Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68results about How to "Accurate determination of concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for measuring NaNO2 concentration in phosphating solution
InactiveCN101477017AGood repeatabilityHigh accuracy and precisionMaterial analysisChemistryMeasurement device
The invention relates to the field of chemical detection, in particular to a method for measuring NaNO2 concentration in phosphating solution. The method for measuring the NaNO2 concentration in the phosphating solution carries out measurement by adopting communication of a liquid reaction device and a measurement device under constant temperature, and comprises the following steps: placing the phosphating solution and a magnetic stirrer in a sample bottle, placing sulfaminic acid solution in a liquid storage pipe, placing purified water in a water storage pipe, and well arranging devices; firstly placing a three-way valve in a three-way state, aligning liquid levels of the liquid storage pipe and a liquid measurement pipe, and recording the liquid level height H1 of a gas measurement pipe; then placing the three-way valve in a two-way state, communicating the reaction device and the measurement device, opening an electromagnetic stirrer to stir the solution, opening a dropper passage and dripping the sulfaminic acid solution into the sample bottle until no bubble generates; adjusting an adjusting knob to align the liquid levels of the liquid storage pipe and the liquid measurement pipe again, and recording the liquid level height H2 of the gas measurement pipe; and calculating the difference of the liquid level heights H1 and H2 as a volume number of N2, and then calculating the NaNO2 concentration.
Owner:FUDAN UNIV
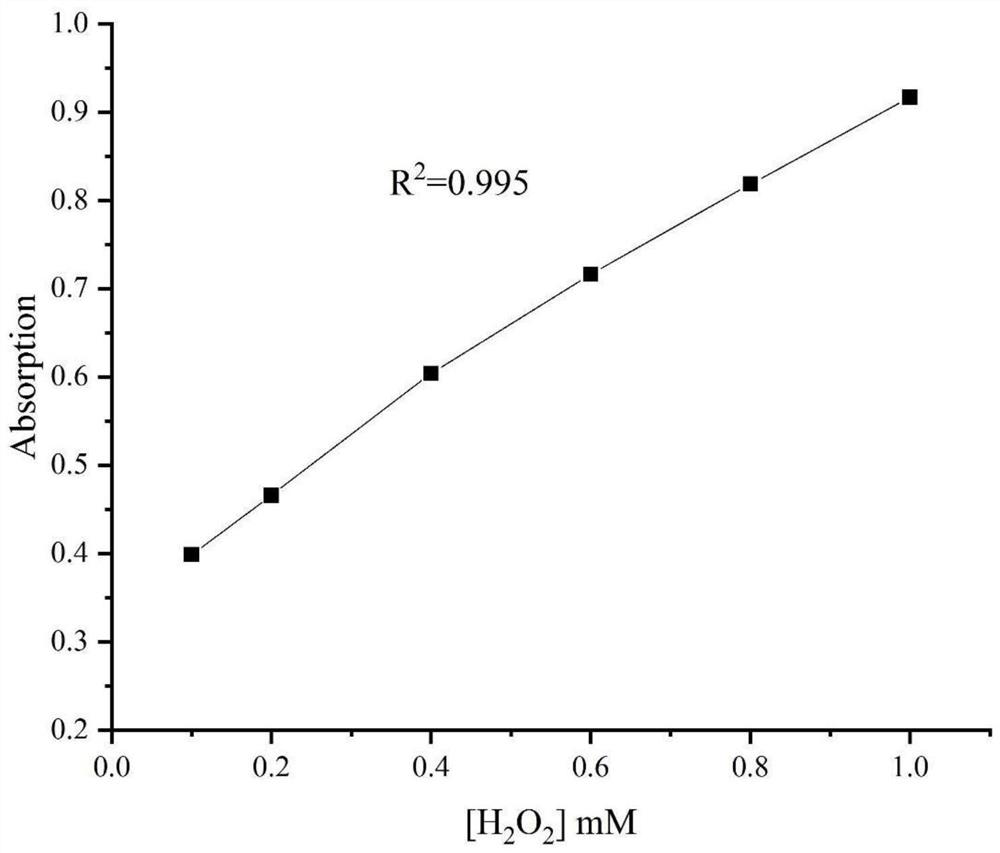
Method for detecting hydrogen peroxide and relevant target object on the basis of nanoprobe
ActiveCN110411990AAccurate determination of concentrationEasy to operateFluorescence/phosphorescenceOxidation reductionUric acid
The invention belongs to the technical field of biological detection, and discloses a method for detecting a hydrogen peroxide and a relevant target object on the basis of a nanoprobe. The method adopts a water-soluble rare earth doped NaCeF4 nanomaterial as a fluorescence probe, rare earth ion luminescence is quenched through the oxidation-reduction reaction of the hydrogen peroxide and a ceriumion, and the change of doped rare earth ion fluorescence intensity is used for realizing the detection of hydrogen peroxide concentration. The method can be used for detecting the hydrogen peroxide instandard solution or reactants in an enzymatic reaction for generating the hydrogen peroxide, and the detection of the hydrogen peroxide, biological enzymes or zymolyte (such as uric acid) in serumscan be realized. The method has the advantages of being convenient in operation, good in anti-interference performance, quick, sensitive, economic, practical and the like, a theoretical foundation andtechnical support are provided for solving the real-time detection of the relevant substances of uric acid and hydrogen peroxide generation systems in a complex system, and the method has certain clinic application potential.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Method for determining concentration of tadalafil in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927307AThe pretreatment method is simpleGood peak shapeComponent separationChemistryChromatography column
The invention discloses a method for determining the concentration of tadalafil in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by usinga chromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of tadalafil; according to the method, the linear range of a plasma standard curve is 1-600ng / mL, the intra-batch precision RSD and theinter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of tadalafil in plasma.
Owner:徐州立兴佳正医药科技有限公司
Method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry
InactiveCN109541107AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventGas chromatography–mass spectrometry
The invention discloses a method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry. A liquid chromatography mass spectrometry system is used for determining. A sample to be determined is added with a certain amount of mixed organic solvent to extract twice, is separated through a chromatographic column after pretreatment, and is detected by a massspectrometer. The method is rapid, accurate, high in sensitivity, and simple and convenient to operate. The method provides a basis for the blood drug concentration determination of carbamazepine. Thelinear range of a plasma standard curve in the method is 5 to 1000 ng / mL. The intra-batch and inter-batch precision RSD are less than + / -15%. The method is suitable for determining the concentrationof carbamazepine in the plasma.
Owner:徐州立顺康达医药科技有限公司
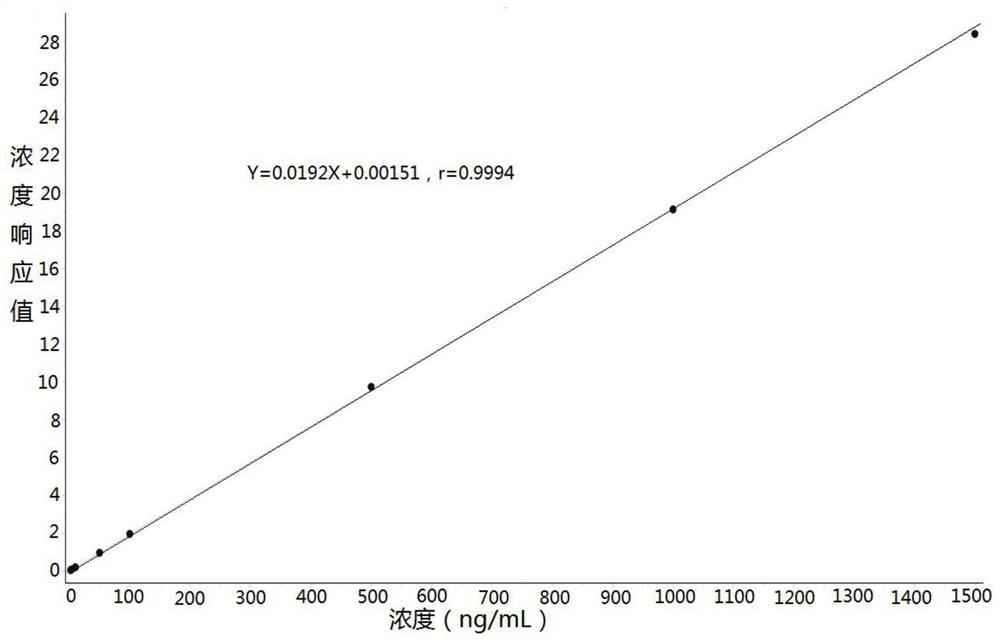
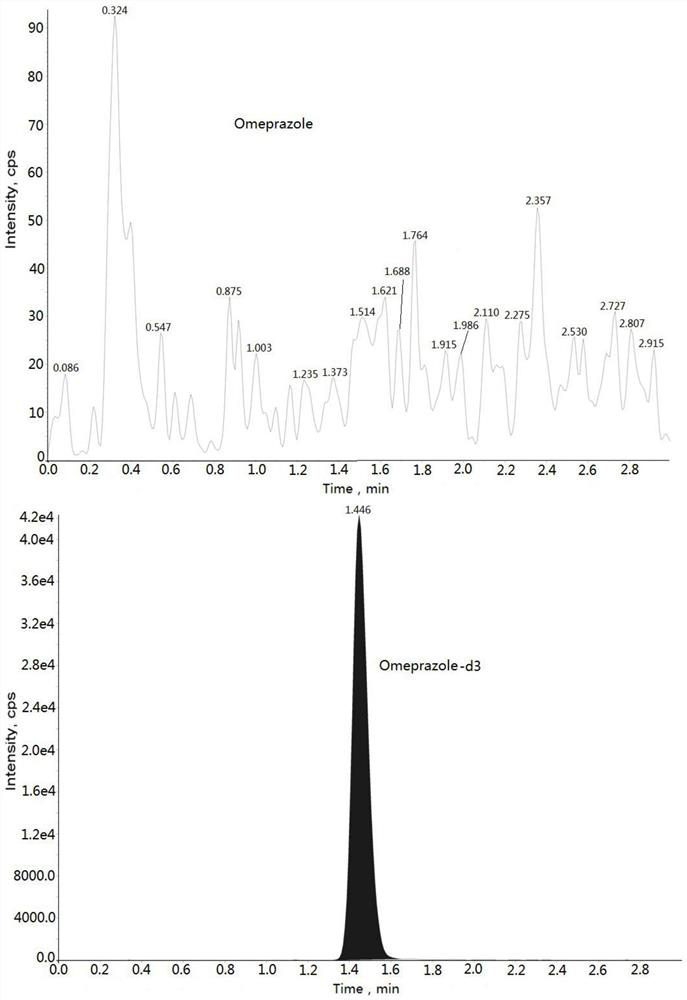
Method for determining concentration of omeprazole in plasma by liquid chromatography-mass spectrometry
InactiveCN112782323AThe method is simpleStrong specificityComponent separationChromatography columnOmeprazole
The invention discloses a method for determining the concentration of omeprazole in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the steps of firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of omeprazole; the linear range of a plasma standard curve of the method is 2 to 1500ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for determining the concentration of omeprazole in plasma.
