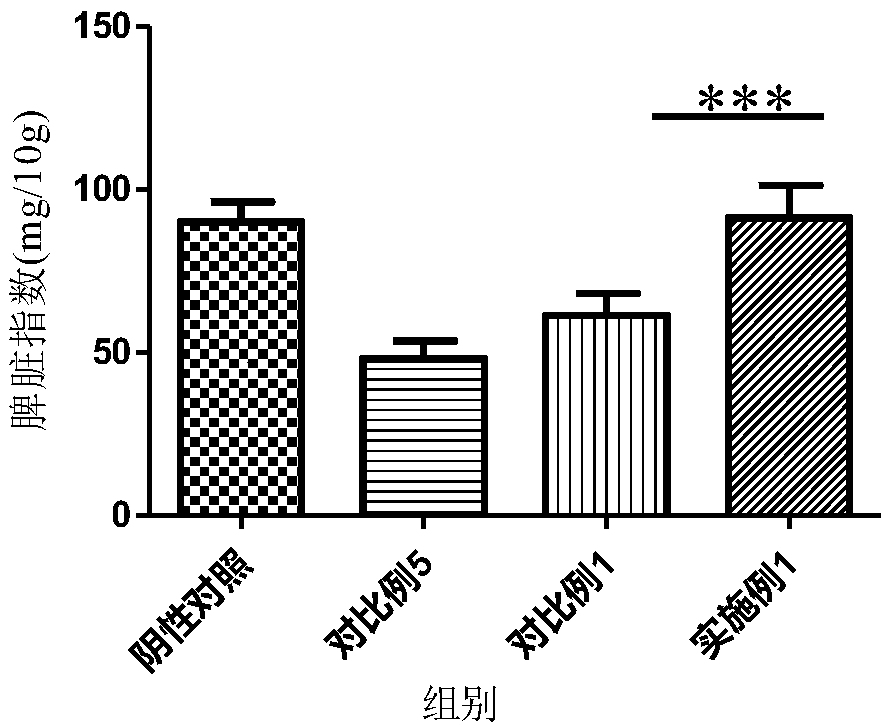
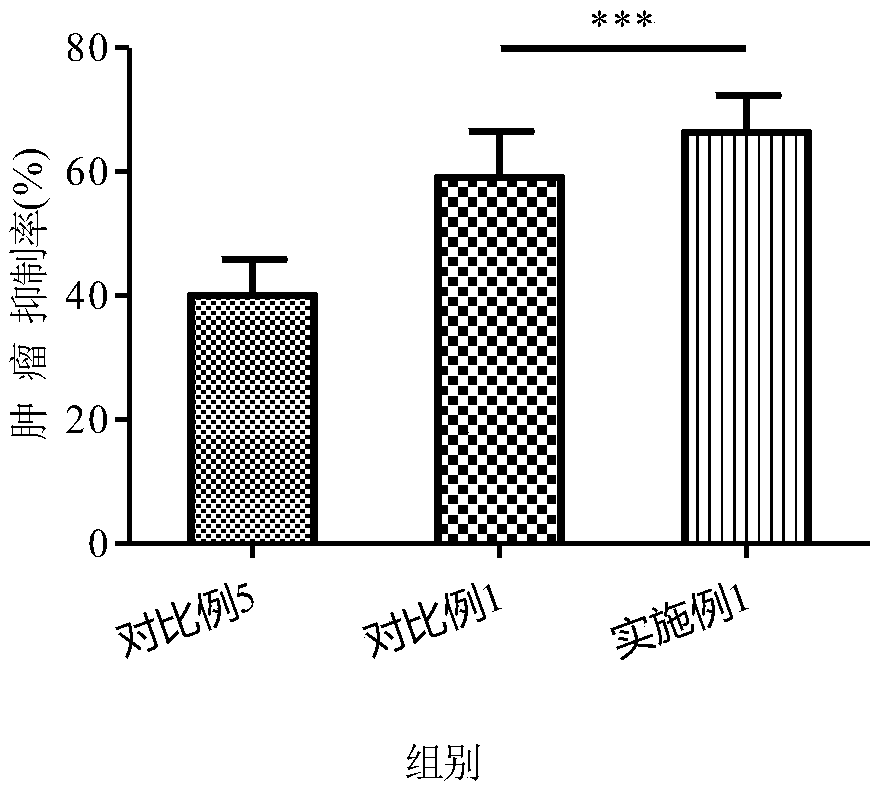
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
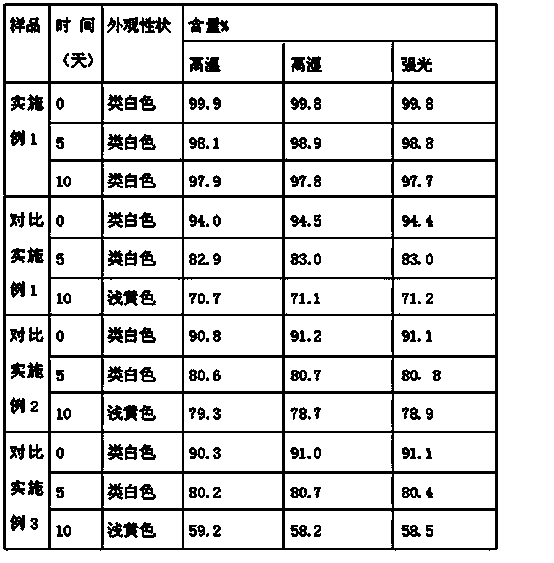
45 results about "Tamoxifen Citrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
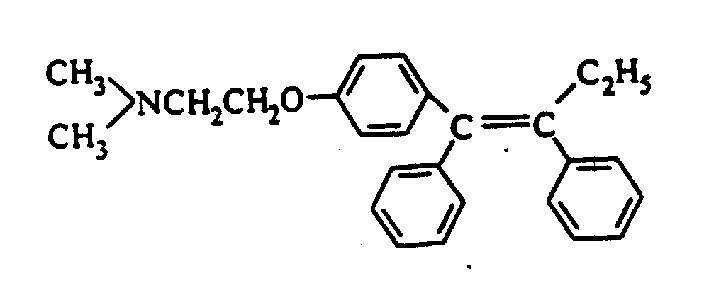
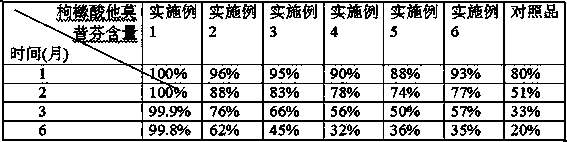
The citrate salt of an antineoplastic nonsteroidal selective estrogen receptor modulator (SERM). Tamoxifen competitively inhibits the binding of estradiol to estrogen receptors, thereby preventing the receptor from binding to the estrogen-response element on DNA. The result is a reduction in DNA synthesis and cellular response to estrogen. In addition, tamoxifen up-regulates the production of transforming growth factor B (TGFb), a factor that inhibits tumor cell growth, and down-regulates insulin-like growth factor 1 (IGF-1), a factor that stimulates breast cancer cell growth. Tamoxifen also down-regulates protein kinase C (PKC) expression in a dose-dependant manner, inhibiting signal transduction and producing an antiproliferative effect in tumors such as malignant glioma and other cancers that overexpress PKC.
