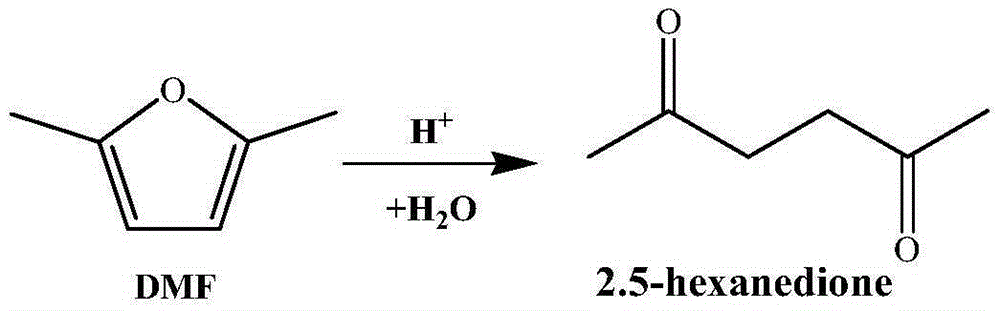
Method for synthesizing 2,5-hexanedione by two-phase process
A technology of hexanedione and organic phase, which is applied in the field of hydrolysis to prepare 2,5-hexanedione, which can solve the problems of limited technology application, low yield, and many side reactions, and achieve the effect of improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
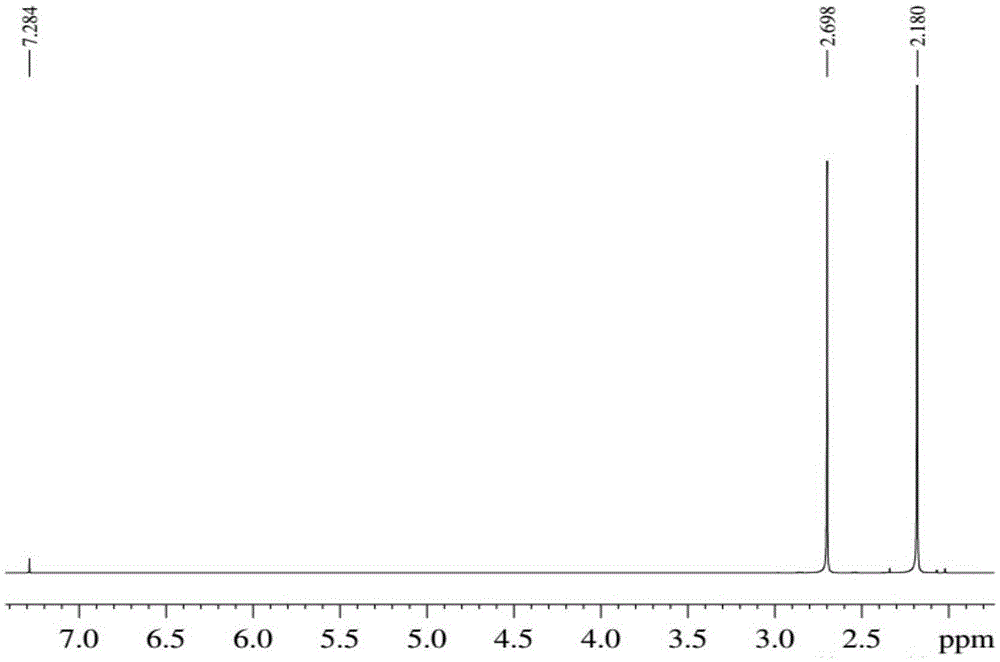
[0027] Add 1ml of 2,5-dimethylfuran into the container, then add 10ml of methyl isobutyl ketone, 7ml of dilute sulfuric acid (9.8% by mass), and mix well. The container was placed in a constant temperature reactor at 120°C, and reacted for 24 hours. After the reaction, the container was put into an ice bath to quench the reaction, and the temperature of the system dropped rapidly to room temperature. The reaction product was taken out, the two phases were separated by centrifugation, and the organic phase was collected. The organic phase obtained is distilled under reduced pressure, removes organic solvent, obtains product (see attached image 3 with 4 ). The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography. The results showed that the conversion rate of the raw material 2,5-dimethylfuran was 97%, the purity of the product wa...
Embodiment 2
[0029] Into the container, sequentially add: 1ml 2,5-dimethylfuran, 10ml cyclohexane, 7ml dilute hydrochloric acid (mass fraction 3.7%), and mix evenly. The container was placed in a constant temperature reactor and reacted at 100° C. for 12 hours. After the reaction, the system was rapidly dropped to normal temperature. The reaction solution is centrifuged, and the obtained organic phase is distilled under reduced pressure to remove the organic solvent to obtain the product. The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography. The results showed that the conversion rate of the raw material 2,5-dimethylfuran was 89%, the purity of the product was 90%, and the yield of 2,5-hexanedione was 85%.
Embodiment 3
[0031] Add 1ml of 2,5-dimethylfuran into the container, then add 15ml of dichloromethane, 10ml of dilute phosphoric acid (mass fraction 8.5%), and mix well. The container was placed in a constant temperature reactor at 180°C, and reacted for 12 hours. After the reaction, the container was put into an ice bath to quench the reaction, and the temperature of the system dropped rapidly to room temperature. The reaction product is taken out, the aqueous phase and the organic phase are separated by centrifugation, the organic phase is collected, and the organic phase is subjected to vacuum distillation to remove the organic solvent to obtain the product. The conversion rate of the raw material and the yield of the product were determined by gas chromatography external standard method, and the purity of the product was determined by liquid chromatography. The results showed that the conversion rate of the raw material 2,5-dimethylfuran was 87%, the purity of the product was 92%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com