Owner:徐州立顺康达医药科技有限公司
Preparation method of peroxidase-like nano-enzyme beta-FeOOH and application of peroxidase-like nano-enzyme beta-FeOOH in H2O2 detection
ActiveCN111965136AAccurate measurementImprove responseMaterial analysis by observing effect on chemical indicatorMetal/metal-oxides/metal-hydroxide catalystsPeroxidaseHydrolysis
The invention discloses a preparation method of peroxidase-like nano-enzyme beta-FeOOH and application of peroxidase-like nano-enzyme beta-FeOOH in H2O2 detection, and belongs to the technical field of inorganic nano-materials. The peroxidase nano-enzyme beta-FeOOH is obtained through a simple one-step low-temperature hydrolysis precipitation method, H2O2 can be catalyzed to generate hydroxyl freeradicals with high oxidability, and TMB is catalyzed to generate a chromogenic reaction in an extremely short time. Compared with a natural enzyme, the peroxidase-like nano-enzyme beta-FeOOH preparedby the method has the advantages of high stability, easiness in storage and simple process, in addition, the peroxidase-like nano-enzyme beta-FeOOH prepared by the method has higher affinity with H2O2, and is expected to be applied to detection of low-concentration H2O2.
Owner:SHAANXI UNIV OF SCI & TECH
Method for detecting carcinogenic dye in plastic packaging material
InactiveCN109060975AEasy to handleAccurate determination of concentrationComponent separationPlastic packagingMass Spectrometry-Mass Spectrometry
The invention discloses a method for detecting a carcinogenic dye in a plastic packaging material, and the method combines high performance liquid chromatography and mass spectrometry. The method adopts the plastic packaging material as an object, sample pretreatment is simple, no special purification treatment is required, the concentration of the high content carcinogenic dye in a target productcan be accurately determined, and the method is suitable for standardization.
Owner:厦门市产品质量监督检验院
Reaction device for photometric colorimetric detection and detection method
PendingCN111141681AReasonable structural designGuaranteed uptimeMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsEngineeringChemistry
The invention provides a reaction device for photometric colorimetric method detection and a detection method. The reaction device comprises a reaction main body unit, a detection unit, a liquid inletunit and a stirring unit. The detection unit comprises a light source emitting part and a light source signal collecting part; two opposite openings are formed in the side wall of the bottom of a reaction cup base; and the light source emitting part and the light source signal collecting part are embedded into the two openings respectively. A light source emitted by the light source emitting partirradiates a solution in the reaction cup to send a light intensity signal to the light source signal collecting part. According to the invention, the end point, namely a chemical variable point, oftitration analysis is accurately judged through a photometric colorimetric method, so that the concentration of liquid medicine is accurately and automatically analyzed.
Owner:安徽帝业分析仪器有限公司
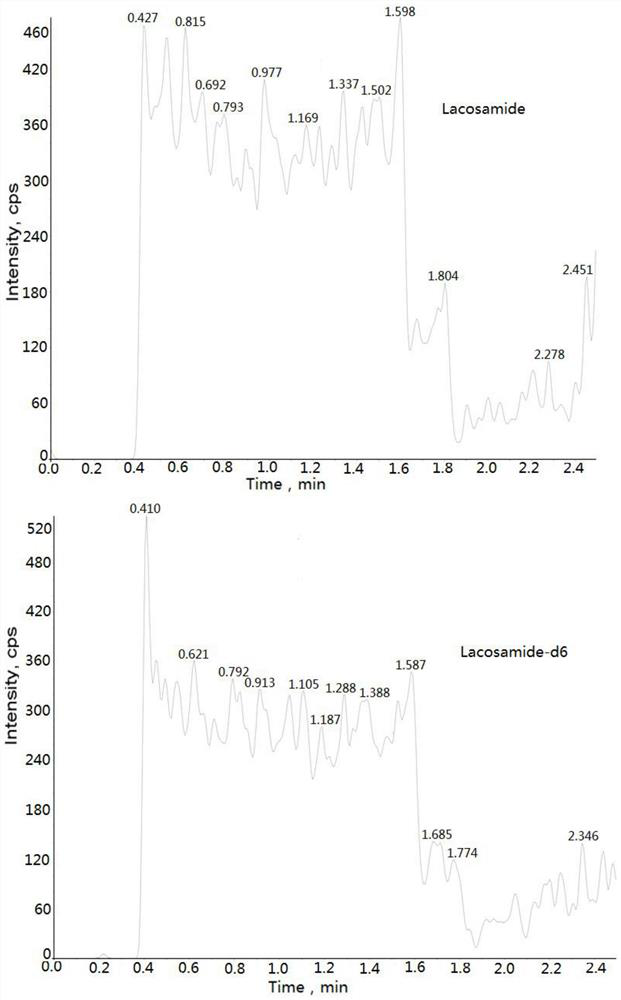
Method for determining concentration of lacosamide in blood plasma by liquid chromatography-mass spectrometry
InactiveCN112630352AThe pretreatment method is simpleGood peak shapeComponent separationChromatography columnBlood plasma
The invention discloses a method for determining the concentration of lacosamide in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the blood concentration of lacosamide; and according to the method, the linear range of the plasma standard curve is 100-15000 ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of lacosamide in plasma.
Owner:徐州立顺康达医药科技有限公司
Method for measuring concentration of terazosin in plasma by liquid chromatography-mass spectrometry
InactiveCN112748205AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventTerazosin
The invention discloses a method for measuring the concentration of terazosin in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the plasma concentration of terazosin; and the linear range of a plasma standard curve of the method is 1-200 ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for measuring the concentration of terazosin in plasma.
Owner:徐州立顺康达医药科技有限公司
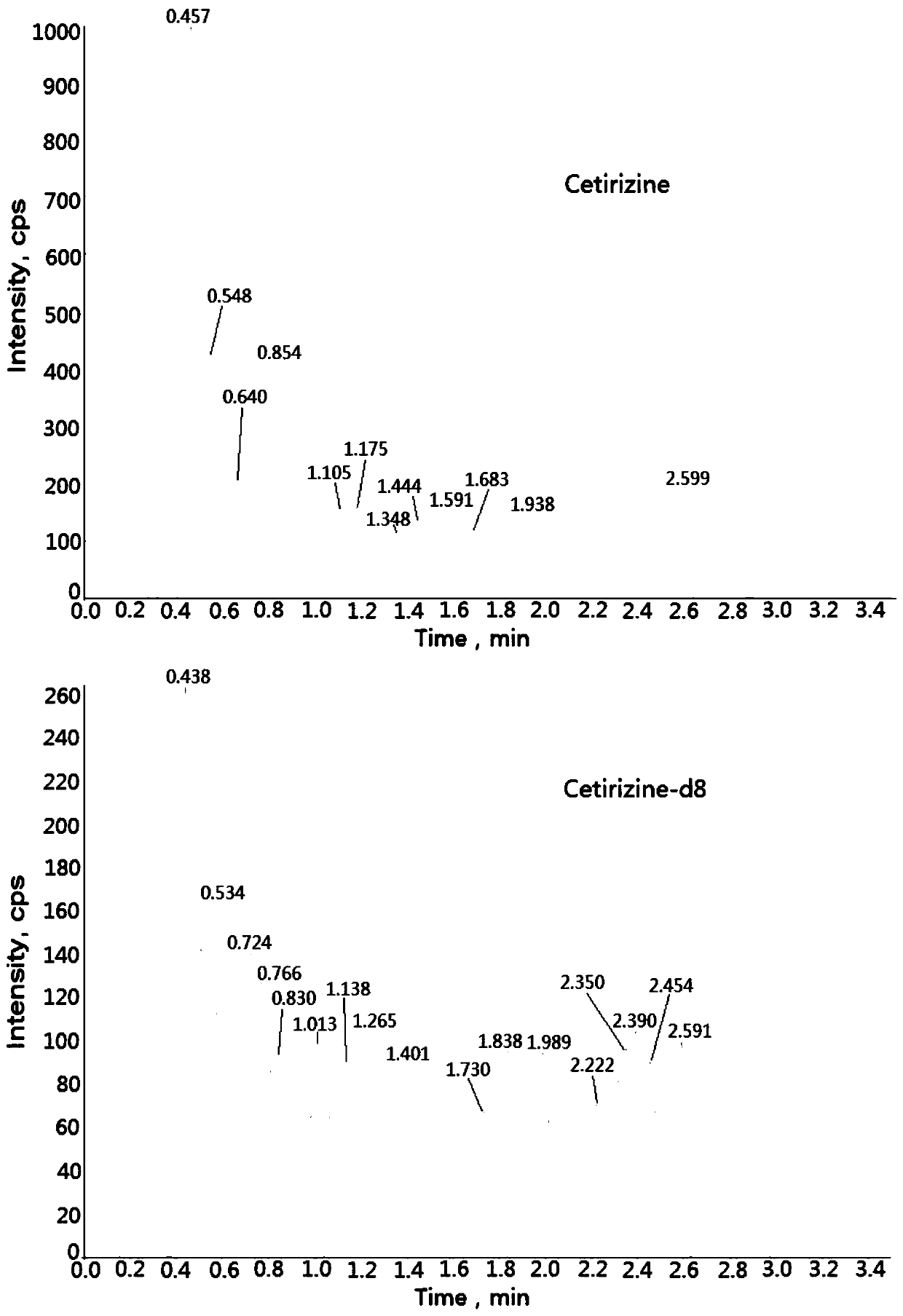
Method for determining concentration of cetirizine in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927308AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of cetirizine in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by using a chromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of cetirizine; according to the method, the linear range of the plasma standard curve is 1-600ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of cetirizine in plasma.