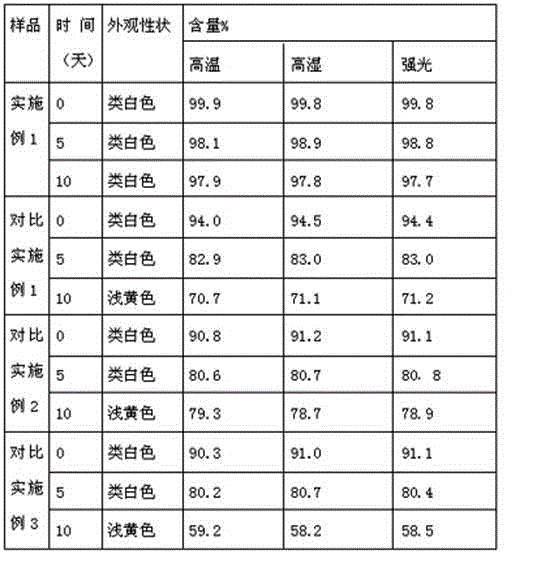
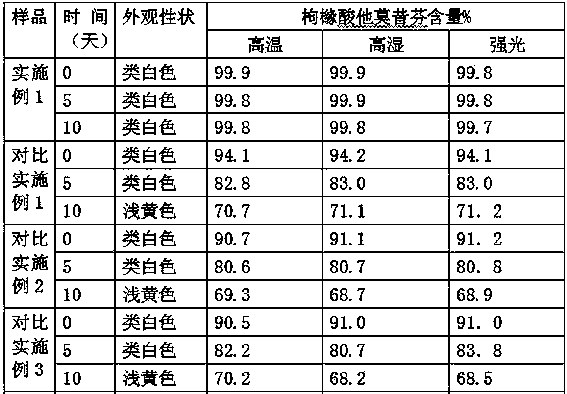
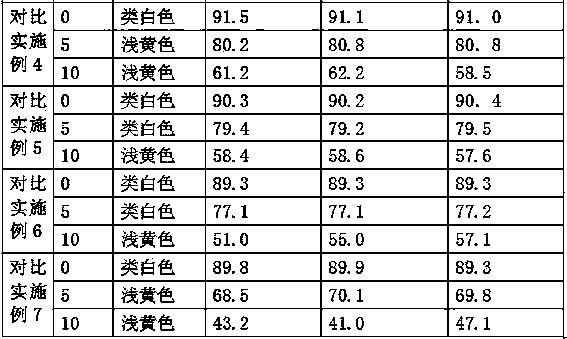
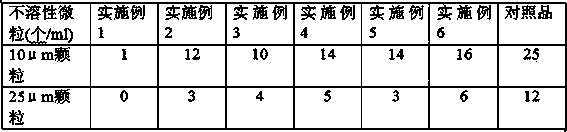
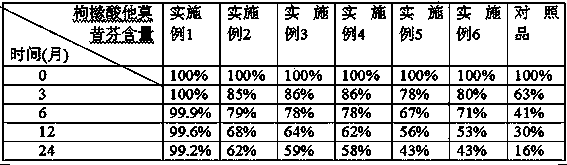
Dispersible tablet of tamoxifen citrate and preparation method thereof
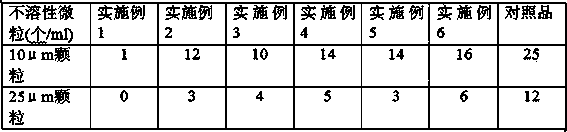
The dispersive tamosxifen citrate tablet is prepared through mixing tamosxifen citrate powder of 25 micron below size, microcrystal cellulose, lactose, PVP, sodium dodecyl sulfate and partial sodium carboxymethyl cellulose; pelletizing the mixture into disintegrated pellets of 0.5 mm below size; adding magnesium stearate and the rest sodium carboxymethyl cellulose; and tabletting into the dispersive tablet. The dispersive tamosxifen citrate tablet can disintegrate fast within 2 min and has fast medicine digesting speed and high bioavailability.
Owner:马晶
Dispersion tablet of Tamoxifen(Nolvedox) of cedrat acid as well as preparationmethod and usage
InactiveCN1539411AReduce stimulationInhibition formationOrganic active ingredientsPill deliveryAlcoholMedicine
A dispersing tablet of tamoxifen citrate is prepared from tamoxifen citrate, disintegrant, filler, lubricant and alcohol through sieving by 100 meshes, proportionally mixing, wet granulating, drying, sieving by 18-24 meshes, adding lubricant, and tabletting. It can be used for treating mastadenoma, melanocarcinoma, etc.
Owner:SHENYANG PHARMA UNIVERSITY
Solid dispersion of tamoxifen citrate, method for preparing same and application thereof
ActiveCN101732235AImprove bioavailabilityEasy to manufactureOrganic active ingredientsPeptide/protein ingredientsTamoxifen CitrateMedicine
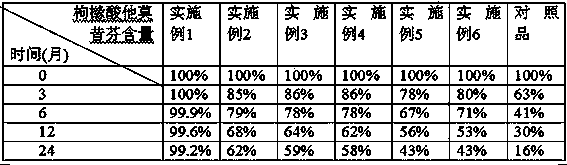
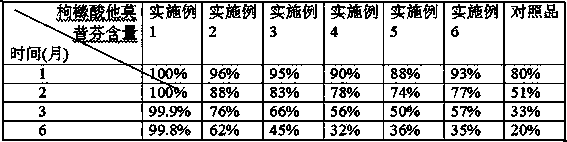
The invention discloses a solid dispersion of tamoxifen citrate. The solid dispersion of tamoxifen citrate consists of tamoxifen citrate, polyethylene glycol-6,000 and a flow aid, wherein the weight ratio of the tamoxifen citrate to the polyethylene glycol-6,000 is 1:4.5-12.5; and the flow aid is micropowder silica gel or talcpowder, and the dosage of the flow aid is 0.5 to 3 percent based on the total weight of the tamoxifen citrate and the polyethylene glycol-6,000. The solid dispersion of tamoxifen citrate can be made into orally taken solid preparations with high bioavailability and good absorbability, such as common tablets, dispersible tables, sustained release tablets and capsules, can be conveniently prepared, and the dissolution of the tamoxifen citrate can stably reach over 90 percent; and the preparation method has the advantages of low cost, easy operation, no special requirement on equipment, suitability for mass production and the like, and establishes a good relationship between the disintegration, dispersion and release speed and the medical dissolution of the solid dispersion of tamoxifen citrate.
Owner:上海复旦复华药业有限公司 +1
Preparation method of high-purity tamoxifen citrate
InactiveCN103450036ASave operating timeHigh yieldOrganic compound preparationCarboxylic acid salt preparationAlkyl transferIsomerization
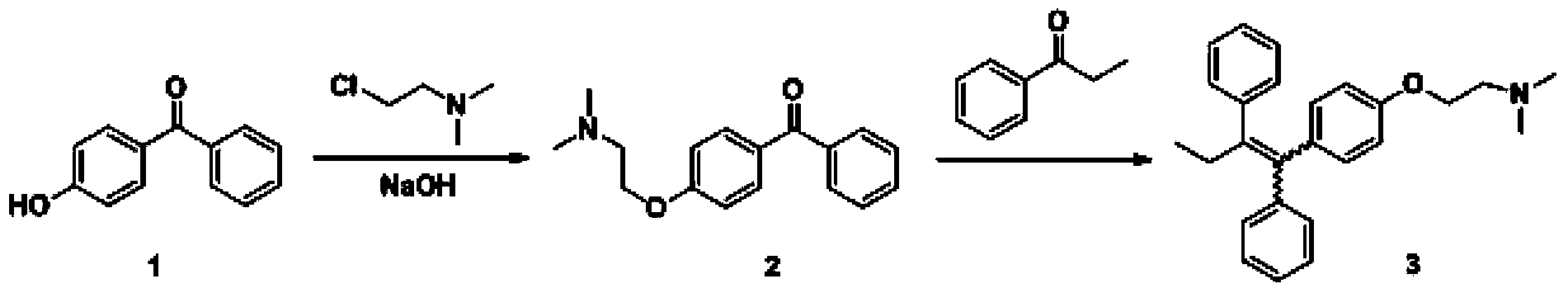
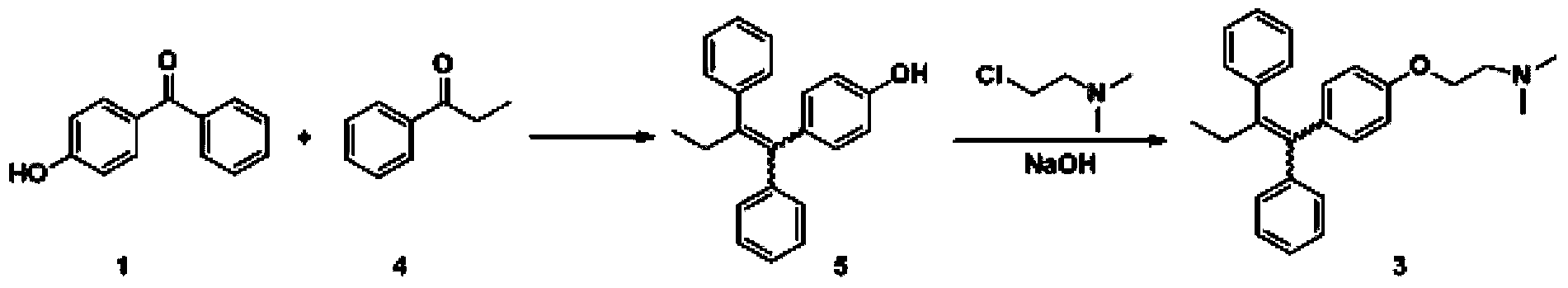
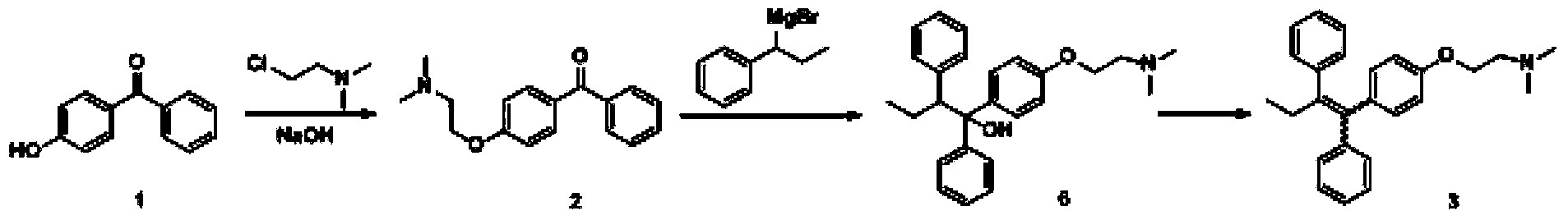
The invention discloses a preparation method of high-purity tamoxifen citrate. By improving the problems in the existing route, the invention discloses a novel synthesis method of high-purity low-E-isomer-content tamoxifen citrate. The method comprises the following steps: by using simple and accessible 4-hydroxybenzophenone as a raw material, carrying out coupling reaction and alkylation reaction to obtain a crude product 2-[4-(1,2-diphenyl1-butenyl)-phenoxy]-N,N-dimethylethylamine; directly carrying out isomerization reaction on the crude product, carrying out simple purification, and salifying with citric acid to conveniently obtain the high-purity tamoxifen citrate of which the E isomer content does not exceed 0.05%. The technique adopts a continuous feed mode, and only needs one-step purification, thereby solving the problems of high cost and difficulty in preparing high-purity tamoxifen citrate in pharmaceutical industry. The technique has the advantages of mild reaction conditions, high stability, high purity and high yield, is simple to operate, and provides an option for large-scale production.
