Preparation method of tamoxifen citrate E isomer
A technology of tamoxifen and citric acid, applied in the field of drug synthesis, can solve the problems of inability to obtain high-purity products, increased cost, and low content of E isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation method of the high-purity tamoxifen citrate E isomer of the present embodiment, the steps are as follows:
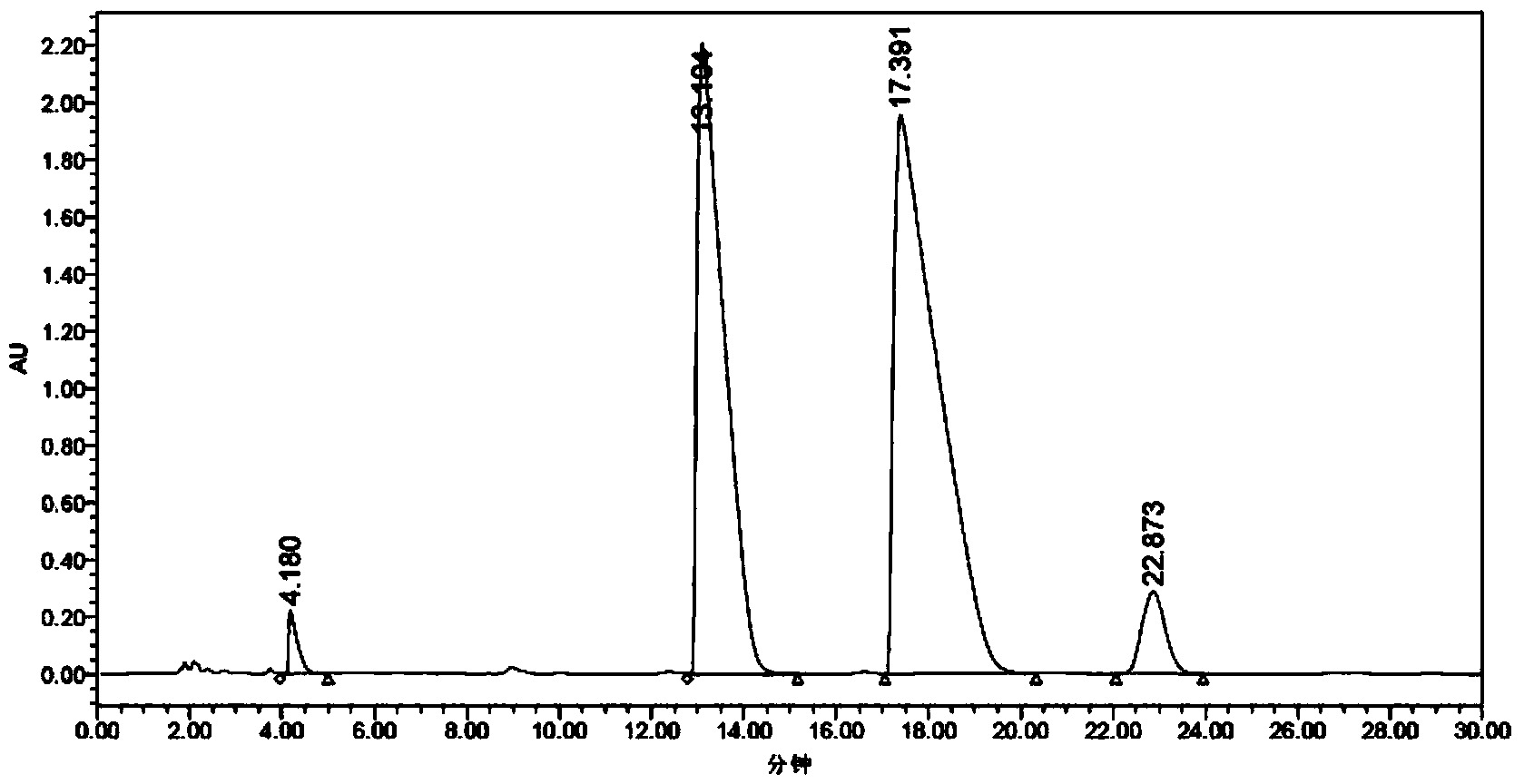
[0057] (1) Preparation of Z-tamoxifen and E-tamoxifen mixture: add 20g of raw material (formula I) to the three-neck flask, add 180ml of isopropanol, stir to dissolve, then add 50ml of hydrochloric acid, heat to reflux for 5h , HPLC monitors that the E isomer is greater than 35% (see HPLC spectrum figure 1), cooled to room temperature and crystallized for 2 hours, filtered, the filter cake can be recycled and reused, and 120ml of water was added to the filtrate, if there were solids, filtered, the filtrate was taken, and the pH was adjusted to strong alkalinity with sodium hydroxide. Extract with ethyl ester until the organic layer has no obvious color, wash with pure water to weak alkalinity, dry over anhydrous sodium sulfate, filter and concentrate to dryness to obtain 10 g of oil, which is a mixture of Z-tamoxifen and E-tamoxifen , E isomer con...