Owner:徐州立兴佳正医药科技有限公司
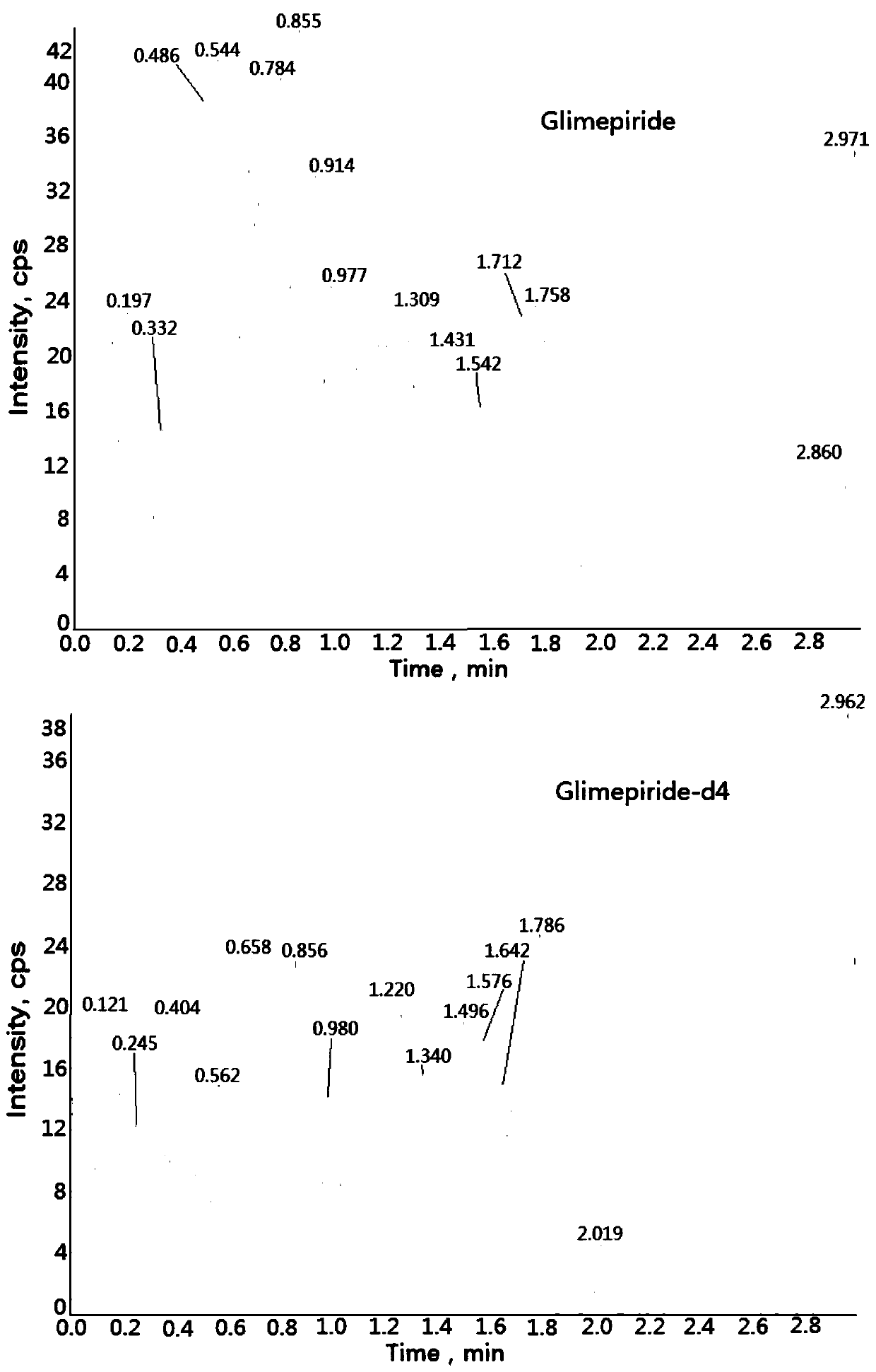
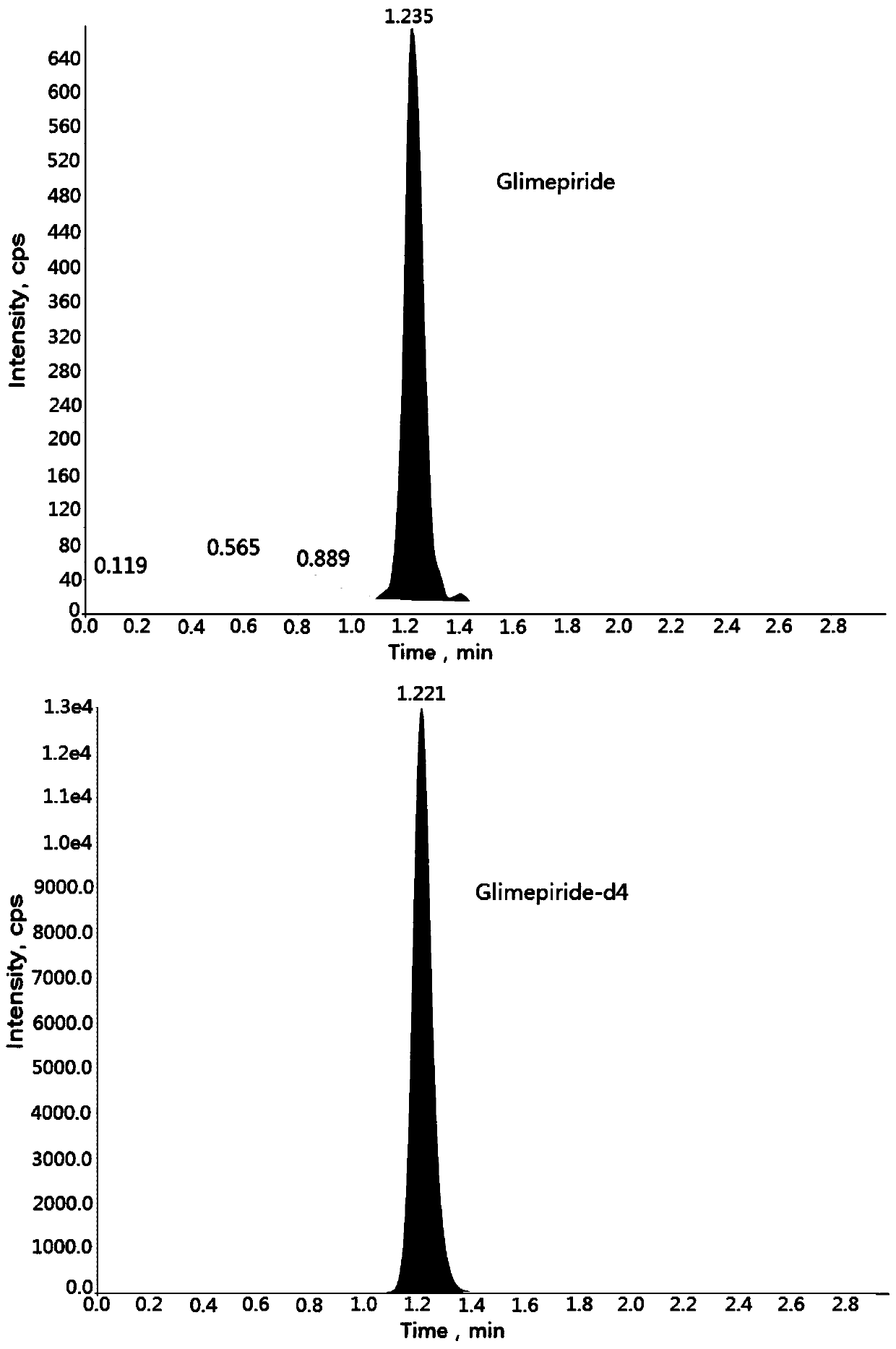
Method for determining concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927304AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be determined, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by achromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of glimepiride; the linear range of a plasma standard curve of the method is 0.5-500 ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of glimepiride in plasma.
Owner:徐州立兴佳正医药科技有限公司
Method for measuring cefadroxil concentration in plasma through hygroplasm combination
InactiveCN109682913AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring cefadroxil concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises the steps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of cefadroxil. The linear range of a plasma standard curve is within 0.1-60 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method is suitable for measurement of cefadroxil concentration in plasma.
Owner:徐州立兴佳正医药科技有限公司
Quantitative monitoring method for hexavalent chromium in foil washing water
InactiveCN109085128AAccurate determination of concentrationConcentration monitoringColor/spectral properties measurementsWastewaterVolumetric flask
The invention discloses a quantitative monitoring method for hexavalent chromium in foil washing water. The quantitative monitoring method comprises the following steps that hexavalent chromium standard color developing solutions of different concentrations are prepared, and a standard curve is made; the foil washing water to be tested is diluted to a required concentration, two 25mL volumetric flasks are taken, one volumetric flask is filled with mixed acid, a color developing agent and the diluted foil washing water to be tested, constant volume and uniform shaking are performed to obtain liquid to be tested, the other volumetric flask is filled with ultrapure water, mixed acid and a color developing agent, constant volume and uniform shaking are performed to obtain a blank sample, and the hexavalent chromium content in the liquid to be tested is determined according to the obtained standard curve; and the actual hexavalent chromium content of the foil washing water to be tested is calculated according to the hexavalent chromium content in the liquid to be tested. The method is simple in operation, low in cost and high in analysis speed, can accurately determine the concentrationof the hexavalent chromium in the foil washing water, and effectively monitors the hexavalent chromium concentration in a water body, thereby reducing the difficulty of treating hexavalent chromium waste water.