Owner:ASYMCHEM LAB TIANJIN +4
Pharmaceutical formulation comprising bicalutamide
Owner:ASTRAZENCA UK LTD
Tamoxifen citrate freeze-dried powder injection
ActiveCN103494776ANo stabilizing effectOrganic active ingredientsPowder deliveryEthylenediamine tetraacetateMedicine
The invention relates to a tamoxifen citrate freeze-dried powder injection which comprises tamoxifen citrate, a composition of sorbitol, dextran and lactose in a weight ratio of 3.2:5.5:2, a composition of arginine and glutathione in a weight ratio of 1.6:5.7, and disodium ethylenediamine tetraacetate.
Owner:NANTONG GUANGTAI BIOCHEM PROD
Preparation of acidum citricum tamoxifen polylactic acid-glycolic co-polymer microballoons
InactiveCN101249082AGood biocompatibilityPromote degradationOrganic active ingredientsPharmaceutical delivery mechanismDrug contentMicrosphere
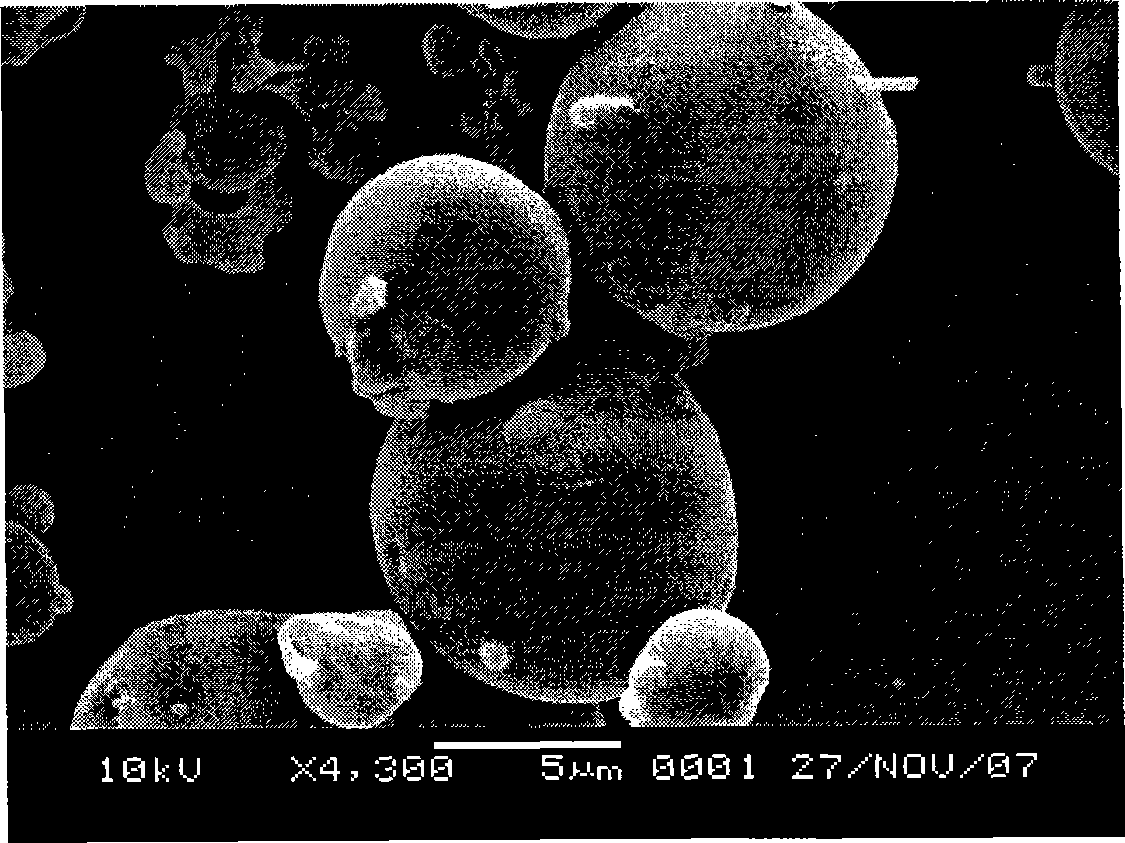
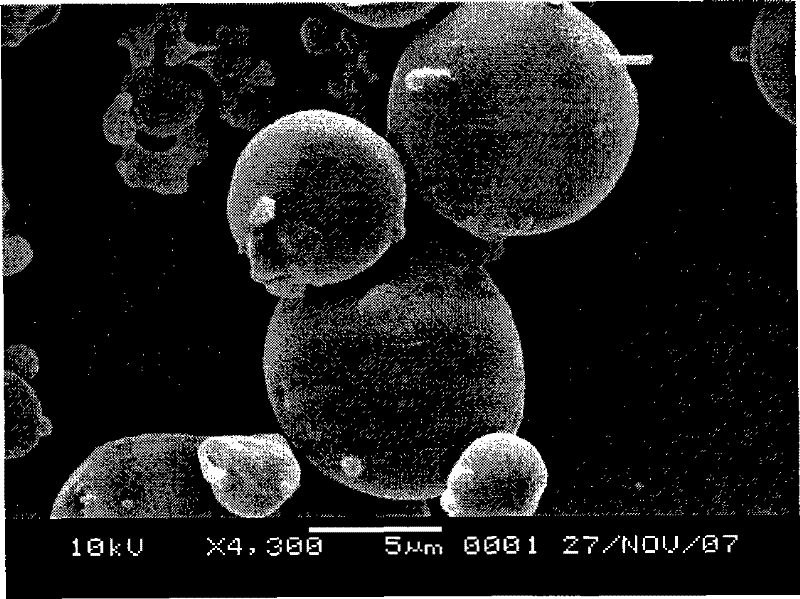
The invention relates to a preparation method of tamoxifen citrate polylactic acid-glycolic acid copolymer microspheres. The method comprises the following steps: 1) dissolving polylactic acid-glycolic acid copolymer (PLGA) in an organic solvent, adding tamoxifen citrate, and dissolving under ultrasonic action to obtain a solution as organic phase; 2) adding slowly the organic phase into aqueous solution of polyvinyl alcohol (PVA) as water phase under stirring, adding electrolytes, stirring at room temperature until the organic solvent volatilizes completely, centrifugating, washing, and freeze-drying to obtain tamoxifen citrate polylactic acid-glycolic acid copolymer microspheres. The microsphere has round shape, uniform particle size distribution (below 10 micrometers), high drug content (above 10%), high encapsulation rate (about 80%), and in vitro release characteristics of long-acting preparations; and is suitable for the use as implant for prevention and treatment of breast cancer.