Embodiment 2
[0062] The preparation method of the high-purity tamoxifen citrate E isomer of the present embodiment, the steps are as follows:
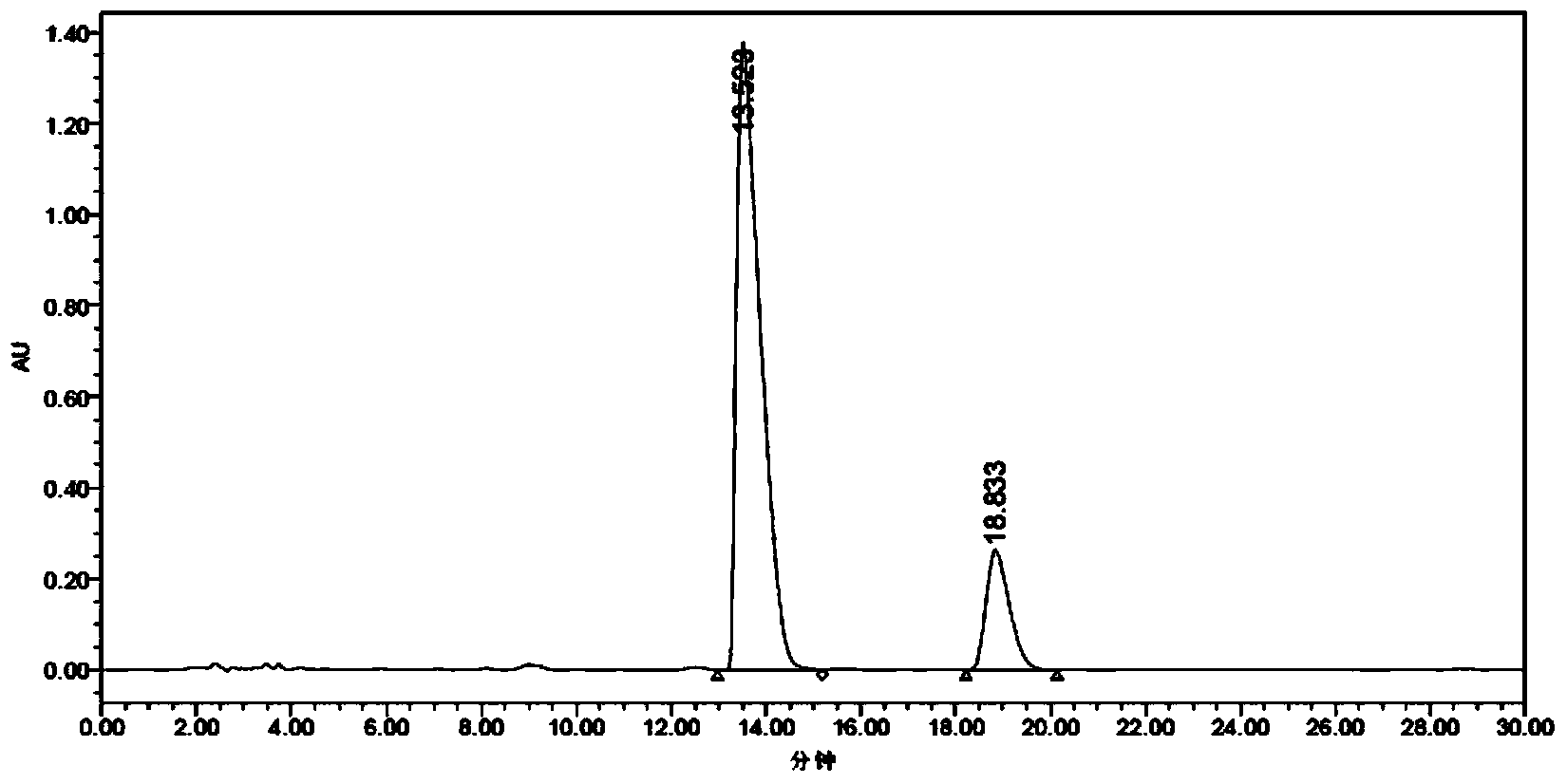
[0063] (1) Preparation of Z-tamoxifen and E-tamoxifen mixture: add 20g of raw material (formula I) to the three-neck flask, add 20ml of methanol, stir to dissolve, then add 20ml of sulfuric acid, heat to reflux for 6h, HPLC Monitoring E isomer greater than 35% (HPLC spectrum see Figure 6 ), cooled to room temperature and crystallized for 5h, filtered, the filter cake can be recycled and reused, add 10ml of water to the filtrate, if there is solid, filter, take the filtrate, adjust the pH to strong alkalinity with sodium carbonate or sodium bicarbonate, and solids are precipitated , extracted with dichloromethane until the organic layer has no obvious color, washed with pure water to weak alkalinity, dried over anhydrous sodium sulfate, filtered and concentrated to dryness to obtain 9g of oily matter, namely Z-tamoxifen and E-tamoxifen A mixture o...