Owner:JIUJIANG TELFORD ELECTRONICS MATERIAL CO LTD
Method for determining concentration of serodosin and KMD-3213G in plasma by using liquid chromatography-mass spectrometry
InactiveCN109541109AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of serodosin and KMD-3213G in plasma by using liquid chromatography-mass spectrometry. The concentration of serodosin and KMD-3213G in the plasma is determined by using a liquid chromatography-mass spectrometry system; taking a sample to be tested first, and adding a certain amount of mixed organic solvent for extraction twice; after pretreatment, performing chromatographic column separation, and then detecting by using a mass spectrometry detector. The method for determining the concentration of serodosin and KMD-3213G in theplasma by using the liquid chromatography-mass spectrometry disclosed by the invention has the advantages of rapid, accurate, high sensitivity and simple operation, and provides a basis for determining the blood concentration of serodosin and KMD-3213G. According to the plasma standard curve of the method, the quantitative range of serodosin is 0.2-60 ng / mL, the quantitative range of KMD-3213G is0.2-40 ng / mL, and the intra-assay and inter-assay precisions RSDs are less than + / -15%, which is suitable for determining the concentration of serodosin and KMD-3213G in the plasma.
Owner:徐州立顺康达医药科技有限公司
Method for determining concentration of azithromycin in plasma by liquid chromatography-mass spectrometry
InactiveCN111044659AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for determining the concentration of azithromycin in plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the steps of taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, separating by a chromatographic column after pretreatment,and detecting by a mass spectrum detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the plasma concentration of azithromycin. According to the method, the linear range of a plasma standard curve is 1-500ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for determining the concentration of azithromycin in plasma.
Owner:南京立顺康达医药科技有限公司
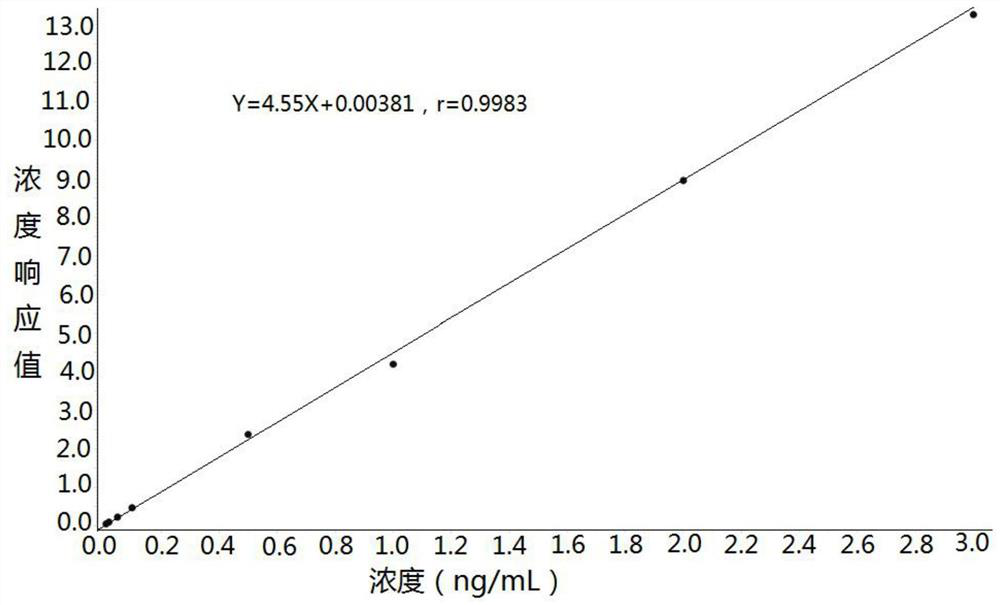
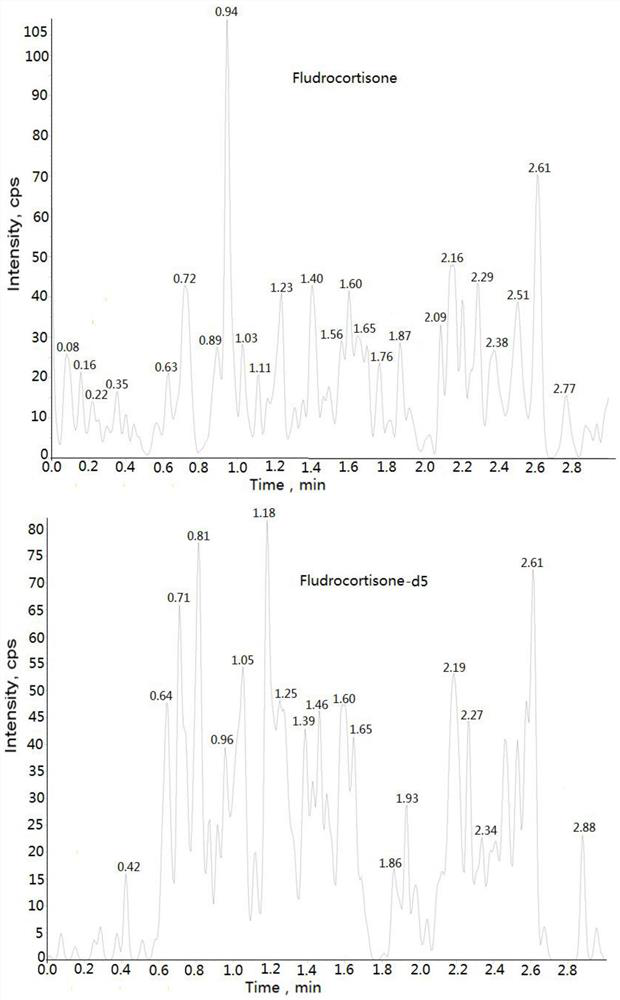
Method for detecting concentration of hydrocortisone in plasma by liquid chromatography-mass spectrometry
InactiveCN112763619AThe pretreatment method is simpleSuitable for routine determinationComponent separationFludrocortisoneBlood concentration
The invention discloses a method for detecting the concentration of hydrocortisone in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, pretreating, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the blood concentration of the hydrocortisone; and the linear range of the plasma standard curve of the method is 0.01-3 ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for measuring the concentration of the hydrocortisone in the plasma.
Owner:徐州立兴佳正医药科技有限公司