Owner:DONGHUA UNIV
Methods of Treating Disorders Associated with Protein Aggregation
ActiveUS20140047569A9Eliminates a major bottleneck in the screening processImprove throughputCompounds screening/testingBiocideHuntingtons choreaCell Aggregations
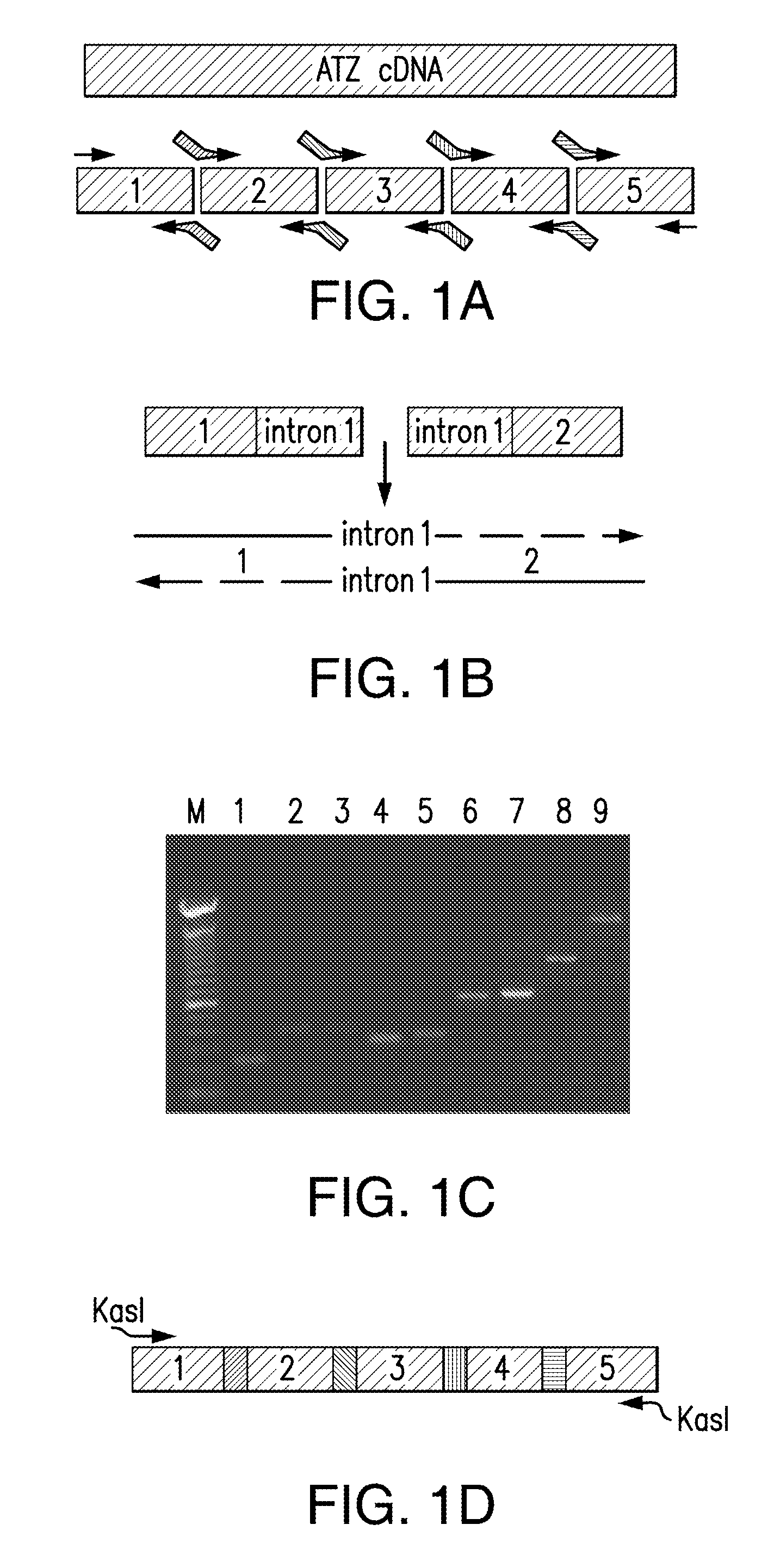
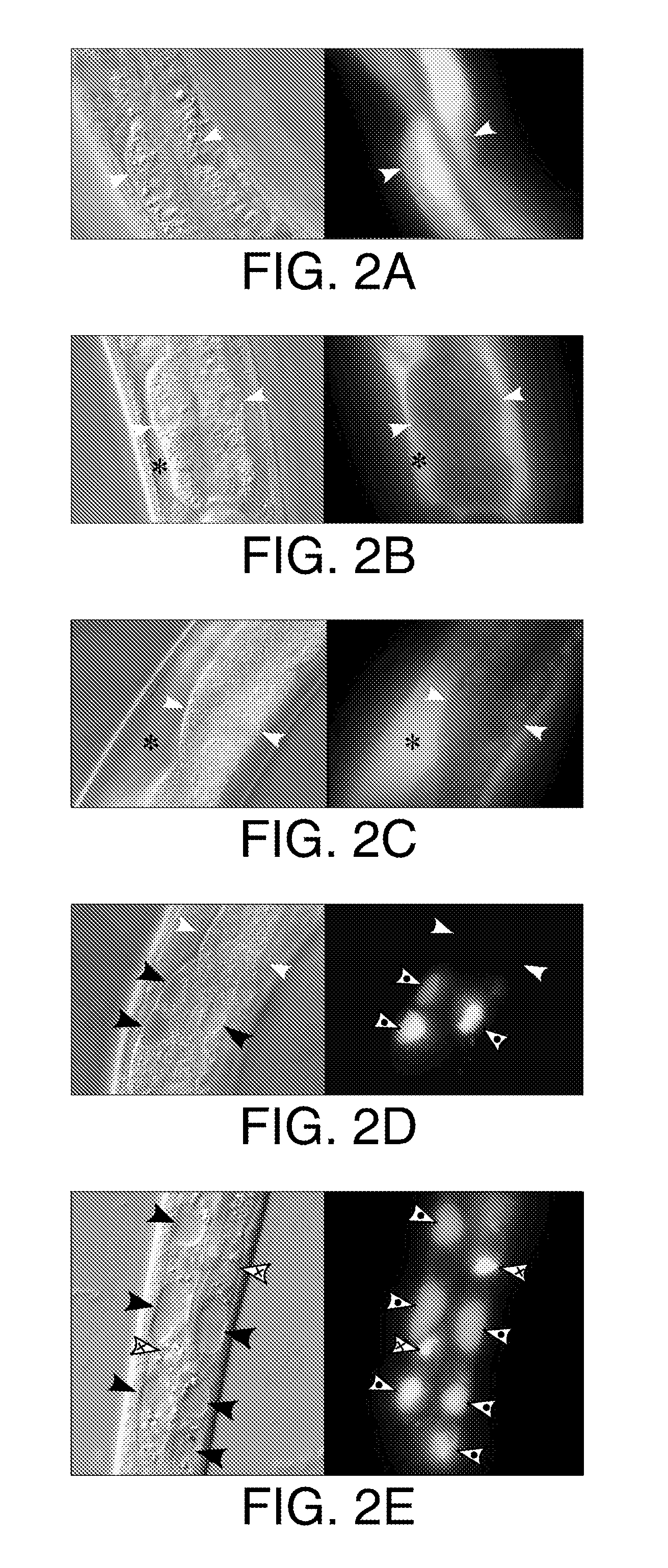
The present invention relates to methods of treatment of clinical disorders associated with protein aggregation comprising administering, to a subject, an effective amount of an anti-protein aggregate (“APA”) compound selected from the group consisting of pimozide, fluphenazine (e.g., fluphenazine hydrochloride), tamoxifen (e.g., tamoxifen citrate), taxol, cantharidin, cantharidic acid, salts thereof and their structurally related compounds. It is based, at least in part, on the discovery that each of the aforelisted compounds were able to promote degradation of aggregated ATZ protein in a Caenorhabditis elegans model system. According to the invention, treatment with one or more of these APA compounds may be used to ameliorate the symptoms and signs of AT deficiency as well as other disorders marked by protein aggregation, including, but not limited to, Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease.
Owner:UNIVERSITY OF PITTSBURGH
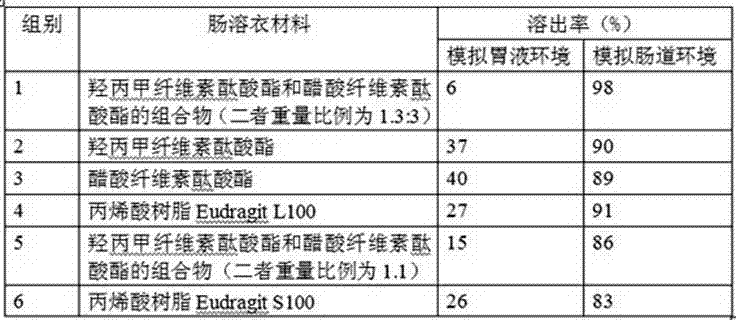
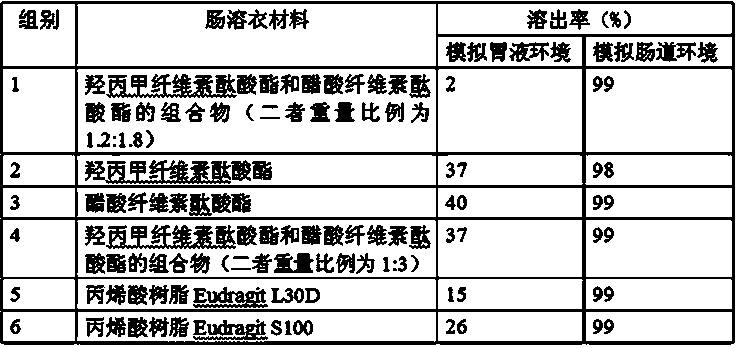
Tamoxifen citrate enteric coated particles
ActiveCN103393604AReduced stabilityImprove stabilityOrganic active ingredientsGranular deliveryTamoxifen CitrateBiochemistry
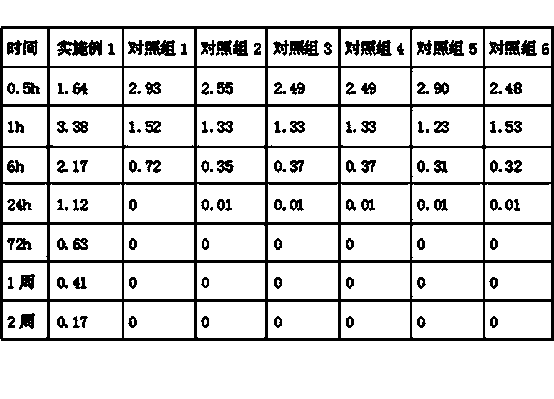
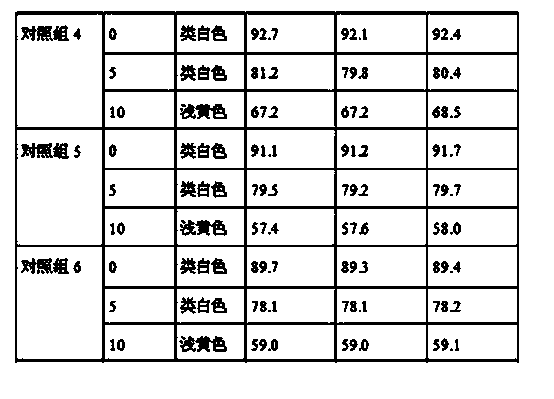
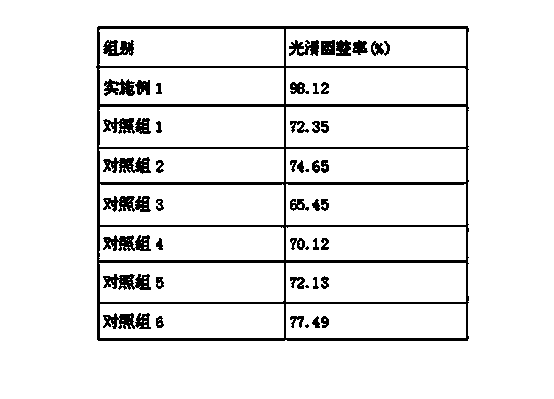
The invention relates to tamoxifen citrate enteric coated particles which are prepared by the following steps: firstly, preparing tamoxifen citrate into inclusion compounds; and then, preparing the enteric coated particles.
