Preparation method of high-purity tamoxifen citrate
A technology of tamoxifen and citric acid, applied in the field of preparation of high-purity tamoxifen citrate, can solve the problems of high cost, difficulty in obtaining high-purity tamoxifen citrate, etc. Yield, mild reaction conditions, and the effect of saving operating time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
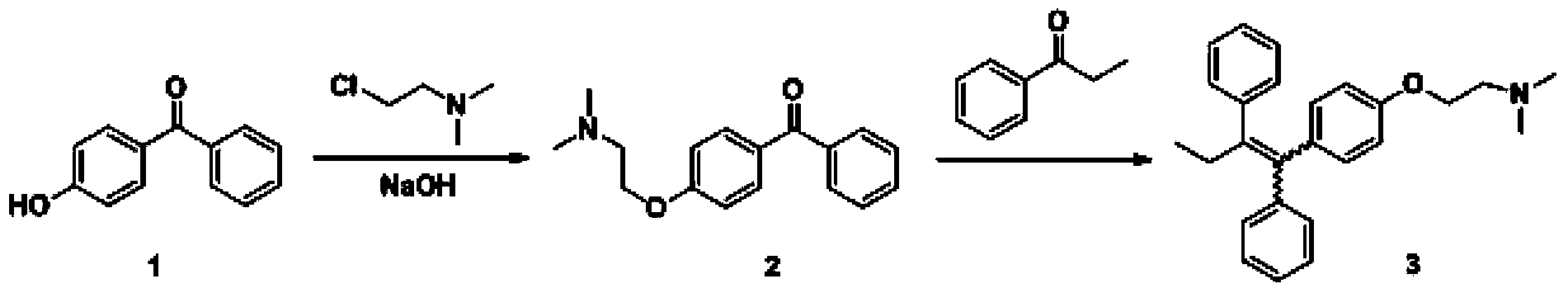
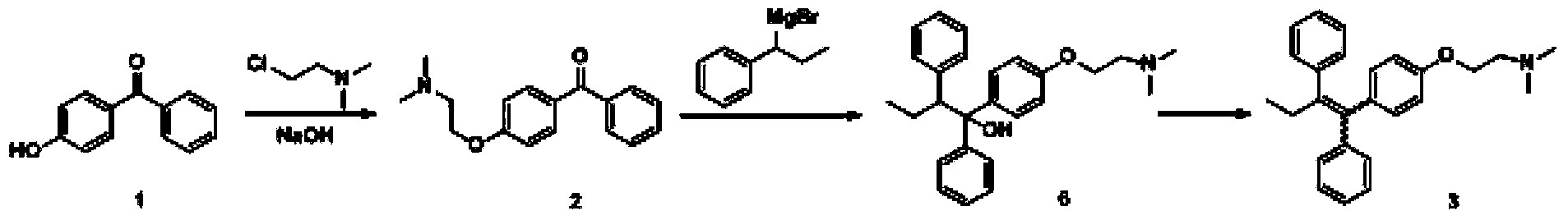
[0037] Embodiment 1: a kind of preparation method of high-purity tamoxifen citrate is characterized in that the specific preparation steps are as follows:
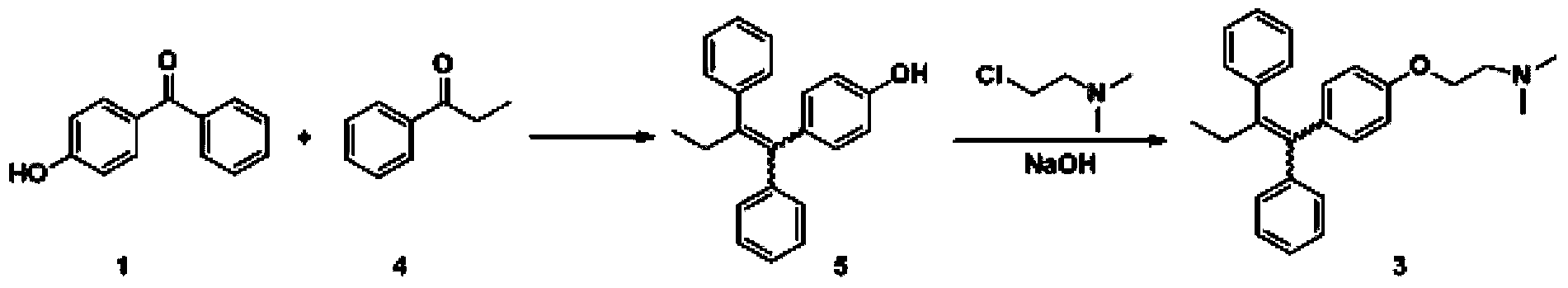
[0038] (1) Coupling reaction: Add 60L tetrahydrofuran (15mL / g) into a 200L reactor, add 6.55kg zinc powder (5 equivalents) under stirring, cool down to -10°C, add dropwise 7.66kg titanium tetrachloride (2 equivalents) ), the temperature was raised to 63-68°C for 1 hour after dropping, and then dropped to 20-25°C after 2 hours of heat preservation. Add dropwise a solution of 20L tetrahydrofuran (5mL / g) dissolved with 4kg 4-hydroxybenzophenone (1.0 equivalent) and 2.71kg propiophenone (1 equivalent). After complete conversion of -hydroxybenzophenone, cool down to 20-25°C, add 60L of 20% potassium carbonate aqueous solution (15mL / g) to the system, separate the organic phase, extract the aqueous phase with 40L of methyl tert-butyl ether, combine the organic phase, concentrated to obtain 7.5kg of crude product 4-(1,2-diphenyl-...
Embodiment 2
[0042] Embodiment 2: a kind of preparation method of high-purity tamoxifen citrate is characterized in that the specific preparation steps are as follows:
