and the molar yield of 2,5-dimethylfuran was 33.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com