Owner:NANTONG GUANGTAI BIOCHEM PROD
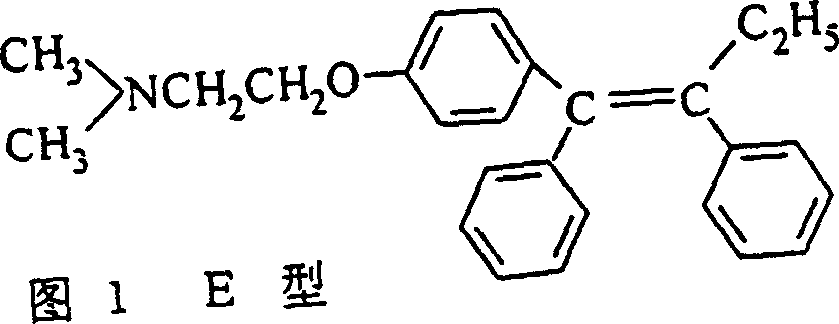
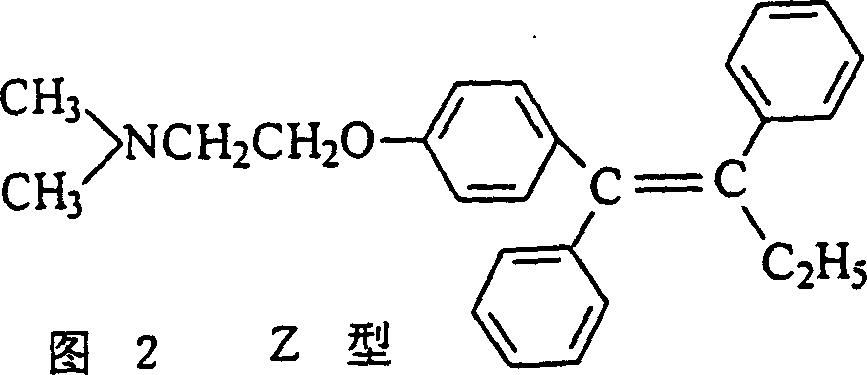
Preparation method of tamoxifen citrate E isomer
ActiveCN103992234AResolve detectionOrganic compound preparationAmino-hyroxy compound preparationOrganic solventTamoxifen Citrate
The invention provides a preparation method of a tamoxifen citrate E isomer, which comprises the following steps: 1) with an intermediate for preparing tamoxifen citrate and having a structural formula as shown in the formula I as a raw material, performing a dehydration reaction in an acid condition in a mixed solution of water and organic solvent at certain proportion to obtain a mixture of an intermediate 1 with a structural formula as shown in the formula II and an intermediate 2 with a structural formula as shown in the formula III; 2) in an organic solvent of certain amount, enabling the intermediate 1 and the intermediate 2 to react with citric acid or hydrate thereof, and cooling for crystallization to obtain a mixture of Z-tamoxifen citrate with a structural formula as shown in the formula IV and E-tamoxifen citrate with a structural formula as shown in the formula V; and 3) in the water and organic solvent at certain proportion, performing twice recrystallization of the mixture of Z-tamoxifen citrate and E-tamoxifen citrate. The method provided by the invention can be used for preparing a high-purity tamoxifen citrate E isomer, provides an impurity reference substance for the National Institutes for Food and Drug Control, and solves the problem in E-isomer detection in a practical production process.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
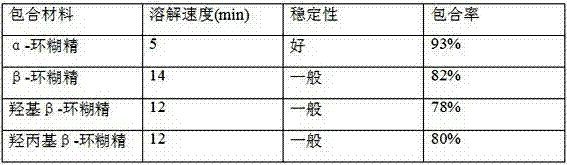
Tamoxifen citrate dropping pill
ActiveCN103494787AReduced stabilityOrganic active ingredientsPharmaceutical delivery mechanismSodium phosphatesTamoxifen Citrate
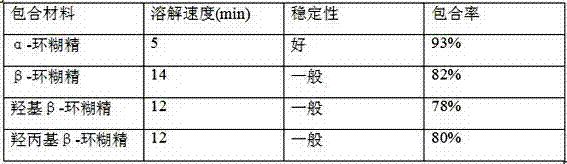
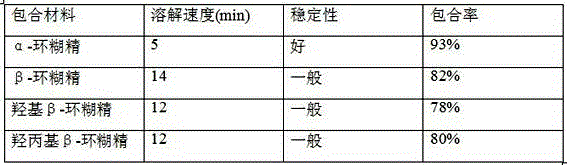
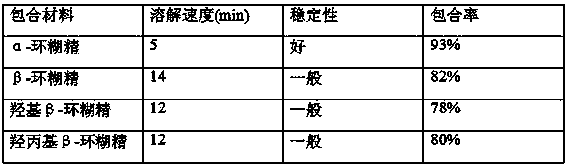
The invention relates to a dropping pill, and particularly relates to a slow-release dropping pill containing tamoxifen citrate clathrates. The tamoxifen citrate is a clathrate with alpha-cyclodextrin as a clathration material, and the weight ratio of tamoxifen citrate to the clathration material is 1:2; the slow-release dropping pill comprises tamoxifen citrate clathrates, a mixture of poloxamer, polyethylene glycol 800, and stearic acid, and a composition of sodium hydrosulphite and sodium phosphate; the condensate liquid is dimethicone.
Owner:NANTONG GUANGTAI BIOCHEM PROD
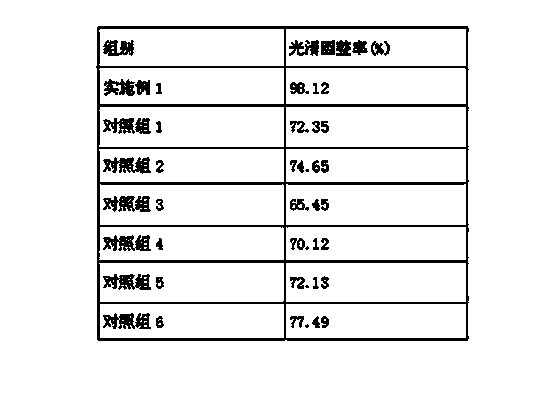
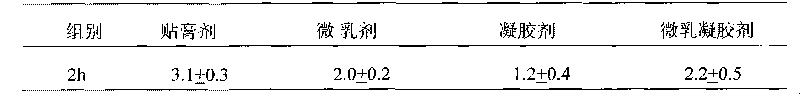
Tamoxifen citrate emplastrum and preparation method thereof
InactiveCN101711754AConvenient treatmentStabilize local blood drug concentrationOrganic active ingredientsSexual disorderTransdermal patchAdditive ingredient
The invention relates to a tamoxifen citrate emplastrum and a preparation method thereof. A tamoxifen citrate micro emulsion is prepared into a transdermal emplastrum, and the transdermal emplastrum is divided into four layers: an anti-adhesion layer, a viscose layer, a back lining layer and a medicine storage layer. The tamoxifen citrate micro emulsion is used as a drug effect ingredient and comprises tamoxifen citrate used as an active ingredient, polysorbate 80 used as a surfactant, propylene glycol used as a cosurfactant, pure water or distilled water, and the like. Besides the drug effect ingredient, some non-polar polymers, a plasticizer, a tackifier, transdermal enhancer and an antioxidant are also added to the emplastrum. Compared with the traditional dosage forms such as tablets, solutions, oral cavity disintegrants, dispersants and external micro emulsion preparations, the tamoxifen citrate emplastrum has the prominent advantages of safety, low toxicity, convenient and simple use and accurate drug dosage.