[0043] (1) Coupling reaction: Add 60L of ethylene glycol dimethyl ether (15mL / g) to a 200L reactor, add 7.87kg of zinc powder (6 equivalents) under stirring, drop the temperature to -10°C and add 11.48kg of tetrachloride Titanium chloride (3 equivalents), after dropping and keeping warm for 1 hour, the temperature of the system was raised to 60-70°C, and after keeping warm for 2 hours, it was lowered to 20-25°C, and then 4kg of 4-hydroxybenzophenone (1.0 equivalent) and 2.84 kg propiophenone (1.05 equivalent) in 20L ethylene glycol dimethyl ether (5mL / g) solution, after the dripping, the system is heated to 60 ~ 65 ° C, kept warm until the conversion of 4-hydroxybenzophenone is complete, then cooled to 20 ~25°C, add 60L 20% potassium carbonate aqueous solution (15mL / g) to the system, separate the organic phase, extract the...
Embodiment 3
[0047] Embodiment 3: a kind of preparation method of high-purity tamoxifen citrate is characterized in that the specific preparation steps are as follows:
[0048] (1) Coupling reaction: Add 60L 2-methyltetrahydrofuran (15mL / g) to a 200L reactor, add 6.6kg zinc powder (5 equivalents) under stirring, cool down to -10°C, and dropwise add 15.4kg tetrachloride Titanium (4 equivalents), the temperature of the system was raised to 60-70°C after the drop and kept for 1 hour, and the temperature was lowered to 20-25°C after 2 hours of heat preservation, and then 4kg of 4-hydroxybenzophenone (1.0 equivalent) and 4.06kg of 20L of 2-methyltetrahydrofuran (5mL / g) of propiophenone (1.5 equivalents), after the dripping is completed, the temperature of the system is raised to 65-70°C, and the temperature is maintained until the conversion of 4-hydroxybenzophenone is complete. Cool down to 20-25°C, add 60L of 20% potassium carbonate aqueous solution (15mL / g) to the system, separate the organi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com