Embodiment 3
[0067] The preparation method of the high-purity tamoxifen citrate E isomer of the present embodiment, the steps are as follows:
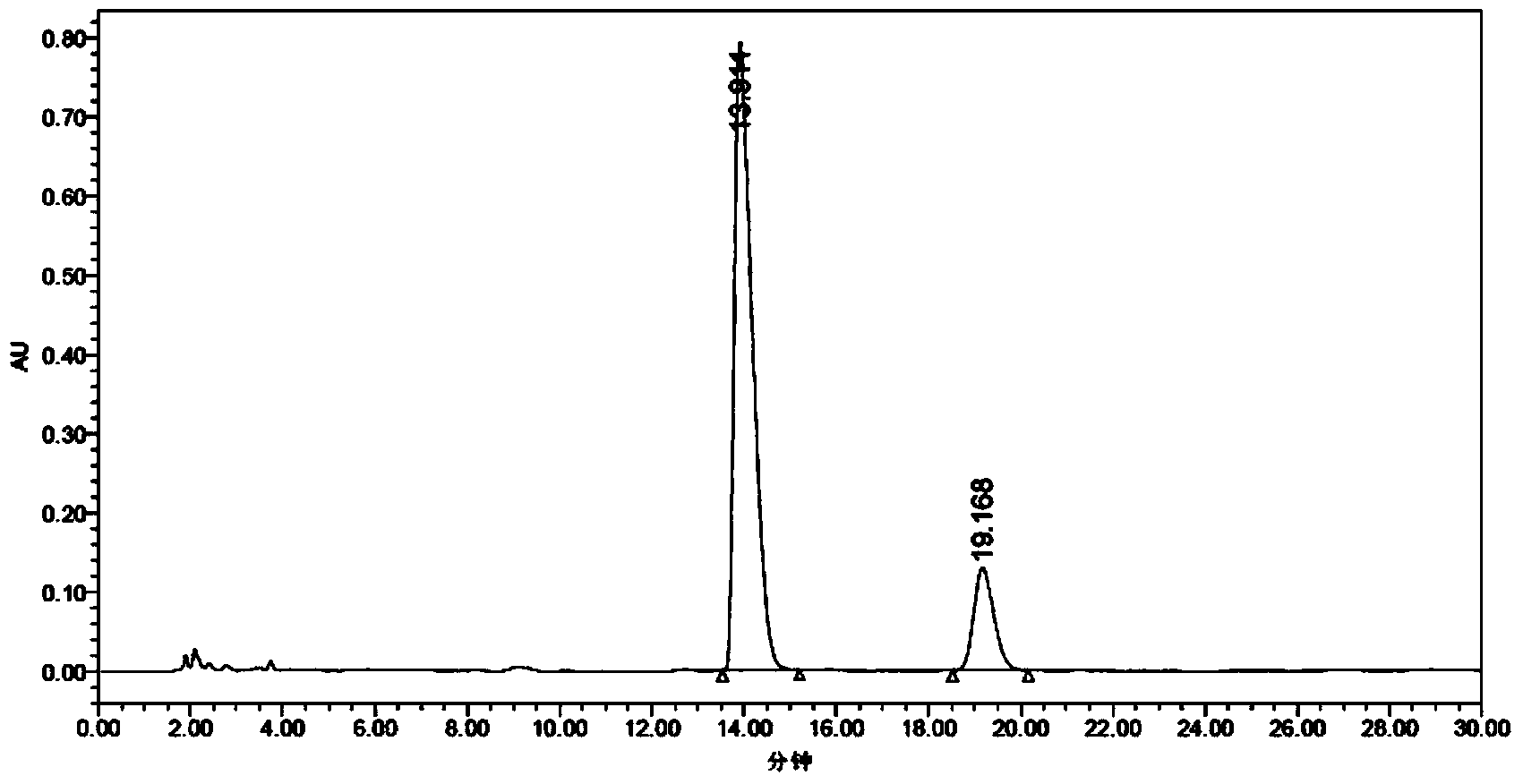
[0068] (1) Preparation of Z-tamoxifen and E-tamoxifen mixture: add 20g of raw material (formula I) to the three-necked flask, add 200ml of toluene, stir, then add 200ml of hydrochloric acid, heat to reflux for 6h, and monitor by HPLC Obtained E isomer greater than 35% (HPLC spectrum sees Figure 11 ), cooled to room temperature and crystallized for 5 hours, filtered, the filter cake can be recycled and reused, and 160ml of water was added to the filtrate, if there were solids, filtered, separated, the water layer was taken, and the pH was adjusted to strong alkalinity with sodium carbonate or sodium bicarbonate, Solids were precipitated, extracted with dichloromethane until the organic layer had no obvious color, washed with pure water until weakly alkaline, dried over anhydrous sodium sulfate, filtered and concentrated to dryness to obtain 6 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com