Owner:河南省生物工程技术研究中心
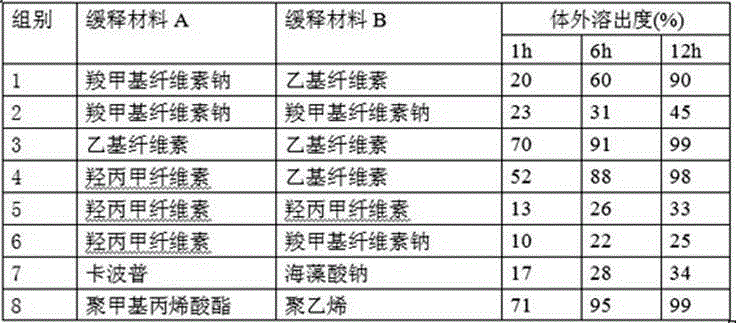
Slow-releasing Tamoxifen citrate tablet
InactiveCN1169523CUniform release rateSimple production processPeptide/protein ingredientsPharmaceutical delivery mechanismTamoxifen CitrateMedicine
Owner:BEIJING CREATE FORTUNE TECH IND GRP CO LTD
Process for preparing high purity low E type Tamoxifen citrate
InactiveCN1554641ASolve long-standing problemsEasy to operateOrganic compound preparationAmino-hyroxy compound preparationAlcoholTamoxifen Citrate
The preparation process of high purity low type-E content Tamoxifen citrate includes dissolving Tamoxifen in dilute acid solution for refining and forming salt with citric acid; or dissolving Tamoxifen citrate in alcohol for refining, and can obtain Tamoxifen citrate with content of type-E Tamoxifen citrate isomer not higher than 0.05%. The process has the advantages of simple operation, short separation process and low cost.
Owner:SHANGHAI NEW HUALIAN PHARMA
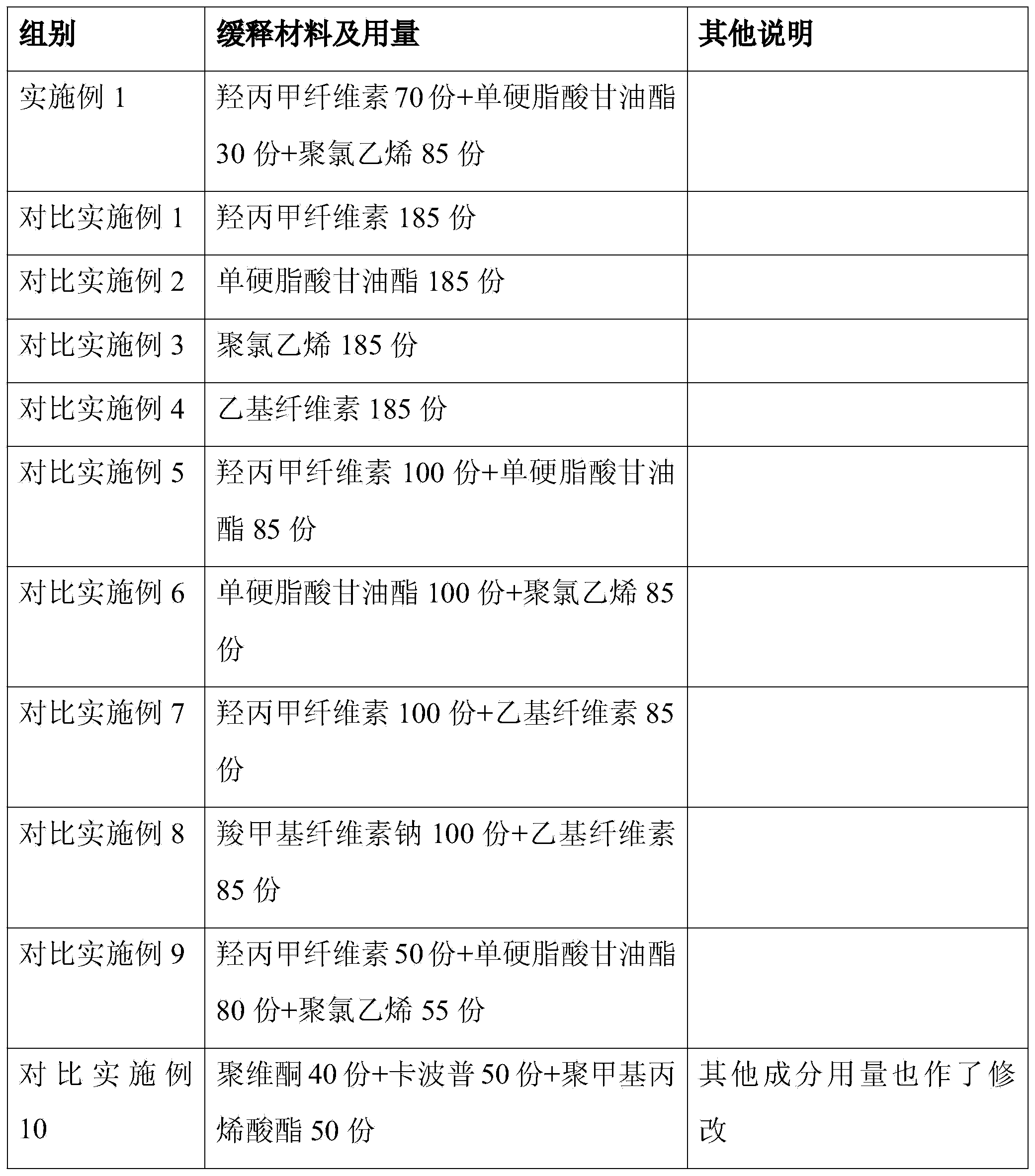
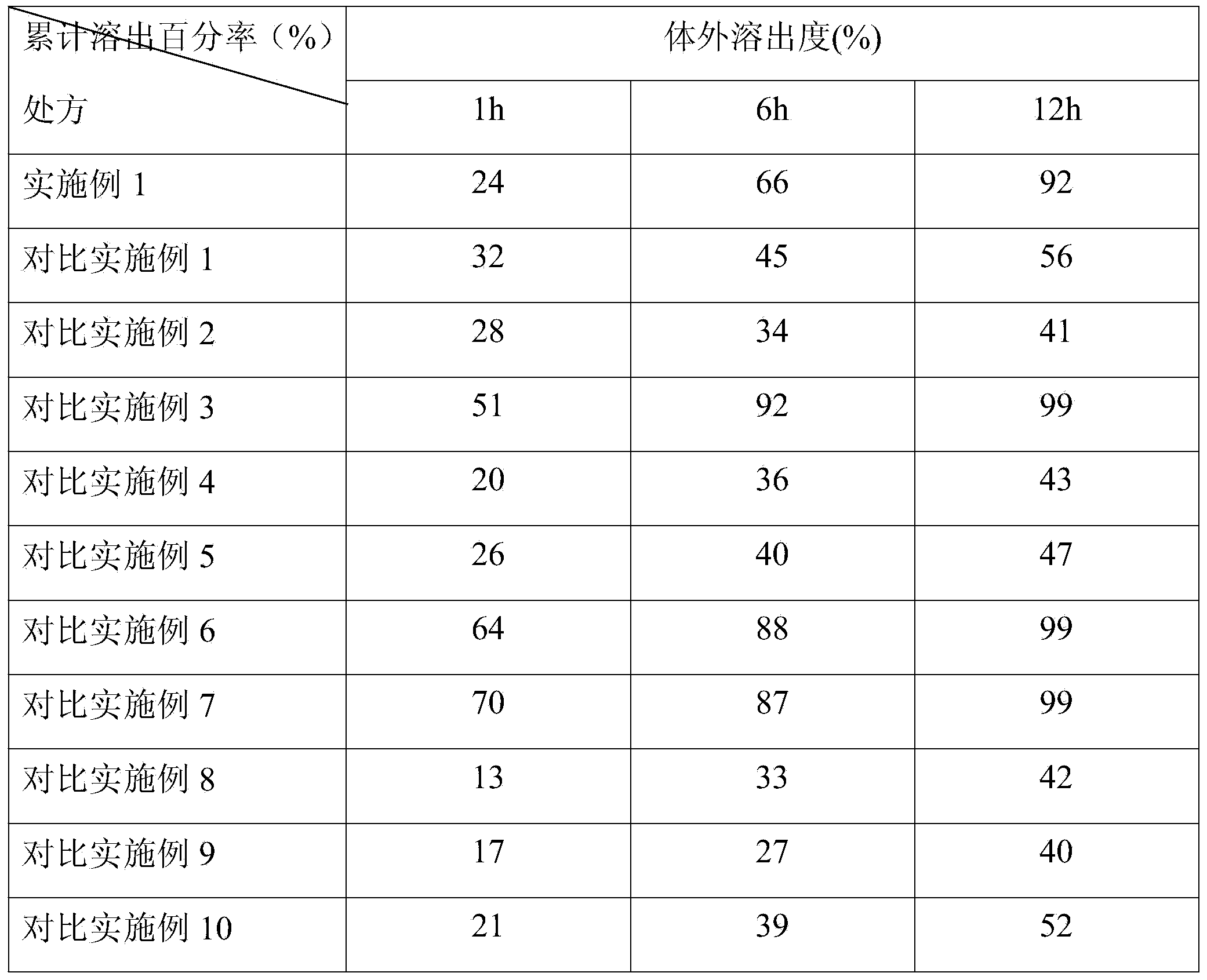
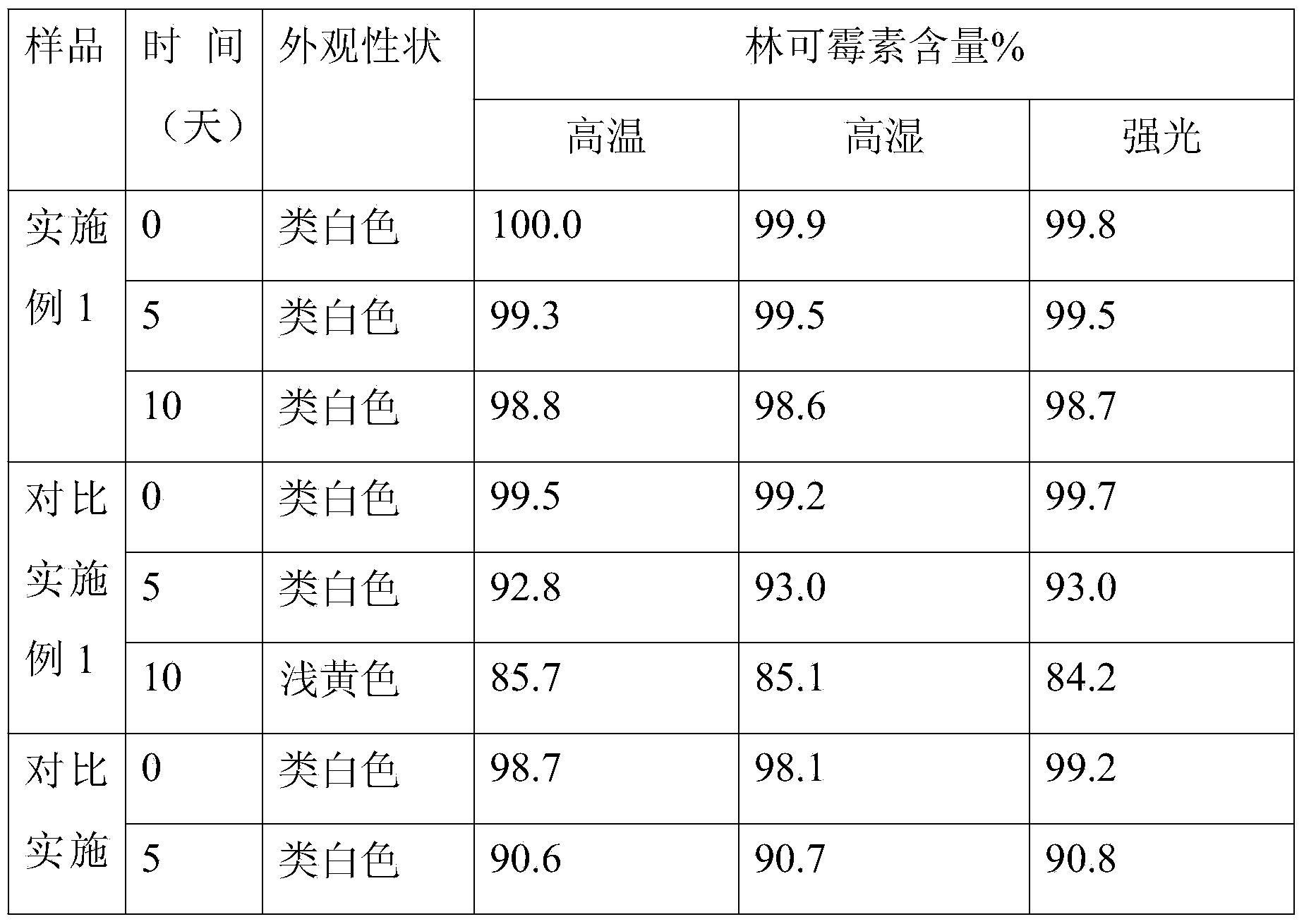
Controlled-release lincomycin granules
The invention relates to controlled-release lincomycin granules. Firstly, tamoxifen citrate is prepared into a clathrate compound and then the clathrate compound is prepared into the controlled-release granules.
Owner:邳州舜邦生物科技有限公司
Tamoxifen citrate enteric-coated tablets
ActiveCN103349648AReduced stabilityOrganic active ingredientsPill deliveryCoated tabletsTamoxifen Citrate
The invention relates to tamoxifen citrate enteric-coated tablets, the preparation method of which comprises the steps of preparing tamoxifen citrates into clathrate compounds and further preparing the clathrate compounds into enteric-coated tablets.
Owner:NANTONG GUANGTAI BIOCHEM PROD
Methods of Treating Disorders Associated with Protein Aggregation
ActiveUS20130024953A1Accurately define objectHigh throughput screeningCompounds screening/testingBiocideDiseaseHuntingtons chorea
The present invention relates to methods of treatment of clinical disorders associated with protein aggregation comprising administering, to a subject, an effective amount of an anti-protein aggregate (“APA”) compound selected from the group consisting of pimozide, fluphenazine (e.g., fluphenazine hydrochloride), tamoxifen (e.g., tamoxifen citrate), taxol, cantharidin, cantharidic acid, salts thereof and their structurally related compounds. It is based, at least in part, on the discovery that each of the aforelisted