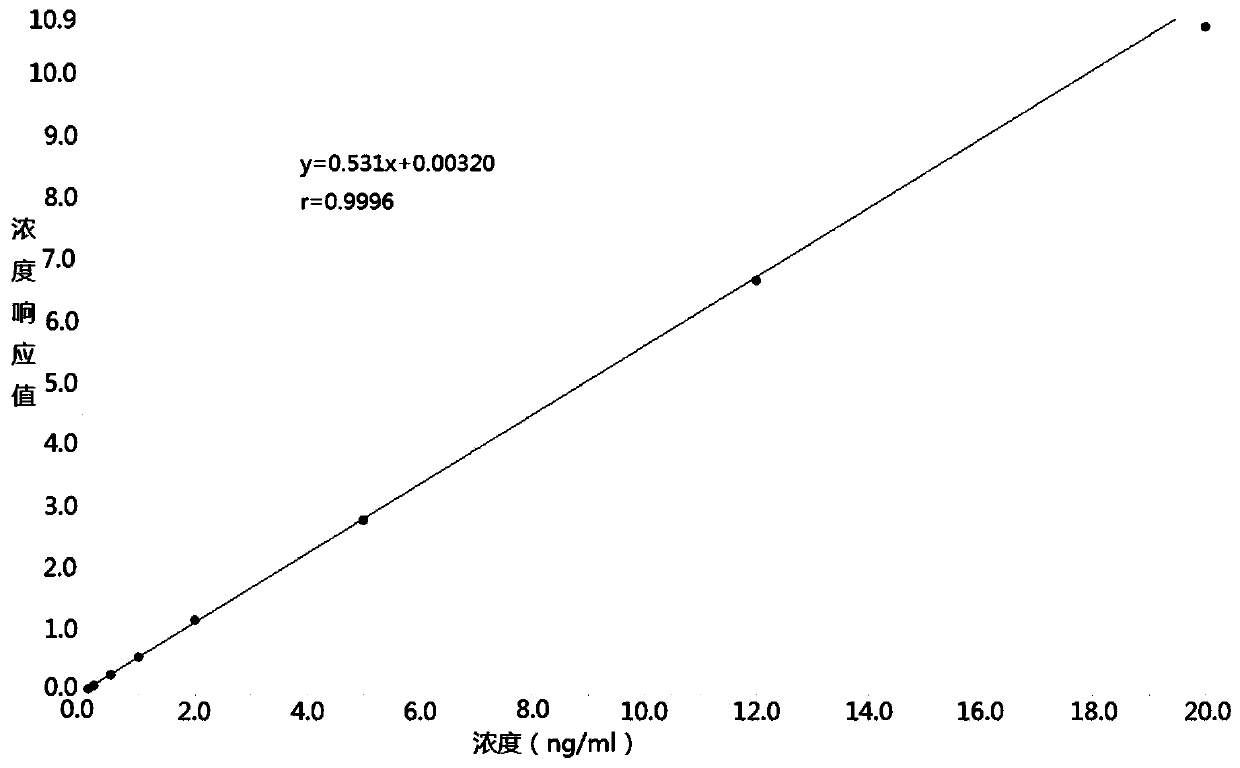
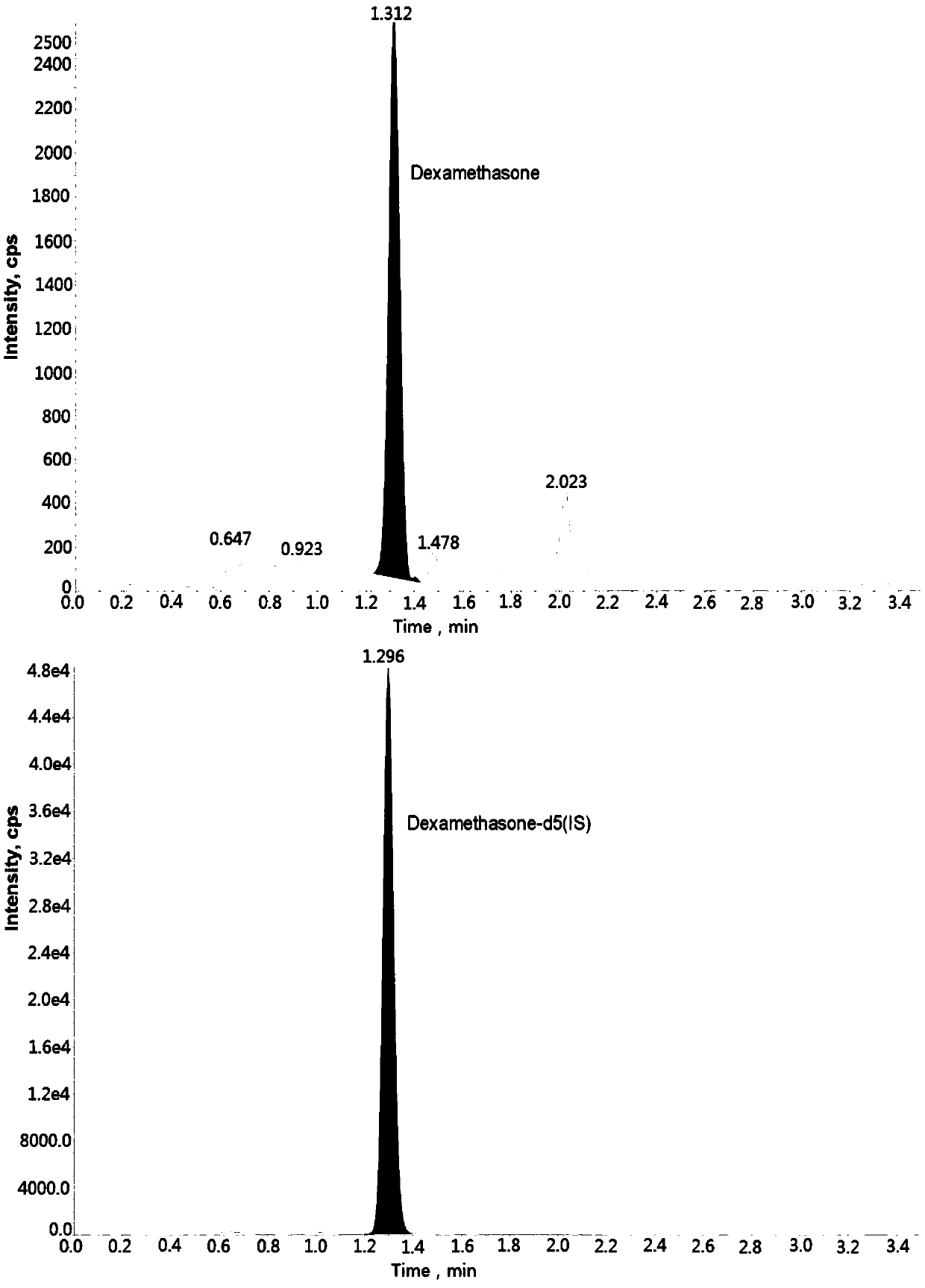
Method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry
InactiveCN109541108AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventGas chromatography–mass spectrometry
The invention discloses a method for determining concentration of dexamethason in plasma by liquid chromatography mass spectrometry. A liquid chromatography mass spectrometry system is used for determining. A sample to be determined is added with a certain amount of mixed organic solvent to extract twice, is separated by a chromatographic column after pretreatment, and is detected by a mass spectrometer. The method is rapid, accurate, high in sensitivity, and simple and convenient to operate. The method provides a basis for the blood drug concentration determination of dexamethason. The linearrange of a plasma standard curve in the method is 0.1 to 20 ng / mL. The intra-batch and inter-batch precision RSD are less than + / -15%. The method is suitable for determining the concentration of dexamethason in the plasma.
Owner:徐州立顺康达医药科技有限公司
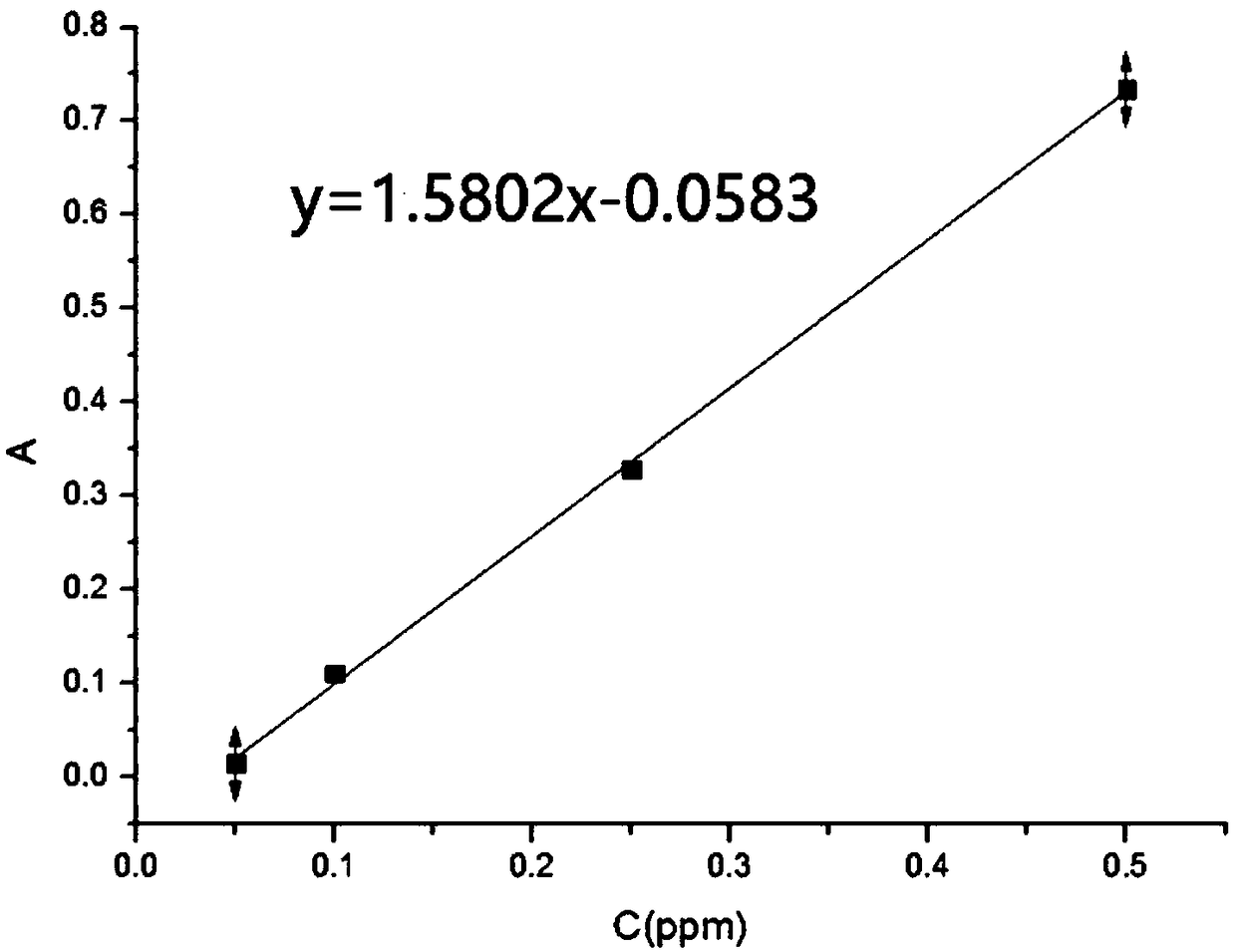
Analytical measurement method for didecyl dimethyl ammonium chloride in seawater
ActiveCN103760123AAccurate determination of concentrationAvoid large deviationPreparing sample for investigationColor/spectral properties measurementsOrganic layerSodium thiosulfate
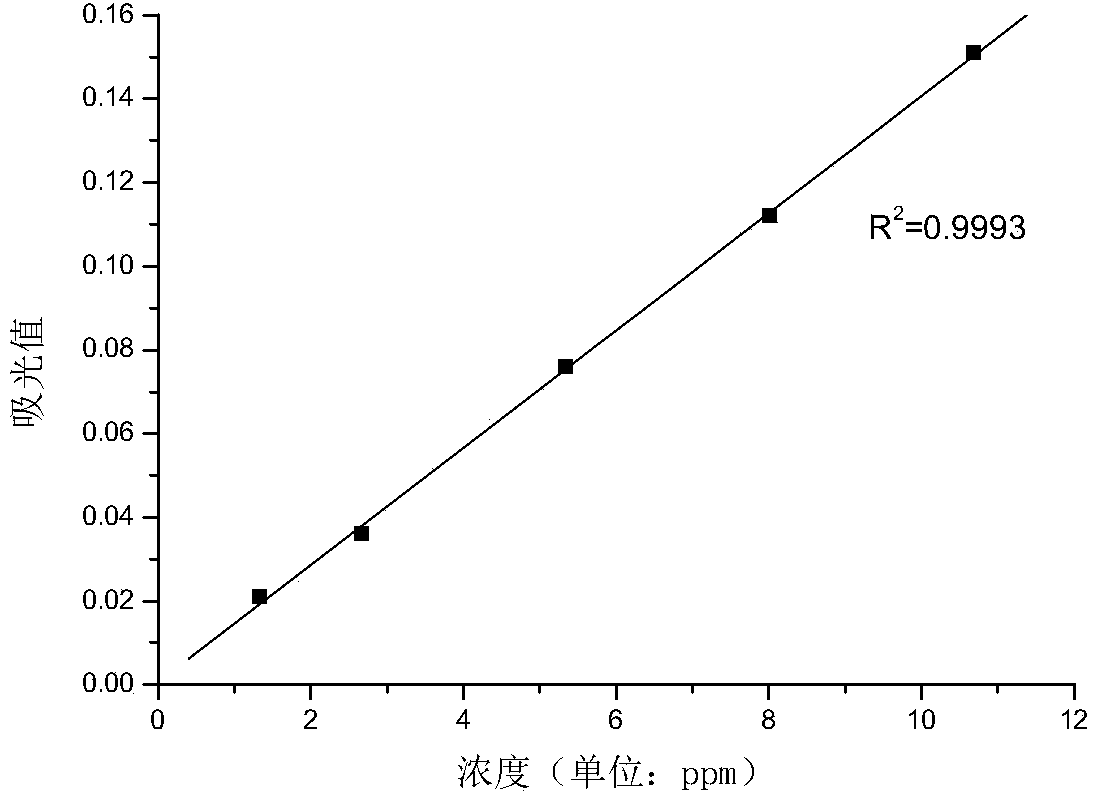
The invention discloses an analytical measurement method for didecyl dimethyl ammonium chloride in seawater. The method comprises the following steps of (1) preparing a standard solution of didecyl dimethyl ammonium chloride, charging gradient volumes of standard solution into separating funnels, adding the seawater, a sodium thiosulfate aqueous solution and a color-developing agent solution into each funnel, controlling the pH, and performing shaking uniformizing and complete standing; (2) adding an organic extracting agent into each separating funnel for extraction; (3) extracting an organic layer solution, measuring a light absorption value of the organic layer solution, performing zero adjustment by using the organic extracting agent, and drawing a standard curve by using the organic layer solution as a blank reference solution; (4) measuring the seawater to be measured into the separating funnels, adding the sodium thiosulfate aqueous solution and the color-developing agent solution into the separating funnels, controlling the pH, and performing shaking uniformizing and complete standing; (5) adding the organic extracting agent into each separating funnel for extraction; and (6) extracting an organic layer solution, measuring a light absorption value of the organic layer solution, and calculating the concentration of the didecyl dimethyl ammonium chloride in the seawater according to the standard curve. The method is accurate, reliable and easy to operate.