compounds were able to promote degradation of aggregated ATZ protein in a Caenorhabditis elegans model system. According to the invention, treatment with one or more of these APA compounds may be used to ameliorate the symptoms and signs of AT deficiency as well as other disorders marked by protein aggregation, including, but not limited to, Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease.
Owner:UNIVERSITY OF PITTSBURGH
Tamoxifen citrate sustained-release tablets
ActiveCN103349650AReduced stabilityReduce releaseOrganic active ingredientsPharmaceutical delivery mechanismTamoxifen CitrateProlonged-release tablet
Owner:NANTONG GUANGTAI BIOCHEM PROD
Preparation of acidum citricum tamoxifen polylactic acid-glycolic co-polymer microballoons
InactiveCN101249082BGood biocompatibilityPromote degradationOrganic active ingredientsPharmaceutical delivery mechanismDrug contentMicrosphere
The invention relates to a preparation method of tamoxifen citrate polylactic acid-glycolic acid copolymer microspheres. The method comprises the following steps: 1) dissolving polylactic acid-glycolic acid copolymer (PLGA) in an organic solvent, adding tamoxifen citrate, and dissolving under ultrasonic action to obtain a solution as organic phase; 2) adding slowly the organic phase into aqueous solution of polyvinyl alcohol (PVA) as water phase under stirring, adding electrolytes, stirring at room temperature until the organic solvent volatilizes completely, centrifugating, washing, and freeze-drying to obtain tamoxifen citrate polylactic acid-glycolic acid copolymer microspheres. The microsphere has round shape, uniform particle size distribution (below 10 micrometers), high drug content (above 10%), high encapsulation rate (about 80%), and in vitro release characteristics of long-acting preparations; and is suitable for the use as implant for prevention and treatment of breast cancer.
Owner:DONGHUA UNIV
Tamoxifen citrate particles
ActiveCN103393603AReduced stabilityOrganic active ingredientsGranular deliveryMedicineTamoxifen Citrate
Owner:NANTONG GUANGTAI BIOCHEM PROD
Tamoxifen citrate tablets
ActiveCN103393616BReduced stabilityOrganic active ingredientsPharmaceutical delivery mechanismTamoxifen CitrateBiochemistry
Owner:NANTONG GUANGTAI BIOCHEM PROD
Lincomycin Sustained Release Granules
The invention relates to controlled-release lincomycin granules. Firstly, tamoxifen citrate is prepared into a clathrate compound and then the clathrate compound is prepared into the controlled-release granules.