Owner:INST OF CHEM ENG GUANGDONG ACAD OF SCI
Traceable polymer and preparation method and application thereof
ActiveCN103694406AEasy to measureAccurate determination of concentrationFluorescence/phosphorescenceLuminescent compositionsSolventPyrene
The invention relates to a traceable polymer and a preparation method and application thereof. By adopting an emulsion polymerization method, water taken as a solvent, lauryl sodium sulfate used as an emulsifier, an ammonium persulfate and tetramethylethylenediamine redox system taken as an initiator, and 8-(4- benzyloxy-vinyl)-1,3,6-pyrene trisulfonic acid trisodium taken as a fluorescent tracer copolymerize with monomers acrylamide and 2-acrylamide-2-methyl propane sulfonic acid under certain temperature and pH conditions, so as to obtain the traceable polymer. The synthetic reaction is carried out under mild conditions, raw materials are easily available, the cost is low, the monomer conversion rate is high, products are easily processed, and the obtained traceable polymer can be applied to accurate determination of the concentration of the polymer, and a metal ion detector and has great potential application value in chemical analysis.
Owner:山东慕尔斯新材料科技有限公司
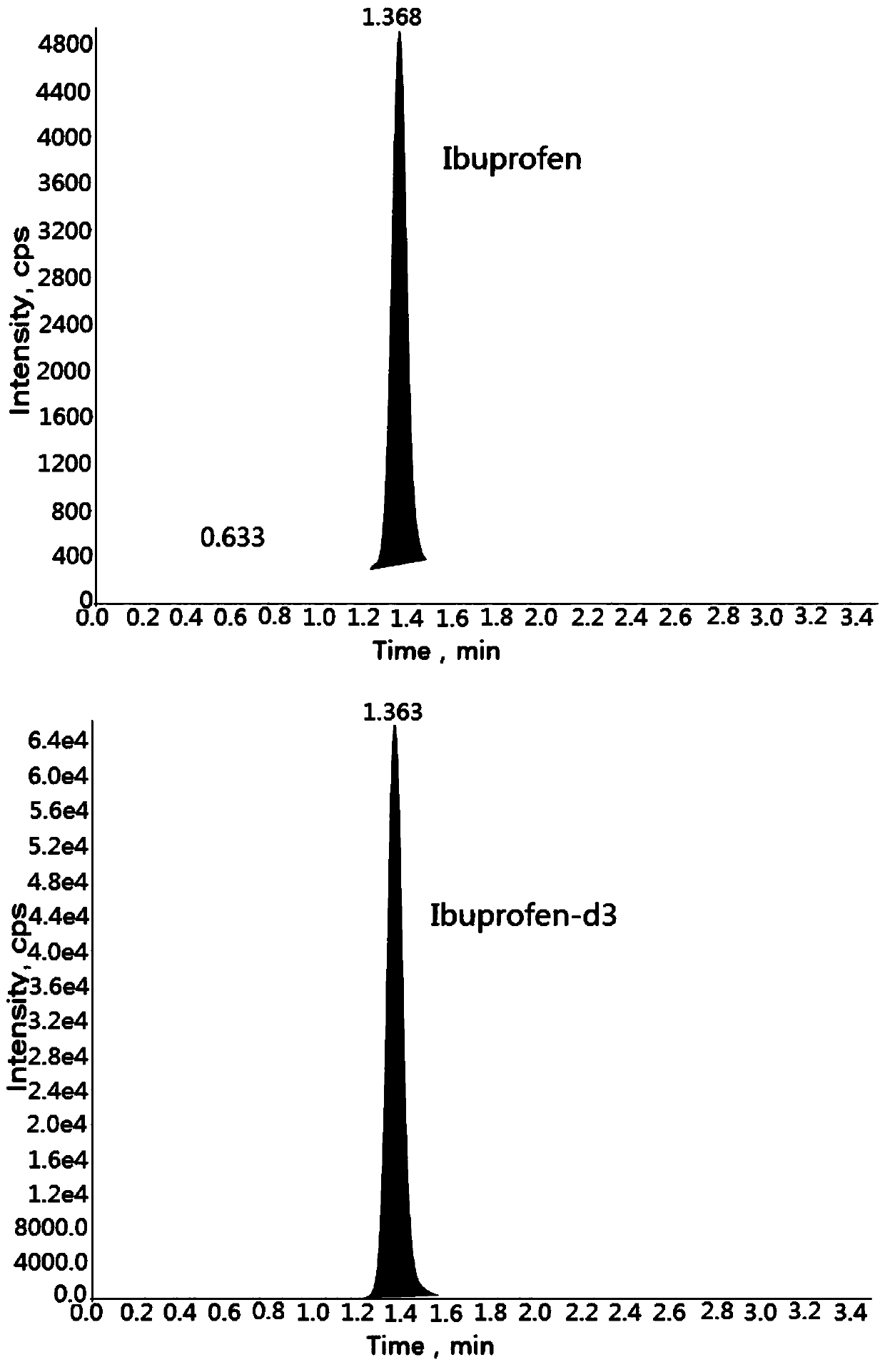
Method for determining concentration of ibuprofen in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927306AThe pretreatment method is simpleSuitable for routine determinationComponent separationBlood concentrationOrganic solvent
The invention discloses a method for determining the concentration of ibuprofen in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be determined, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by using a chromatographic column, and performing detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of ibuprofen; according to the method, the linear range of the plasma standard curve is 0.1-40 mu g / mL, the intra-batch precision RSD andthe inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of ibuprofen in plasma.
Owner:徐州立兴佳正医药科技有限公司
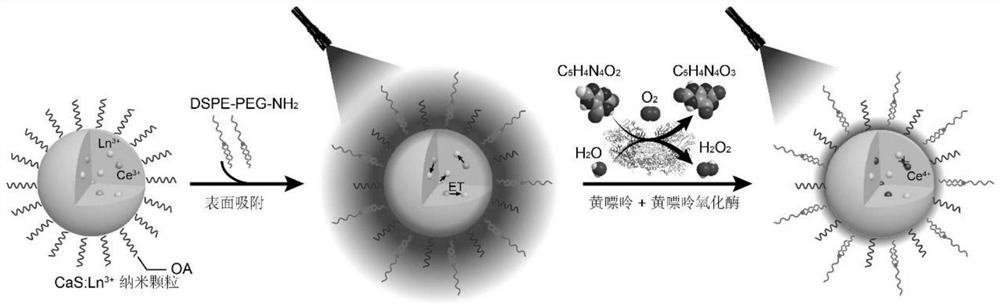
Method for detecting hydrogen peroxide and related targets based on CaS nano fluorescent probe
PendingCN111829993AAccurate determination of concentrationEasy to operateFluorescence/phosphorescenceFluoProbesRare earth ions
The invention belongs to the technical field of biological detection, and discloses a method for detecting hydrogen peroxide and related targets based on a nanoprobe. According to the method, a water-soluble rare earth doped CaS: Ce<3+> / Ln<3+> nano material is used as a fluorescent probe, rare earth ion luminescence is quenched through an oxidation-reduction reaction of hydrogen peroxide and cerium ions, and the concentration of hydrogen peroxide is detected by utilizing the change of the fluorescence intensity of doped rare earth ions. The method can be used for detecting hydrogen peroxide ina standard solution or reactants in an enzymatic reaction for generating hydrogen peroxide, can further realize the detection of hydrogen peroxide, biological enzyme or substrate (such as xanthine) in the serum, has the advantages of being simple and convenient to operate, good in anti-interference performance, rapid, sensitive, economical, practical and the like, can provide theoretical basis and technical support for real-time detection of hydrogen peroxide and hydrogen peroxide generation system related substances in a complex system, and has certain clinical application potential.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
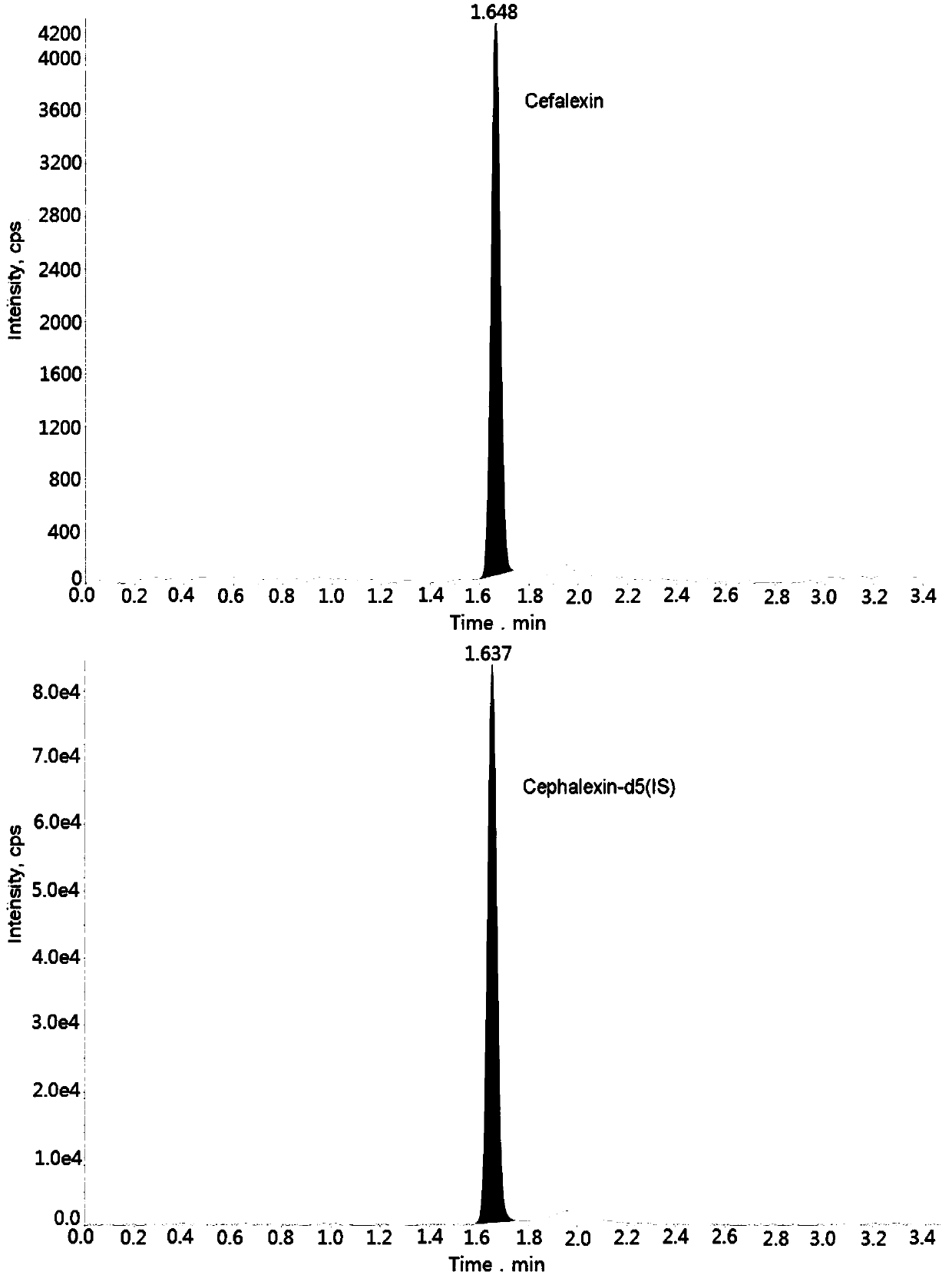
Method for measuring cefalexin concentration in plasma through hygroplasm combination
InactiveCN109682916AThe pretreatment method is simpleSuitable for routine determinationComponent separationCefalexinOrganic solvent
The invention discloses a method for measuring cefalexin concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises the steps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of cefalexin. The linear range of a plasma standard curve is within 0.1-50 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method is suitable for measurement of cefalexin concentration in plasma.
Owner:徐州立兴佳正医药科技有限公司
A kind of polystyrene butyl acrylate and its synthesis method and application
ActiveCN105085781BMeet the needs of profile monitoringAccurate determination of concentrationConstructionsBenzoyl peroxidePolystyrene
Owner:PETROCHINA CO LTD
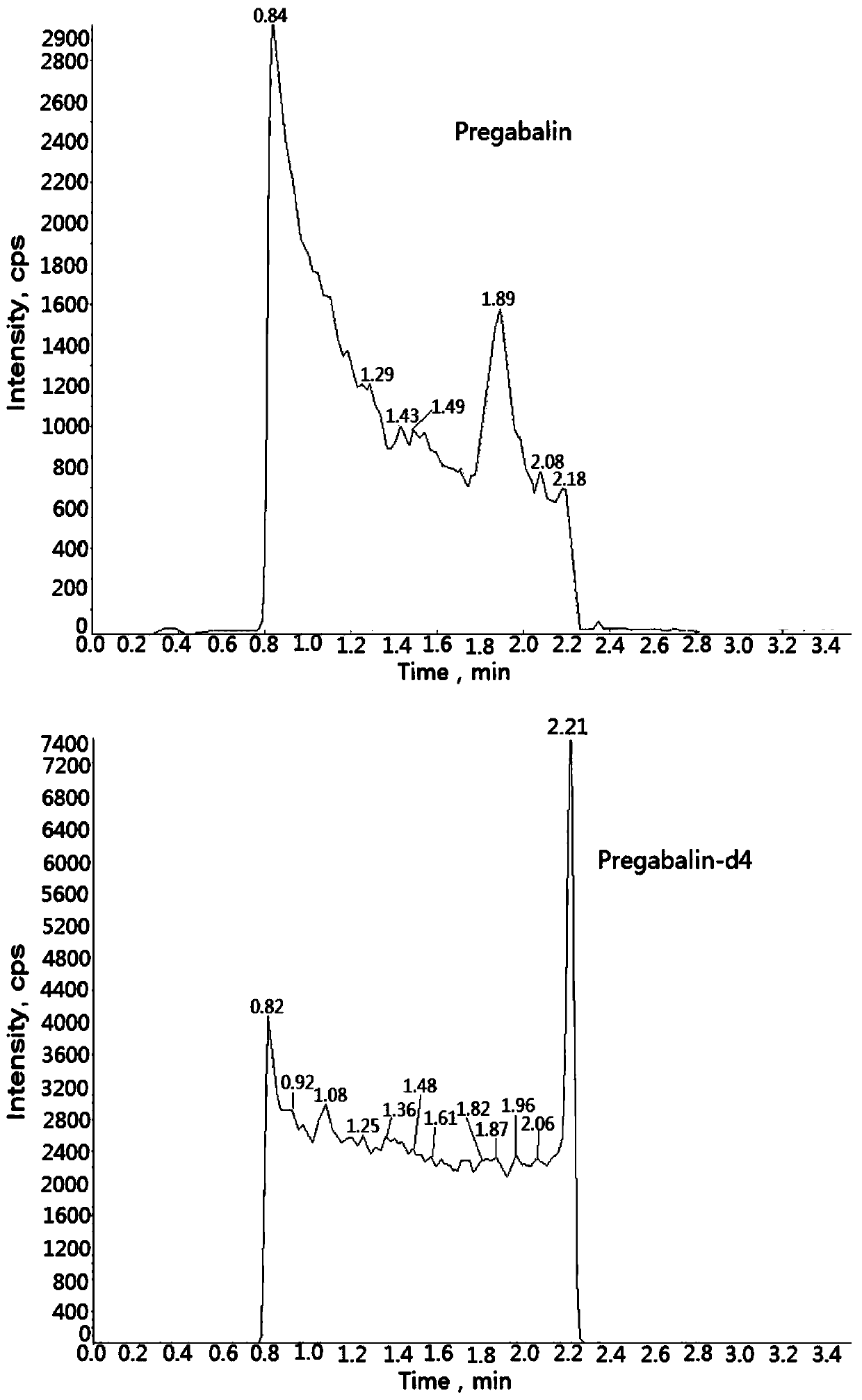
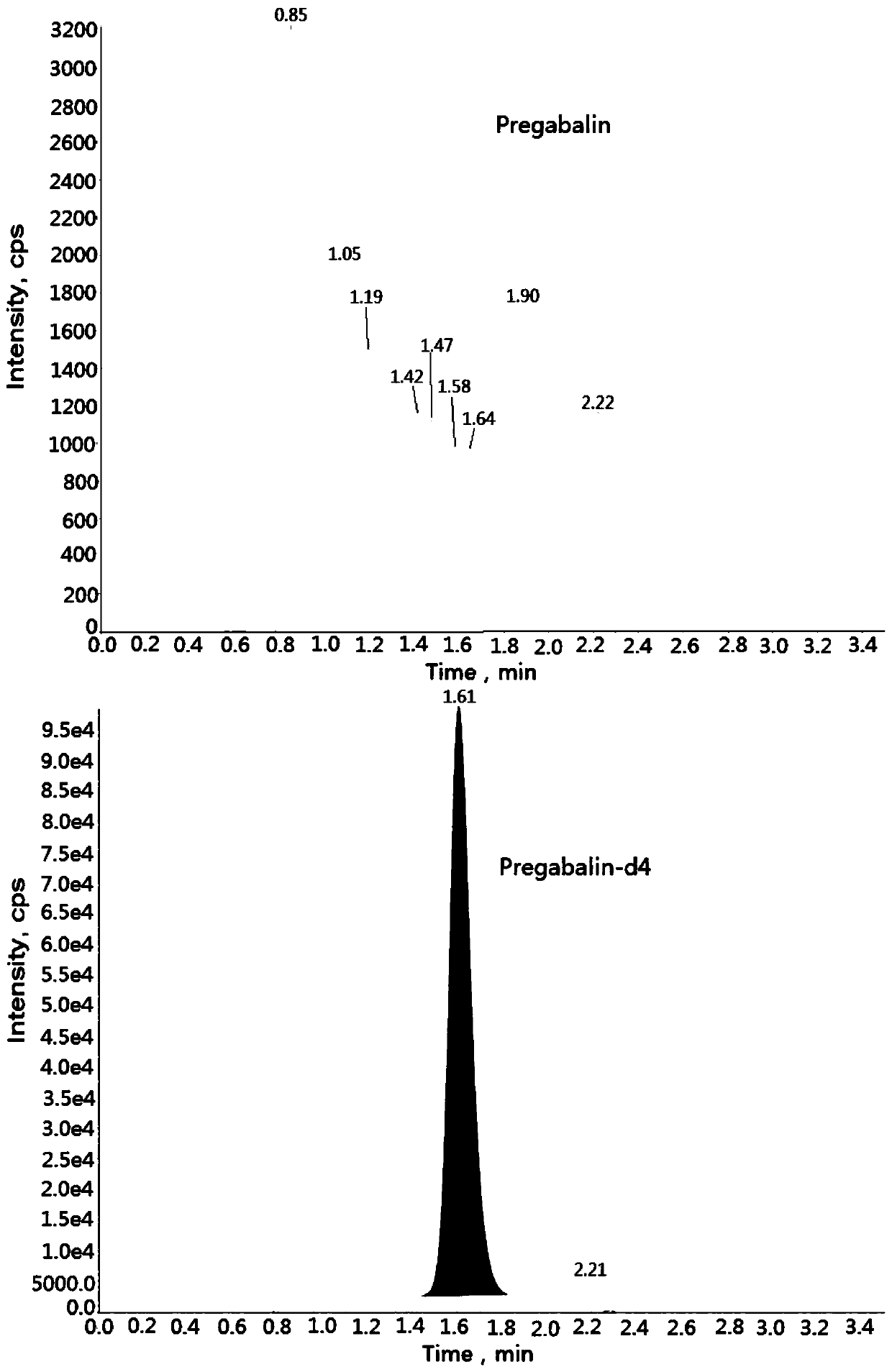
Method for determining concentration of pregabalin in plasma by liquid chromatography-mass spectrometry
InactiveCN111044662AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventPregabalin
The invention discloses a method for determining the concentration of pregabalin in plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system fordetermination and comprises the steps of taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, separating by using a chromatographic column after pretreatment, and detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the plasma concentration of pregabalin; and the linear range of a plasma standard curve of the method is 0.05-15[mu]g / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of pregabalin in plasma.