Owner:邳州舜邦生物科技有限公司
Tamoxifen citrate sustained-release tablets
ActiveCN103349650BReduced stabilityReduce releaseOrganic active ingredientsPharmaceutical delivery mechanismTamoxifen CitrateProlonged-release tablet
Owner:NANTONG GUANGTAI BIOCHEM PROD
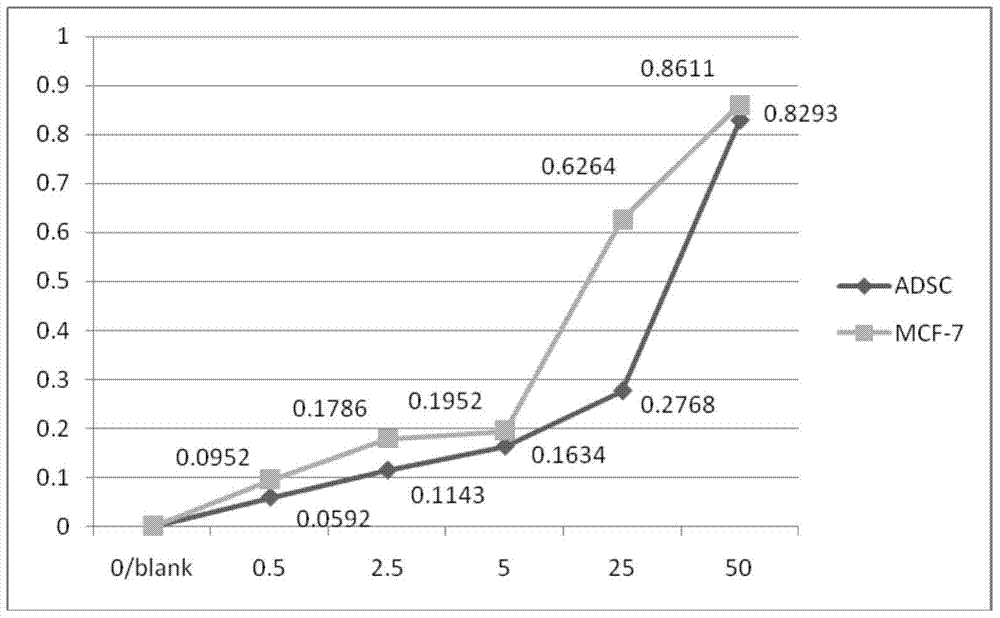
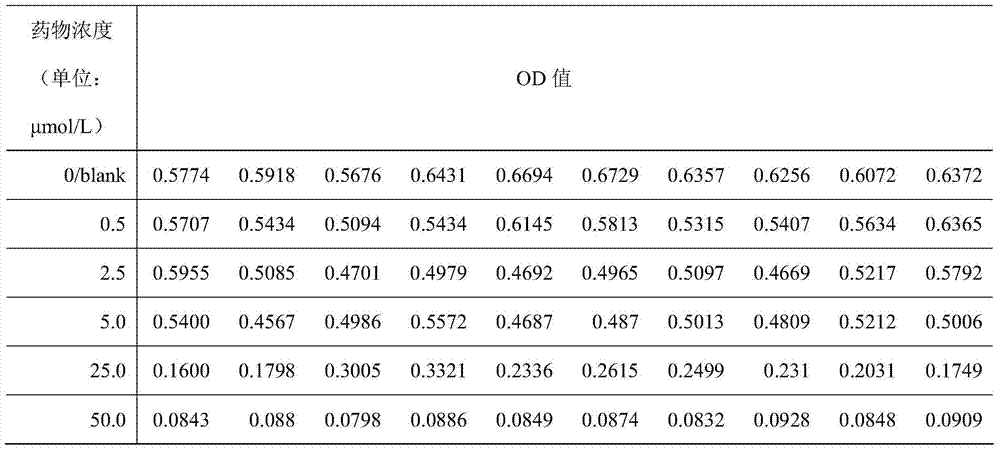
A polypeptide hydrogel scaffold loaded with drugs and fat-derived mesenchymal stem cells and its preparation method
InactiveCN104984403BPlay a sustained release roleFunctionalOrganic active ingredientsMacromolecular non-active ingredientsTamoxifen CitrateMedicine
The invention discloses a polypeptide hydrogel scaffold loaded with drugs and fat-derived mesenchymal stem cells and a preparation method thereof. (1) Use ultrapure water to prepare a tamoxifen citrate solution with a concentration of 1.836×10‑5M~2.651×10‑5M; (2) form a culture plate containing 1.836×10‑5M~2.651×10‑5M The polypeptide hydrogel of 5M tamoxifen citrate; (3) the tamoxifen citrate solution containing 1.836×10‑5M~2.651×10‑5M in the polypeptide hydrogel in the washing step (2) The pH of the solution was 7.0; (4) Take human adipose-derived mesenchymal stem cells at 1.0×105 cells / ml and add 500 μl to each well. After culturing for 3 days, a polypeptide hydrogel scaffold loaded with drugs and adipose-derived mesenchymal stem cells was obtained. The polypeptide hydrogel scaffold of the present invention contains both the anticancer drug tamoxifen citrate and human adipose-derived mesenchymal stem cells, and can be used to construct living tissues.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
Tamoxifen Citrate Dropping Pills
ActiveCN103494787BReduced stabilityOrganic active ingredientsPharmaceutical delivery mechanismSodium phosphatesTamoxifen Citrate
The invention relates to a dropping pill, and particularly relates to a slow-release dropping pill containing tamoxifen citrate clathrates. The tamoxifen citrate is a clathrate with alpha-cyclodextrin as a clathration material, and the weight ratio of tamoxifen citrate to the clathration material is 1:2; the slow-release dropping pill comprises tamoxifen citrate clathrates, a mixture of poloxamer, polyethylene glycol 800, and stearic acid, and a composition of sodium hydrosulphite and sodium phosphate; the condensate liquid is dimethicone.
Owner:NANTONG GUANGTAI BIOCHEM PROD
Tamoxifen citrate sustained-release granules
ActiveCN103349647AReduce releaseReduced stabilityOrganic active ingredientsPharmaceutical non-active ingredientsTamoxifen CitrateOrganic chemistry
Owner:NANTONG GUANGTAI BIOCHEM PROD
Tamoxifen citrate freeze-dried powder injection
ActiveCN103494776BNo stabilizing effectPowder deliveryOrganic active ingredientsEthylenediamine tetraacetateMedicine
The invention relates to a tamoxifen citrate freeze-dried powder injection which comprises tamoxifen citrate, a composition of sorbitol, dextran and lactose in a weight ratio of 3.2:5.5:2, a composition of arginine and glutathione in a weight ratio of 1.6:5.7, and disodium ethylenediamine tetraacetate.
Owner:NANTONG GUANGTAI BIOCHEM PROD
A kind of preparation method of tamoxifen citrate e isomer
ActiveCN103992234BResolve detectionOrganic compound preparationAmino-hyroxy compound preparationOrganic solventTamoxifen Citrate
The invention provides a preparation method of a tamoxifen citrate E isomer, which comprises the following steps: 1) with an intermediate for preparing tamoxifen citrate and having a structural formula as shown in the formula I as a raw material, performing a dehydration reaction in an acid condition in a mixed solution of water and organic solvent at certain proportion to obtain a mixture of an intermediate 1 with a structural formula as shown in the formula II and an intermediate 2 with a structural formula as shown in the formula III; 2) in an organic solvent of certain amount, enabling the intermediate 1 and the intermediate 2 to react with citric acid or hydrate thereof, and cooling for crystallization to obtain a mixture of Z-tamoxifen citrate with a structural formula as shown in the formula IV and E-tamoxifen citrate with a structural formula as shown in the formula V; and 3) in the water and organic solvent at certain proportion, performing twice recrystallization of the mixture of Z-tamoxifen citrate and E-tamoxifen citrate. The method provided by the invention can be used for preparing a high-purity tamoxifen citrate E isomer, provides an impurity reference substance for the National Institutes for Food and Drug Control, and solves the problem in E-isomer detection in a practical production process.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Compositions and methods for topical tamoxifen citrate therapy
ActiveUS9480662B1Adverse drug effectReduce concentrationOrganic active ingredientsSuppositories deliveryMetaboliteN-Desmethyltamoxifen
The present invention provides topical compositions and methods for administering tamoxifen citrate while minimizing the incidence and or severity of adverse drug experiences associated with tamoxifen therapy. In one aspect, these compositions and methods provide a lower plasma concentration of tamoxifen metabolites, such as 4-hydroxytamoxifen and N-desmethyltamoxifen, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient tamoxifen as the sole active agent to benefit a subject with tamoxifen therapy.
Owner:CHOLLET JANET A +1
Tamoxifen citrate liposome and preparation method thereof
ActiveCN110037987AGood film formingLarge particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityFiltration
The invention provides a preparation method of a tamoxifen citrate liposome. The preparation method comprises the steps of enabling tamoxifen citrate, phospholipid and cholesterol to dissolve in a solvent, and performing treatment by a gradient vacuum evaporation method to obtain a liposome film; and performing hydration on the liposome film, and performing filtration so as to obtain the tamoxifencitrate liposome. According to the preparation method, tamoxifen citrate is wrapped with phospholipid and cholesterol mediums to obtain the tamoxifen citrate liposome, so that the toxicity of medicines can be notably reduced, the solubility and the biological availability of the medicines can be improved, and the medicine action time can be prolonged; and besides, the tamoxifen citrate liposome which is obtained by a method for preparing the liposome film through gradient vacuum evaporation of the solvent is better in filming properties, moderate (150-200nm) and uniform in particle diameter,higher in entrapment efficiency (80% or above) and better in stability.
Owner:北京斯利安药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com