Owner:南京立顺康达医药科技有限公司
Method for measuring black carbon concentration and mixed state ratio in snow ice sample
InactiveCN109632593ARetain the original formAvoid the influence of the original stateColor/spectral properties measurementsParticle suspension analysisFreeze-dryingMixed states
The invention provides a sample injection processing device used for measuring the black carbon concentration and the mixed state ratio in a snow ice sample. The device comprises a vacuum pump, a three-way component, a movable adapter, a quartz freeze-drying container, a compressed air source, a cumulative flow meter and a flexible sampling bag. The vacuum pump and the compressed air source are connected with the movable adapter through the three-way component. A first valve is arranged on one end, which is close to the vacuum pump and the compressed air source, of the movable adapter. A cumulative flow meter is connected with the gas path of the compressed air source to measure the air flow output by the compressed air source. A movable joint is connected with the quartz freeze-drying container. A second valve is arranged in one end, which is away from the movable joint, of the quartz freeze-drying container. The quartz freeze-drying container is connected with the flexible samplingbag through the second valve. The sample injection processing device is connected with a single-particle black carbon photometer to detect a sample, which can retain the original form of black carbonin snow ice and can more accurately determine the black carbon concentration and the mixed state ratio.
Owner:中国科学院青藏高原研究所
Polystyrene methyl methacrylate and synthetic method therefor and application thereof
ActiveCN105085772AMeet the needs of profile monitoringAccurate determination of concentrationConstructionsDispersed mediaPolystyrene
The invention provides polystyrene methyl methacrylate and a synthetic method therefor and application thereof. The polystyrene methyl methacrylate is prepared by virtue of the method comprising the following step: by taking methyl methacrylate and polystyrene as reaction monomers, carrying out suspended polymerization reaction by taking water as a dispersing medium in the presence of a dispersant polyvinyl alcohol and an initiator azodiisobutyronitrile. The polystyrene methyl methacrylate provided by the invention has the advantages that the test result shows that the polystyrene methyl methacrylate prepared by the synthetic method can be fully dissolved in crude oil; the concentration of the polystyrene methyl methacrylate can be accurately measured by virtue of a gas chromatograph-mass spectrometer; the dosage is small; and the demand on monitoring an oil producing profile of a horizontal well is satisfied.
Owner:PETROCHINA CO LTD
Polystyrene ethyl isovalerate, and synthetic method and application thereof
ActiveCN105175618AMeet the needs of profile monitoringAccurate determination of concentrationConstructionsPolymer scienceBenzoyl peroxide
The invention provides a polystyrene ethyl isovalerate, and a synthetic method and an application thereof. The polystyrene ethyl isovalerate is prepared through a suspension polymerization reaction of ethyl isovalerate and styrene as reaction monomers with water as a dispersion medium in the presence of a dispersant polyvinyl alcohol and an initiator benzoyl peroxide. A test result shows that the polystyrene ethyl isovalerate prepared through the synthetic method can be completely dissolved in crude oil, the concentration of polystyrene ethyl isovalerate can be accurately determined through a gas chromatograph-mass spectrometer, the use amount is small, and horizontal well oil production profile monitoring needs are met.
Owner:PETROCHINA CO LTD
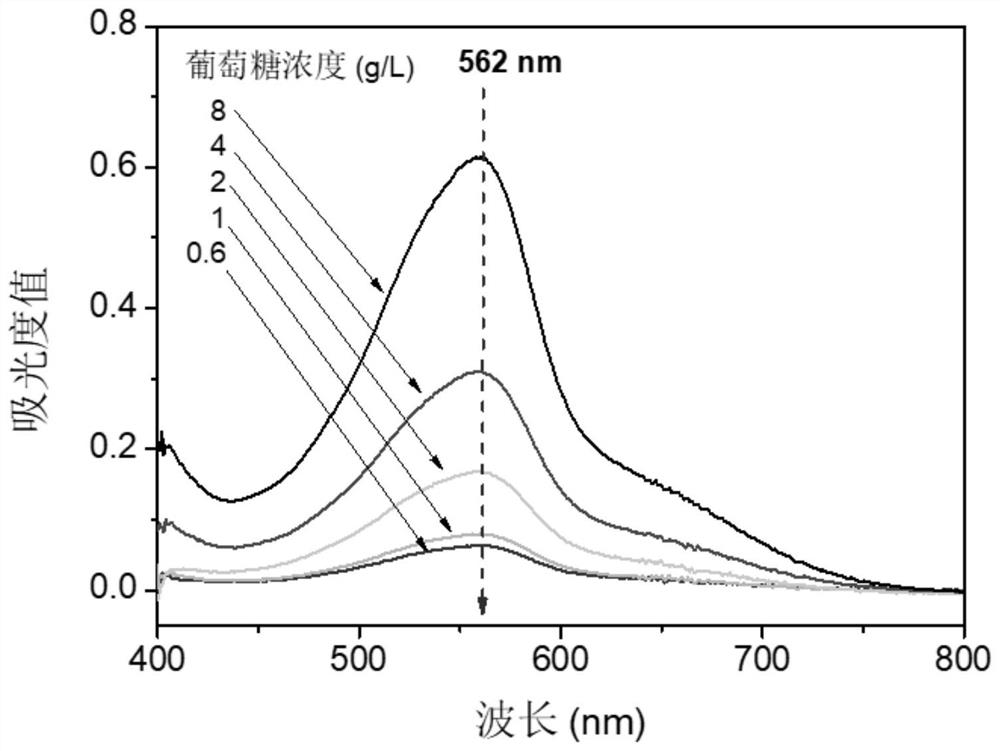
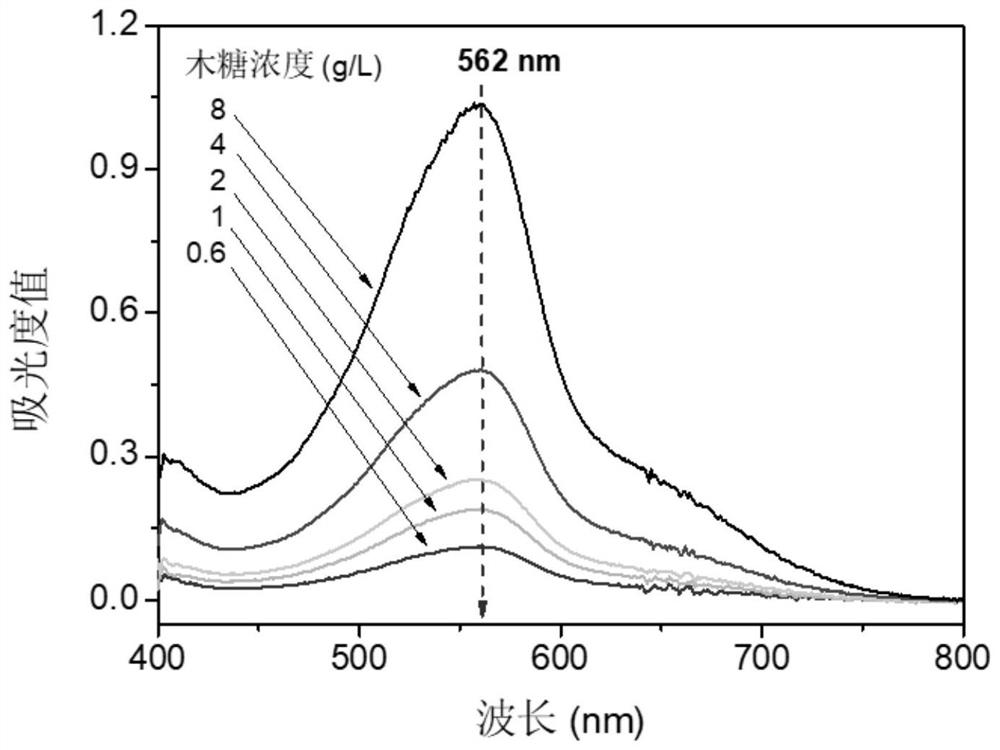
A method for determining the concentration of water-soluble peanut protein in a solution containing reducing monosaccharides
ActiveCN110988249BHigh dissolution rateEliminate distractionsComponent separationBiological material analysisIon chromatographyPhysical chemistry
Owner:FUJIAN AGRI & FORESTRY UNIV
A method for fluorescent detection of trace uranyl ions
ActiveCN107589098BAccurate determination of concentrationFluorescence/phosphorescenceMolecular sieveUranyl
The invention relates to a method for fluorescent detection of trace uranyl ions. The method comprises the following steps: (a) adding aesculin and a mesoporous molecular sieve into an aqueous solution containing uranyl ions and carrying out fluorescent detection to obtain the fluorescence intensity of the aqueous solution; (b) preparing a solution not containing uranyl ions and measuring the fluorescence intensity F<0> of the solution; (c) preparing a solution containing uranyl ions with a different concentration and measuring the fluorescence intensity F<n> of the solution; (d) drafting a curve with F<0> / F<n-1> as a vertical coordinate and the concentration of uranyl ions as a horizontal coordinate; (e) carrying out diluting according to the proportion of the aqueous solution obtained inthe step (a) until the concentration corresponding to the measured fluorescence intensity of the aqueous solution is in a range of 0.001 to 0.05 [mu]mol / L according to the curve drawn in the step (d), and multiplying the concentration by a multiple of the dilution. The method can accurately determine the concentration of uranyl ions in aqueous solutions and has a detection limit of as low as 6 nM.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com